Introduction
Colorectal cancer (CRC), associated with high
morbidity and mortality rates, has become one of the most common
types of cancer (1). Even with the
improvement in diagnostic and therapeutic approaches, the prognosis
of colorectal cancer remains poor primarily due to local
recurrence, and distal metastases (2). Therefore, the identification of novel
reliable prognostic markers to predict the prognosis, and provide
better and more suitable therapy for patients with colorectal
cancer is warranted.
Secreted protein acidic and rich in cysteine-like 1
(SPARCL1), also known as SC1, Hevin and Mast 9, belongs to the
SPARC-associated family of matricellular proteins (3,4). The
SPARCL1 gene is localized at chromosome 4q22 and is expressed in
various normal tissues, including the brain, heart, lung and
lymphoid tissues (5,6). However, SPARCL1 is not expressed or
expressed at a low level in various cancer types, except in
hepatocellular carcinoma (5,7).
Recent studies have suggested that the most
important function of SPARCL1 is modulating high endothelial cell
adhesion to the basement membrane (3). In addition, SPARCL1 is able to suppress
tumor growth by prolonging the G1 phase and inducing
cell differentiation (8,9).
The prognostic role of SPARCL1 in patients with
colorectal cancer appears to be controversial. Certain studies have
suggested that elevated expression of SPARCL1 is associated with a
better prognosis compared with low SPARCL1 expression, and with
reduced odds ratios (ORs) of lymph node involvement and distant
organ metastasis (9,10). Other studies have demonstrated that
SPARCL1 is a negative regulator in the progression of CRC (11). Notably, the expression of SPARCL1 has
been observed to be increased from Dukes' stages A to B, but then
decreased from Dukes' stages B to C and D (11). The aim of the present study was to
clarify the association between SPARCL1 expression and CRC
progression and explore its prognostic value in patients with
colorectal cancer.
Materials and methods
Tissue samples
The clinical and pathological data of 79 patients
who were diagnosed with colorectal cancer at Dukes' stage B or C,
and underwent radical surgery at Kunshan First People's Hospital
(Kunshan, China) between January 2008 and December 2010 were
reviewed. The mean age was 59.5 years, with 38 males and 41
females. None of the patients had received radiotherapy or
chemotherapy prior to surgery. Thirty pairs of fresh-frozen
colorectal tumors and matched normal tissues were also collected
for total protein extraction and stored at −80°C. Written informed
consent was obtained from all patients and the study was approved
by the ethical approval of Kunshan First People's Hospital Ethics
Committee. Follow-up data were available for all patients and the
duration ranged between 3 and 60 months with a mean of 40.29±2.42
months.
Tissue microarray (TMA)
construction
In each case, three representative tumor regions
were selected, from which tissue cylinders with diameters of 0.6 mm
were arrayed into a recipient block using a tissue chip
microarrayer (Beecher Instruments, Inc., Silver Spring, MD, USA).
Subsequently, the recipient block was cut into 5-µm thick sections
on slides pretreated with adhesion agent (Jiangsu Haimen Shitai
Experimental Equipment Co. Ltd., Haimen, China) to support adhesion
of the tissue samples.
Protein extraction and western
blotting
Thirty pairs of fresh-frozen CRC specimens and
corresponding adjacent normal tissues were used for western
blotting. Total protein of each tissue was extracted using RIPA
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China)
for 10–15 min at 0°C, and then the supernatant was collected, in
which the concentration of protein was measured using a BCA protein
assay kit (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Supernatants from each sample were all mixed with 5X SDS-PAGE
Sample Loading Buffer (Beyotime Institute of Biotechnology, Haimen,
China) and boiled for 8–10 min at 96°C. A total of 15 mg sample was
resolved per lane of 6–12% SDS-PAGE, transferred to polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA), and then
blocked in 5% non-fat dry milk with TBS-Tween 20 for 1–2 h at room
temperature (depending on seasonal changes in room temperature; 1 h
in summer, 2 h in winter). Subsequently, the primary antibodies,
mouse anti-human SPARCL1 polyclonal antibody (1:500; cat. no.
ab107533, Abcam, Cambridge, UK) and mouse β-actin monoclonal
antibody (1:500; cat. no. AA128, Beyotime Institute of
Biotechnology), were used to incubate the membranes in 4°C
overnight. Accordingly, horseradish peroxidase (HRP)-conjugated
goat anti-mouse IgG secondary antibody (1:1,000; cat. no. A0216,
Beyotime Institute of Biotechnology) were used to incubate the
membranes for 1 h at 37°C. Membranes were then detected using an
Enhanced Chemiluminescence Detection system (Beyotime Institute of
Biotechnology). The relative densities of proteins were quantified
using ImageJ software (version 1.8.0; National Institutes of
Health, Bethesda, MD, USA). The formula using to calculate the
relative SPARCL1 expression was as follows: Gray value
(SPARCL1)/gray value (β-actin).
