Introduction
Obesity is a major risk factor for a variety of
diseases, including cancer, diabetes and cardiovascular diseases
(1,2),
and is particularly associated with an increased risk of developing
breast cancer in post-menopausal females (3,4). Obese
subjects exhibit more aggressive breast tumors and higher risk of
recurrence. A high body mass index demonstrates a predictive value
for poorer outcome in pre- and post-menopausal patients with breast
cancer (4). In post-menopausal
patients with breast cancer, >50% of mortalities are likely
attributable to obesity (5). Evidence
also suggests that exercise to reduce body weight during and
following medical treatment decreases the risk of mortality by 30%
in patients with breast cancer (6).
Although the association between breast cancer and obesity is well
documented epidemiologically, the molecular mechanisms underlying
this correlation remain incompletely understood.
Normal adult female breast tissue is largely
composed of adipocytes, which significantly out-number epithelial
cells, thereby allowing adipocytes to exert a critical role in
breast development (4,6). It has been demonstrated that adipose
tissue contains specific cells that share characteristics of
pluripotent mesenchymal stem cells isolated from other tissues
(7). These adipose tissue-derived
stem cells (ASCs) include a number of types of cells such as
fibroblasts, pericytes, myofibroblasts, endothelial or
hematopoietic cells and macrophages, which are programmed to
produce different kinds of cytokines, including chemokines and
growth factors (8), and form a
microenvironment that tightly controls the proliferation of
epithelial cells (9). During the
initiation and progression of breast cancer, the cancer cells
reorganize the microenvironment to support their proliferation and
invasion into the surrounding tissue (10). Previous studies have suggested that
cytokine expression in primary human breast cancers, including
interleukin (IL)-6 (11,12), IL-8 (13,14) and
C-C motif ligand 5 (CXCL5) (15)
expression, is associated with reduced differentiation and poor
clinical outcomes. However, it remains to be identified if specific
factors that are involved in the interactions between ASCs and
breast cancer cells may contribute to the tumorigenesis of breast
cells.
To determine if and how ASCs affect the
tumorigenesis and development of breast cancer, different human
breast cancer cell lines (MCF-7 and MDA-MB-231) were used in the
present study to evaluate the effects of ASCs and their products on
cell proliferation, and to identify ASC-secreted cytokines with
cytokine array analysis. To the best of our knowledge, the present
study demonstrated for the first time that CXCL5 secreted by ASCs
in a co-culture medium with human breast cancer cell lines may
mediate the effect of ASCs on breast tumor cell proliferation.
Materials and methods
Ethics
The protocol for the present study was approved by
the Ethics Committee of Harbin Medical University (Harbin, China)
and Heilongjiang Province Institution of Higher Education (Harbin,
China), and it conforms to the provisions of the Declaration of
Helsinki in 1995 (16). All
participants (10 females, aged 25–35 years old) provided their
written informed consent to participate in the study.
Cell culture and reagents
All the culture cells were incubated at 37°C in a
humidified atmosphere containing 5% CO2. The human MCF-7
and MDA-MB-231 breast cancer cell lines and the human WI-38
fibroblast cell line were obtained from the Cell Bank of Chinese
Academy of Sciences (Shanghai, China), where they were
characterized for isozyme, mycoplasma and cell viability detection.
If the cells did not pass these examinations, no further
investigation would be conducted. WI-38 cells were used as a
control for breast cancer cells based on a previous study (17). Human mammary epithelial cells (HMECs;
catalog no., CC-2551), also used as a control, and their culture
medium (catalog no., CC-3150) were purchased from Lonza Group AG
(Basel, Switzerland). MCF-7, MDA-MB-231 and WI-38 cells were grown
in α minimal essential medium (αMEM; Thermo Fisher Scientific,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS,
catalog no., F9665; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
and 100 U/ml penicillin-streptomycin. Cell number was counted using
a hemocytometer, and the number of viable cells present in the
culture was assessed with the Trypan Blue exclusion method
according to the manufacturer's protocol (Trypan Blue Solution was
obtained from Thermo Fisher Scientific, Inc., Waltham, MA, USA;
catalog no., 15250061).
The co-culture of ASCs and cancer cells was
performed using a 24-mm (diameter) chamber with filter inserts
(pore size, 0.4 µm) in 24-well dishes (Corning Costar;
Sigma-Aldrich; Merck KGaA). A total of 5×104 MCF-7 and
MDA-MB-231 tumor cells in 500 µl culture medium were placed in the
upper chamber, while 1×105 ASCs or WI-38 cells were
placed in the lower chamber. Since the pore size of the filter was
smaller than the diameter of either ASCs or cancer cells, these
cells could not pass to the other side of the filter. Accordingly,
cancer cells and ASCs can be separated physically. Cells were grown
in αMEM supplemented with 10% FBS, 10 ng/ml basic fibroblast growth
factor (bFGF, catalog no., 8910; Cell Signaling Technology, Inc.,
Danvers, MA, USA) and 100 U/ml penicillin/streptomycin.