Immunohistochemistry (IHC)
A mouse anti-human SPARCL1 polyclonal antibody (cat.
no. ab107533; 1:200; Abcam, UK) was used as the primary antibody. A
Streptavidin-HRP kit (CW2069A; CWBio, Beijing, China) was used for
IHC according to the manufacturer's protocol. The slides were
deparaffinized, rehydrated and heated in a microwave for 10–15 min
in 10 mmol/l citrate buffer (Sigma-Aldrich; Merck KGaA, Darmstadt
Germany). After the slides were cooled to room temperature, the
slides were treated with reagent1 and reagent2 to block
non-specific staining, and then treated with the primary antibody
for 12 h. Slides were then incubated with reagent3 and reagent4
(anti-rabbit/mouse IgG) for 5 min, then treated with DAB and
hematoxylin for visualization.
Evaluation of immunohistochemical
staining
A stained TMA slide was scanned using electron
microscopy and analyzed with ImageScope software (version 11; Leica
Microsystems GmbH, Wetzlar, Germany). Protein expression was
assessed in a semi-quantitative manner by two pathologists (Dr
Xiao-jiao Gao and Dr Fang Chen; Department of Pathology, Kunshan
First People's Hospital Affiliated to Jiangsu University), who were
unaware of any patient information. The percentage of tumor cells
with staining of the cytoplasm was evaluated as follows: 0%, 0;
1–10%, 1; 11–50%, 2; 51–80%, 3; 81–100%, 4. The staining intensity
was also evaluated as follows: No expression, 0; weak, 1; moderate,
2; or strong, 3. The values were multiplied, resulting in an
immunoreactivity score (IRS) ranging between 0 and 12. Mean scores
calculated from three TMAs were the final numeric values. Samples
were divided into negative/under-expressed (IRS<3) and
positive/over-expressed (IRS>2) levels according to SPARCL1
expression. Any disagreement was resolved by discussion.
Statistical analysis
Data are presented as the means ± standard
deviation. The SPSS software (version 20.0; IBM Corp., Armonk, NY,
USA) was used for statistical analyses and P<0.05 was considered
to indicate a statistically significant difference. Paired
Student's t-tests were used to compare SPARCL1 protein expression
in tumors with normal tissues. Pearson's Chi squared and Fisher's
tests were used to analyze the associations of SPARCL1 expression
with clinicopathological characteristics. In addition, the Cox
univariate and multivariate regression analyses, and Kaplan-Meier
curves with log rank test were also used to analyze overall
survival (OS). Hazard ratios (HRs) with 95% confidence intervals
(CI) were used as the main indicate for OS.
Results
SPARCL1 protein expression in tumors
and normal tissues
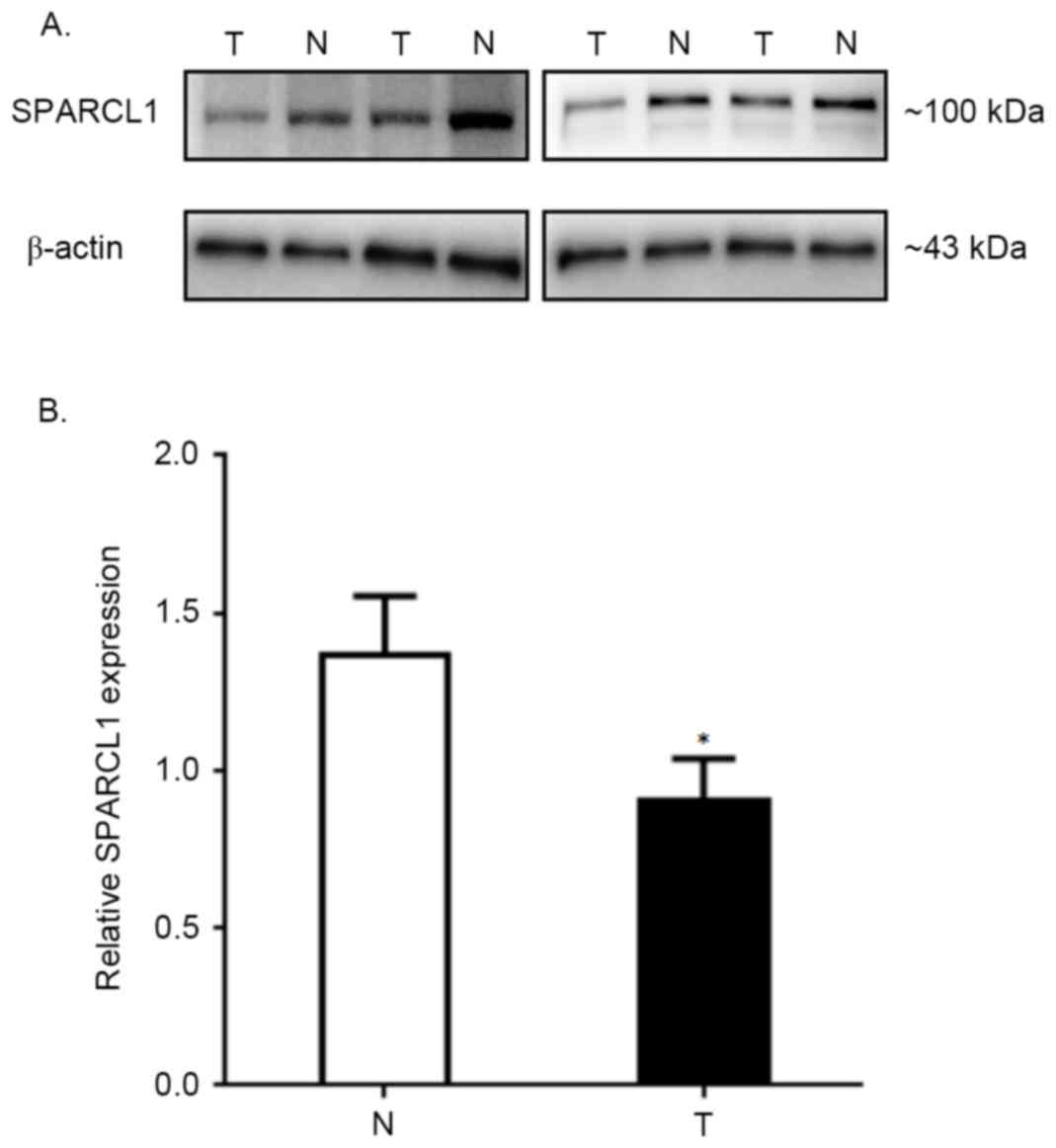
SPARCL1 protein expression in thirty paired CRC and
normal tissues was visualized by western blot analyses. The data of
four representative samples derived from a total of thirty paired
specimens are presented in Fig. 1A.
The comparison of average relative SPARCL1 expression rates between
total thirty tumors and normal tissues are summarized in Fig. 1B (the relative SPARCL1 expression of
tumors vs. normal tissues, was 1.370±0.185 vs. 0.901±0.136;
P<0.0001), which indicated a significantly lower expression
pattern of SPARCL1 protein in colorectal tumors compared with
corresponding normal tissues.
Association between
clinicopathological factors and SPARCL1 expression
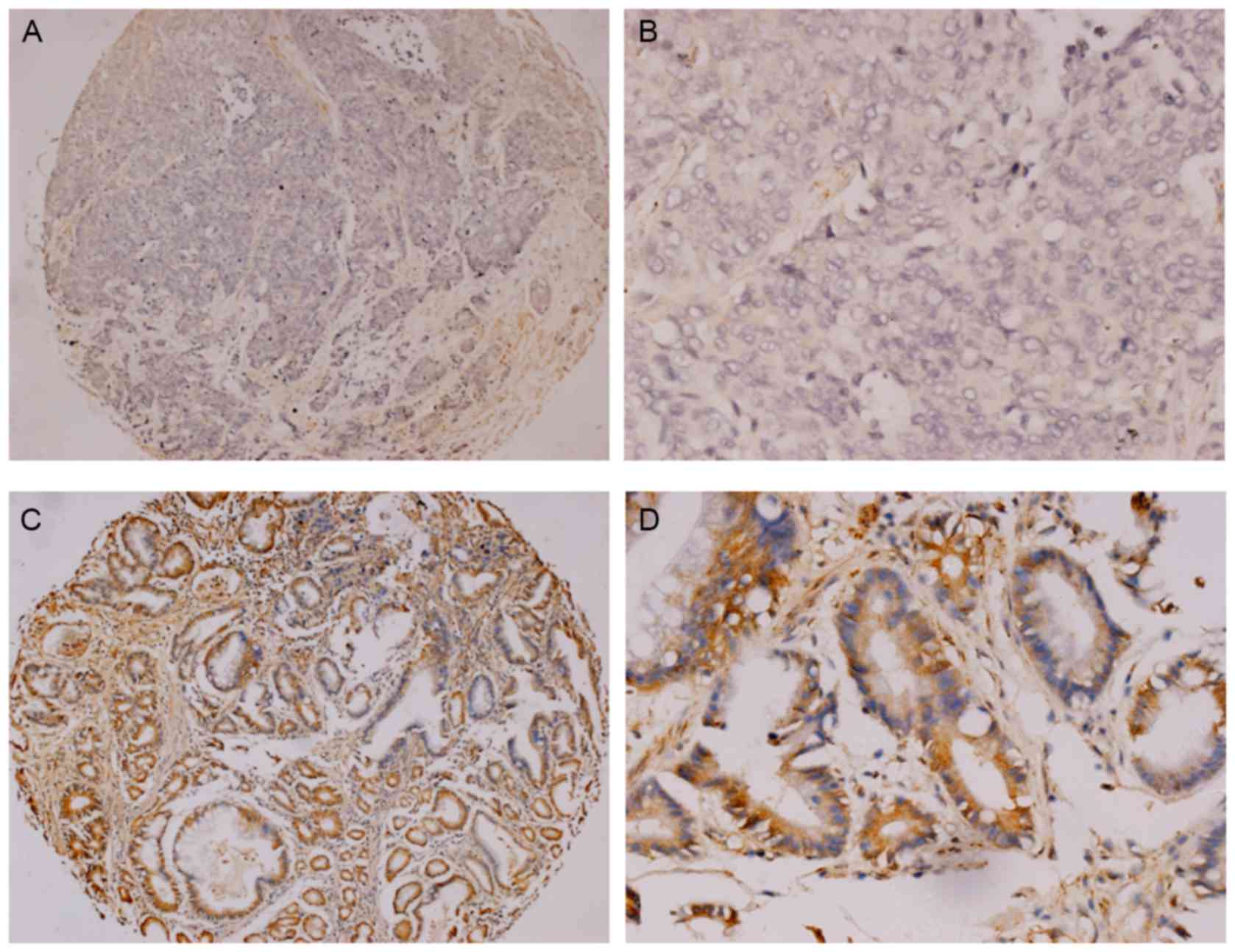
A total of 79 patients with radically resected
Duke's stage B or C tumors, were included in the present study. Of
the 79 patients, 48 cases (60.76%) were negative expression for
SPARCL1 (Fig. 2A and B) and the other
31 (39.24%) were positive (Fig. 2C and
D). Significant differences were identified in the SPARCL1
expression status in relation to differentiation and Duke's stages
(P<0.05; Table I), but no
significant differences were identified among other parameters. The
associations between clinicopathological features of patients with
colorectal cancer and SPARCL1 expression are presented in Table I.
 | Table I.Association between SPARCL1 expression
and clinicopathological variables. |
Table I.
Association between SPARCL1 expression
and clinicopathological variables.
|
|
| SPARCL1 expression
(%) |
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | N | Positive | Negative | χ2
value | P-value |
|---|
| Total | 79 | 31 (39.24) | 48 (60.76) |
|
|
| Age, years |
|
|
|
|
|
| ≤60 | 44 | 21 (47.73) | 23 (52.27) | 3.00 | >0.05 |
|
>60 | 35 | 10 (28.57) | 25 (71.43) |
|
|
| Gender |
|
|
|
|
|
| Male | 38 | 18 (47.37) | 20 (52.63) | 2.03 | >0.05 |
|
Female | 41 | 13 (31.71) | 28 (68.29) |
|
|
| Tumor size, cm |
|
|
|
|
|
| ≤2 | 12 | 3 (25.00) | 9 (75.00) | 1.20 | >0.05 |
| 2–5 | 43 | 18 (41.86) | 25 (58.14) |
|
|
|
>5 | 24 | 10 (41.67) | 14 (58.33) |
|
|
| Location |
|
|
|
|
|
| Right
colon | 37 | 13 (35.14) | 24 (64.86) | −4.69 | >0.05 |
| Left
colon | 18 | 9 (50.00) | 9 (50.00) |
|
|
|
Rectum | 24 | 9 (37.50) | 15 (62.50) |
|
|
| Differentiation |
|
|
|
|
|
| I | 13 | 7 (53.85) | 6 (46.15) | 8.54 | <0.05 |
| II | 44 | 21 (47.73) | 23 (52.27) |
|
|
| III | 22 | 3 (13.64) | 19 (86.36) |
|
|
| Dukes stage |
|
|
|
|
|
| B | 44 | 19 (43.18) | 25 (56.82) | 4.81 | <0.05 |
| C | 35 | 12 (34.29) | 23 (65.71) |
|
|
| Serum CEA, ng/ml |
|
|
|
|
|
|
<5 | 32 | 16 (50.00) | 16 (50.00) | 2.61 | >0.05 |
| ≥5 | 47 | 15 (31.91) | 32 (68.09) |
|
|
| Serum CA19-9,
U/ml |
|
|
|
|
|
|
<37 | 68 | 26 (38.24) | 42 (61.76) | 0.01 | >0.05 |
|
≥37 | 11 | 5 (45.45) | 6 (54.55) |
|
|
| Serum CA12-5,
U/ml |
|
|
|
|
|
|
<35 | 52 | 21 (40.38) | 31 (59.62) | 0.08 | >0.05 |
|
≥35 | 27 | 10 (37.04) | 17 (62.96) |
|
|
|
Complicationa |
|
|
|
|
|
|
Yes | 23 | 7 (30.43) | 16 (69.57) | 1.06 | >0.05 |
| No | 56 | 24 (42.86) | 32 (57.14) |
|
|
| Survival |
|
|
|
|
|
|
Yes | 37 | 17 (45.95) | 20 (54.05) | 1.31 | >0.05 |
| No | 42 | 14 (33.33) | 28 (66.67) |
|
|
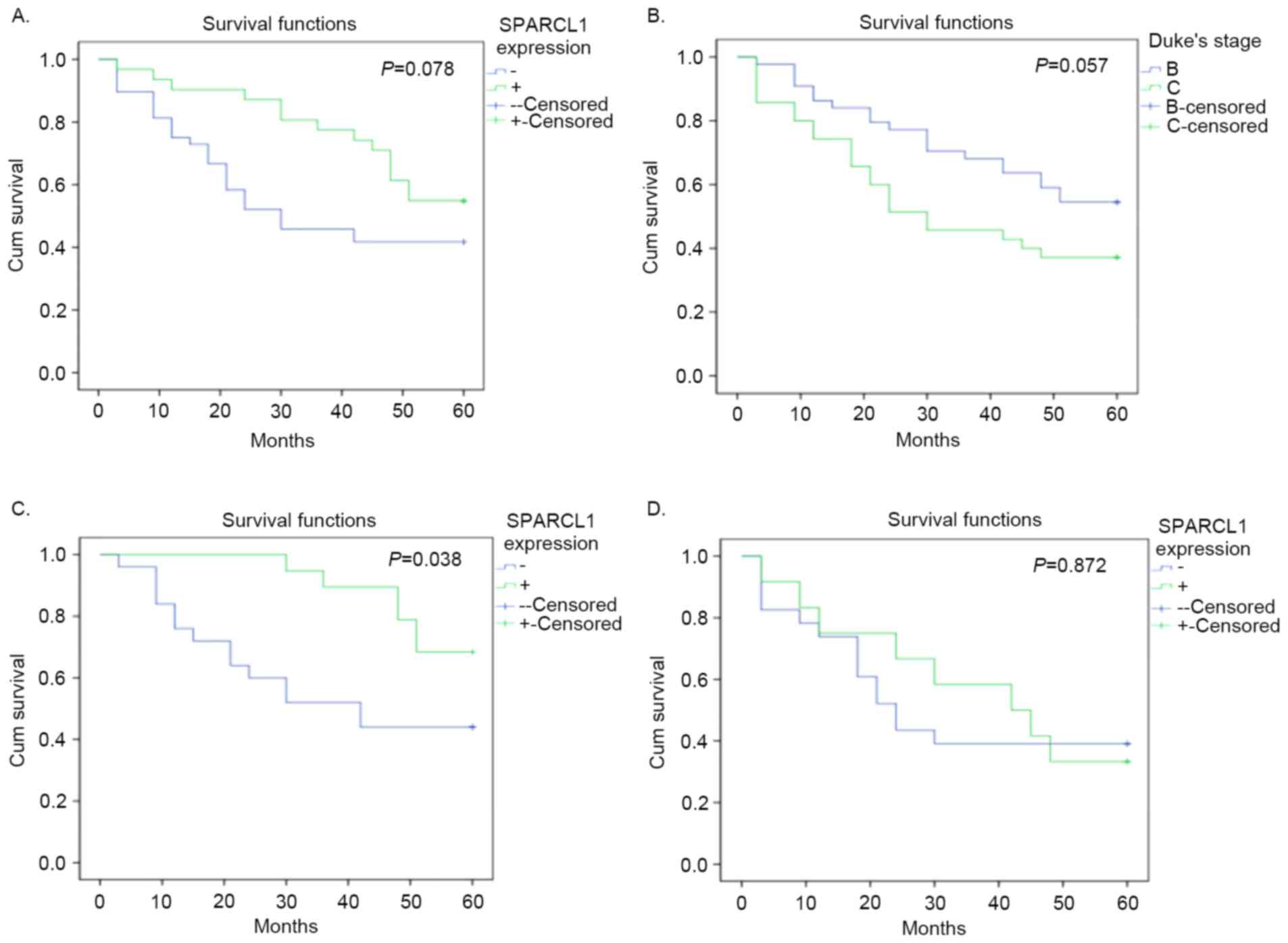
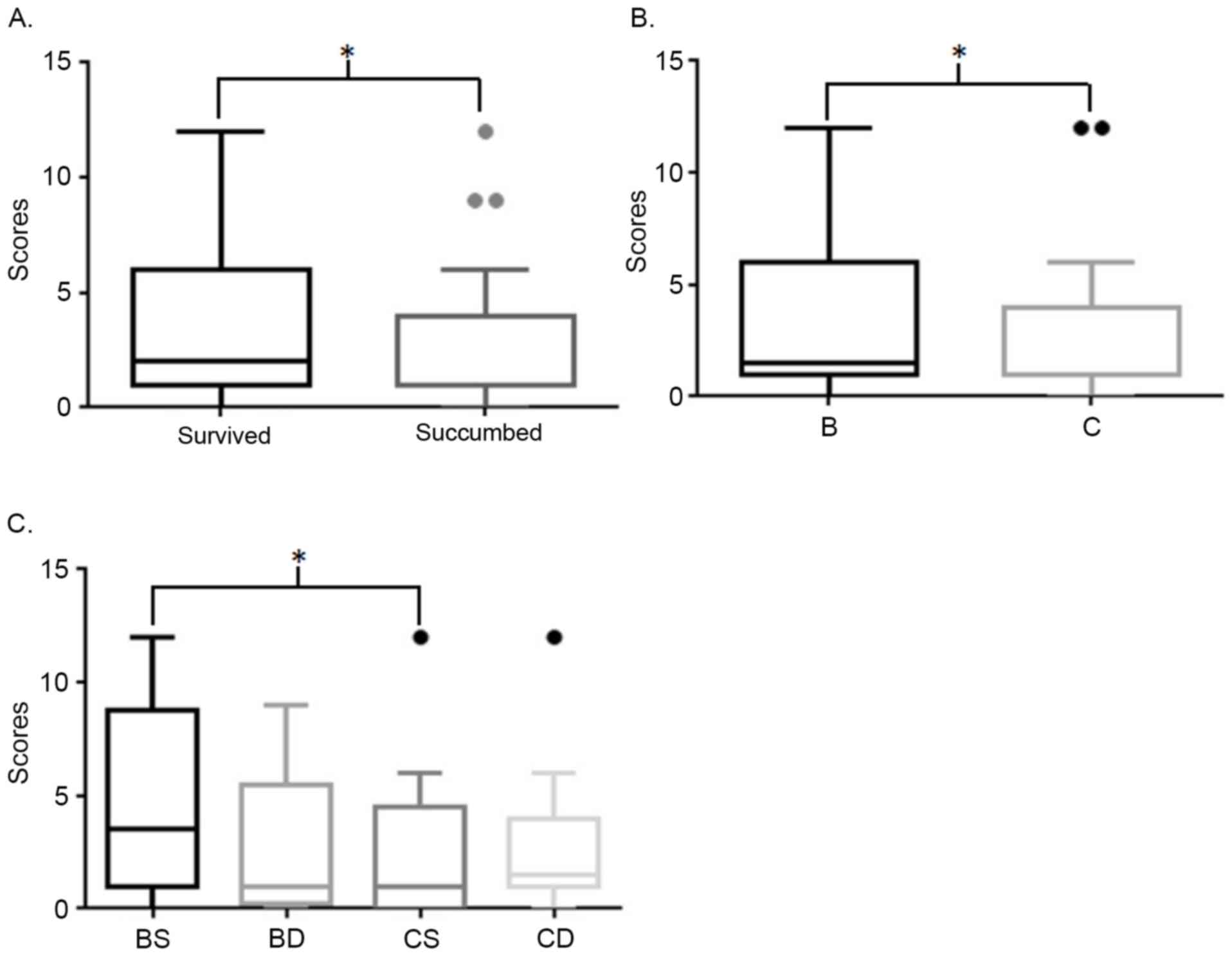
Survival analysis
Although no difference between positive and negative
expression status was identified in overall survival analysis, and
no significance in SPARCL1 expression was detected in the Cox
regression analysis (P>0.05; Tables
I and II; Fig. 4A), immunohistochemical staining scores
of patients who survived were significantly higher compared with
those who succumbed (Fig. 3A). In
addition, scores of patients at Duke's stage B who survived were
significantly higher compared with patients with stage C (Fig. 3). Furthermore, the following Kaplan
Meier curves demonstrated that positive expression of SPARCL1 was a
potentially good indicator of prognosis at Duke's stage B, but not
at stage C (P=0.038 and P=0.872, respectively; Fig. 4). The HR of overall survival
calculated by log rank test, was 0.5755 (95% CI, 0.3108–1.045;
P=0.078). The HR of patients at stage B was 0.3850 (95% CI,
0.1603–0.9277; P=0.038). The HR of patients at stage C was 0.9337
(95% CI, 0.3928–2.198; P=0.872).
 | Table II.Univariate and multivariate analyses
of factors in terms of overall survival. |
Table II.
Univariate and multivariate analyses
of factors in terms of overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
|
| 95% CI |
|
| 95% CI |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Factors | HR | Lower | Upper | P-value | HR | Lower | Upper | P-value |
|---|
| SPARCL1 (+ vs.
-) | 0.572 | 0.301 | 1.090 | 0.090 | 0.613 | 0.313 | 1.203 | 0.155 |
| Differentiation (I
vs. II&III) | 0.378 | 0.122 | 1.173 | 0.092 | 0.529 | 0.158 | 1.774 | 0.303 |
| Differentiation
(I&II vs. III) | 0.937 | 0.472 | 1.860 | 0.851 | 1.084 | 0.532 | 2.212 | 0.824 |
| Duke's stage (B vs.
C) | 0.567 | 0.309 | 1.041 | 0.067 | 1.385 | 0.725 | 2.646 | 0.324 |
Discussion
In the present study, the expression of SPARCL1
protein was investigated in colorectal carcinoma tissues through
western blot analysis and detected a significantly lower expression
in colorectal tumors compared with in adjacent normal tissues. The
associations between SPARCL1 expression, clinicopathological
factors and overall survival in patients with colorectal cancer
were also analyzed. Since it was markedly decreased from
differentiation I to III, it was hypothesized that the
downregulation of SPARCL1 serves an essential role during the
development and progression of colorectal cancer. Due to
significantly higher survival in patients with positive SPARCL1
expression at Duke's stage B, SPARCL1 may be used as a potential
biomarker for the prognosis of colorectal cancer. Despite a recent
study demonstrated that SPARCL1 was a possible biomarker for the
diagnosis of colorectal cancer, Zhang et al (11) reported that upregulated SPARCL1 was
associated with poor survival. This conclusion was different the
results of the present study, whereby SPARCL1 was an indicator of
good prognosis at stage B, but not at C. In addition, another study
reported that high SPARCL1 expression was associated with better
prognosis in patients with rectal cancer with radiotherapy, but not
in patients without radiotherapy (12). Therefore, high SPARCL1 expression may
indicate better prognosis in patients with colorectal cancer at
early stage or with neoadjuvant therapy, implying that SPARCL1 is a
valuable target, and a significant marker of diagnosis. In previous
studies, downregulated SPARCL1 expression was demonstrated to be
significantly associated with lymphatic metastasis and poor grade
of breast cancer, gastric adenocarcinoma, prostate cancer, and lung
adenocarcinoma (13–16). In hepatocellular carcinoma, the
expression of SPARCL1 was elevated in tissues, but overexpression
of Hevin and SPARC significantly delayed tumor growth in
vivo (7).
Gene mutations, loss of heterozygosity (LOH), and
aberrant promoter methylation were three common possible mechanisms
of gene inactivation (17). To the
best of our knowledge, SPARCL1 gene was mapped to chromosome 4,
which involves numerous hotspots for LOH in various tumor types,
and a previous report, which included 10 paired tumor tissues with
matched normal tissues, demonstrated that LOH may be responsible
for the downregulation of SPARCL1 (8,18).
However, following the transfection of SPARCL1 into colorectal
cancer cells, including RKO and SW620 cells, overexpressed SPARCL1
protein significantly inhibited the growth, migration, and invasion
of cancer cells (9). The
aforementioned studies support the results of the current study,
indicating that SPARCL1 may be an indicator of good prognosis.
However, the molecular signaling pathway underlying SPARCL1
interaction remains unclear.
SPARCL1, also known as ‘SPARC-like 1’, exhibits
functions similar or opposite to SPARC to a certain extent
(3,4).
SPARCL1 is putatively counter-adhesive to dermal fibroblasts in a
similar way to that of SPARC; but compared with SPARC, SPARCL1 does
not significantly inhibit the proliferation of cells (19). Likewise, high expression of SPARC and
SPARCL1 may be two independent indicators of good prognosis in
colorectal, and hepatocellular carcinomas (7,9,20); however, in other tumor types, their
prognostic value appeared to be the opposite (18,21–23). In
addition, the SLF (SPARC-like fragment), split from SPARCL1,
similar to SPARC, may antagonize SPARCL1 and regulate synaptogenic
activity (19). However, no previous
study reported the influence of SPARC expression on prognosis of
SPARCL1. In the present study, the prognosis of SPARCL1
overexpression was discussed, but the value of SPARC was not
evaluated. In spite of the result that elevated SPARCL1 expression
in patients at Duke's stage B predicted a better prognosis, the
level of SPARC expression may also influence the outcome.
In the present study, though with no significant
value in Cox regression analysis, it was demonstrated that the
level of SPARCL1 expression was associated with the overall
survival of patients with Duke's stage B. Overexpression of SPARCL1
indicated the patients at Duke's stage B with a longer survival.
This result further suggested that SPARCL1 is an ‘early
oncoprotein’ in colorectal carcinoma. However, in view of the
sample size with 79 patients, which was small, further studies
should be performed to reveal the potential values of SPARCL1.
In conclusion, elevated SPARCL1 expression in
patients with Duke's stage B colorectal cancer, presented an
association with better prognosis. Herein, upregulated SPARCL1 may
be a candidate biomarker of good prognosis for early colorectal
carcinoma.
Acknowledgements
The present study was supported by Jiangsu General
University Program of Practical Innovation of Professional
Postgraduate Degree (grant no. SJLX16_0450), Jiangsu University
Program of Scientific Research (grant no. 15A358), Suzhou Youth
Science and Technology Program of ‘Science and Education’ (grant
no. KJXW2015053), Kunshan Science and Technology Program of Social
Development (grant no. KS1654) and Jiangsu University Science and
Technology Program of Clinical Medicine (grant no.
JLY20160040).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ng L, Poon RT and Pang R: Biomarkers for
predicting future metastasis of human gastrointestinal tumors. Cell
Mol Life Sci. 70:3631–3656. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Girard JP and Springer TA: Cloning from
purified high endothelial venule cells of hevin,a close relative of
the antiadhesive extracellular-matrix protein SPARC. Immunity.
2:113–123. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hambrock HO, Nitsche DP, Hansen U,
Bruckner P, Paulsson M, Maurer P and Hartmann U: SC1/hevin. An
extracellular calcium-modulated protein that binds collagen I. J
Biol Chem. 278:11351–11358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Isler SG, Schenk S, Bendik I, Schraml P,
Novotna H, Moch H, Sauter G and Ludwig CU: Genomic organization and
chromosomal mapping of SPARC-like 1, a gene down regulated in
cancers. Int J Oncol. 18:521–526. 2001.PubMed/NCBI
|
|
6
|
Bendik I, Schraml P and Ludwig CU:
Characterization of MAST9/Hevin, a SPARC-like protein, that is
down-regulated in non-small cell lung cancer. Cancer Res.
58:626–629. 1998.PubMed/NCBI
|
|
7
|
Lau CP, Poon RT, Cheung ST, Yu WC and Fan
ST: Sparc and hevin expression correlate with tumour angiogenesis
in hepatocellular carcinoma. J Pathol. 210:459–468. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Claeskens A, Ongenae N, Neefs JM, Cheyns
P, Kaijen P, Cools M and Kutoh E: Hevin is down-regulated in many
cancers and is a negative regulator of cell growth and
proliferation. Br J Cancer. 82:1123–1130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu H, Zhang H, Ge W, et al: Secreted
protein acidic and rich in cysteines-like 1 suppresses
aggressiveness and predicts better survival in colorectal cancers.
Clin Cancer Res. 18:5438–5448. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu SJ, Yu JK, Ge WT, Hu HG, Yuan Y and
Zheng S: SPARC1, Shp2, MSH2, E-cadherin, p53, ADCY-2, and MAPK are
prognosis-related in colorectal cancer. World J Gastroenterol.
17:2028–2036. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang H, Widegren E, Wang DW and Sun XF:
SPARCL1: A potential molecule associated with tumor diagnosis,
progression and prognosis of colorectal cancer. Tumour Biol.
32:1225–1231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kotti A, Holmqvist A, Albertsson M and Sun
XF: SPARCL1 expression increases with preoperative radiation
therapy and predicts better survival in rectal cancer patients. Int
J Radiat Oncol Biol Phys. 88:1196–1202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao F, Wang K, Zhu R, Hu YW, Fang WZ and
Ding HZ: Clinicopathological significance of reduced SPARCL1
expression in human breast cancer. Asian Pac J Cancer Prev.
14:195–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jakharia A, Borkakoty B and Singh S:
Expression of SPARC like protein 1 (SPARCL1), extracellular
matrix-associated protein is down regulated in gastric
adenocarcinoma. J Gastrointest Oncol. 7:278–283. 2016.PubMed/NCBI
|
|
15
|
Xiang Y, Qiu Q, Jiang M, Jin R, Lehmann
BD, Strand DW, Jovanovic B, DeGraff DJ, Zheng Y, Yousif DA, et al:
SPARCL1 suppresses metastasis in prostate cancer. Mol Oncol.
7:1019–1030. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Isler SG, Ludwig CU, Chiquet-Ehrismann R
and Schenk S: Evidence for transcriptional repression of SPARC-like
1, a gene downregulated in human lung tumors. Int J Oncol.
25:1073–1079. 2004.PubMed/NCBI
|
|
17
|
Lerebours F, Olschwang S, Thuille B,
Schmitz A, Fouchet P, Buecher B, Martinet N, Galateau F and Thomas
G: Fine deletion mapping of chromosome 8p in non-small-cell lung
carcinoma. Int J Cancer. 81:854–858. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li P, Qian JX, Yu GZ, Chen Y, Liu K, Li J
and Wang J: Down-regulated SPARCL1 is associated with clinical
significance in human gastric cancer. J Surg Oncol. 105:31–37.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bradshaw AD: Diverse biological functions
of the SPARC family of proteins. Int J Biochem Cell Biol.
44:480–488. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu QZ, Gao XH, Chang WJ, Wang HT, Wang H,
Cao GW and Fu CG: Secreted protein acidic and rich in cysteine
expression in human colorectal cancer predicts postoperative
prognosis. Eur Rev Med Pharmacol Sci. 19:1803–1811. 2015.PubMed/NCBI
|
|
21
|
Wang Z, Hao B, Yang Y, Wang R, Li Y and Wu
Q: Prognostic role of SPARC expression in gastric cancer: A
meta-analysis. Arch Med Sci. 10:863–869. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Esposito I, Kayed H, Keleg S, Giese T,
Sage EH, Schirmacher P, Friess H and Kleeff J: Tumor-suppressor
function of SPARC-like protein 1/Hevin in pancreatic cancer.
Neoplasia. 9:8–17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han W, Cao F, Chen MB, Lu RZ, Wang HB, Yu
M, Shi CT and Ding HZ: Prognostic value of SPARC in patients with
pancreatic cancer: A systematic review and meta-analysis. Plos One.
11:e01458032016. View Article : Google Scholar : PubMed/NCBI
|