The co-culture medium from the upper chamber was
harvested, centrifuged at 1,842 × g for 5–6 min at room
temperature, and passed through an sterile filter (catalog no.,
SLHV033RS; EMD Millipore, Billerica, MA, USA). The supernatant was
stored at −80°C in aliquots for subsequent use.
Preparation of human ASCs
Using previously described protocols (18), human ASCs were isolated from breast
tissue obtained from reduction mammoplasty procedures, while
abdominal adipose tissue was obtained from abdominal liposuction
procedures in cancer-free donors at the Fourth Affiliated Hospital
of Harbin Medical University (Harbin, China) between March 2011 and
August 2011.
These subjects exhibited no family history of
diabetes or other chronic diseases, had normal glucose tolerance
and body mass index, were free from any major organ diseases, and
demonstrated a stable body weight for at least 1 year. During the
surgical procedures, a hollow blunt-tipped cannula was inserted
into the subcutaneous space through small (~1 cm) incisions. The
cannula was attached to a vacuum device for gentle suction and
moved through the adipose region, while a mixed solution containing
saline and the vasoconstrictor epinephrine (catalog no., E4375;
Sigma-Aldrich; Merck KGaA) was infused into the adipose region to
minimize blood loss and tissue contamination.
The harvested lipoaspirate (~1 g/patient) was washed
extensively with PBS and digested at 37°C for 45–60 min with 0.075%
collagenase (catalog no., 17454; Bio-Sun Sci&Tech Co., Ltd.,
Shanghai, China). Then, the dissociated tissue was filtered by a
100-µm nylon mesh (catalog no., F613463; Sangon Biothech Co., Ltd.,
Shanghai, China) to remove the debris, and the adipocytes were
separated from the stromal vascular fraction by centrifugation at
room temperature at 7,371 × g for 10 min. The pelleted cells were
resuspended and washed with PBS three times. The ASCs were plated
at a density of 2×103/cm2 on 10-cm tissue
culture petri dishes, and incubated for 24 h at 37°C and 5%
CO2 in αMEM supplemented with 10% FBS, 2 mM L-glutamine
(catalog no., ST083; Beyotime Institute of Biotechnology, Haimen,
China) and 100 U/ml penicillin-streptomycin. Following incubation
at 37°C for 30 min, cultures were washed three times with PBS,
provided with fresh medium, and maintained at 37°C and 5%
CO2. Daily washing with PBS was performed to remove
non-attached and red blood cells, and the medium was changed every
3 days. The ASCs were sub-cultured every 5–7 days, and cells
between passages 2 and 6 were used for all experiments. In
preliminary observations, it was revealed that ASCs from
liposuction and breast reduction exhibited the same effect on the
proliferation of breast tumor cell lines. Thus, these cells were
mixed in a 1:1 ratio and used in all the experiments.
Flow cytometric analysis of the
phenotype of ASCs
The ASCs of the third or fourth passage were
harvested by treatment with trypsin-EDTA (catalog no., 25200072;
Thermo Fisher Scientific, Inc.) and then fixed in 1%
paraformaldehyde-PBS. Following fixation, cells were washed three
times with PBS. Cell aliquots (1,200 cells/ml) were stained with
primary antibodies for 30 min at room temperature in the dark. The
primary antibodies were fluorescein isothiocyanate-conjugated
anti-human CD44 (dilution, 1:50; catalog no., MABF1556; EMD
Millipore, Billerica, MA, USA), CD34 (dilution, 1:25; catalog no.
030848; United States Biological, Salem, MA, USA), CD90 (dilution,
1:25; catalog no., C2441-60; United States Biological, Salem, MA,
USA), CD11b (dilution, 1:50; catalog no., 11-0113-42; Thermo Fisher
Scientific, Inc.), CD105 (dilution, 1:50; catalog no., MA5-11854;
Thermo Fisher Scientific, Inc.), CD14 (dilution, 1:25; catalog no.,
033460; United States Biological, Salem, MA, USA) and CD45
(dilution, 1:25; catalog no., 040667; United States Biological)
antibodies. Any unbound antibodies were removed by washing the
cells in Flow Cytometry Staining Buffer (R&D Systems,
Minneapolis, MN, USA). The suspended cells were centrifuged at 300
× g for 5 min at 4°C and the buffer was decanted. Cells were then
resuspended by adding 2 ml of Flow Cytometry Staining Buffer.
Isotype-matched normal mouse immunoglobulin G (IgG) molecules were
used as controls. Flow cytometry was performed on a
fluorescence-activated cell sorter (BD Biosciences, Franklin Lakes,
NJ, USA) in samples from 5 donors from whom ASCs were obtained.
Briefly, ASCs were positive for CD90 (96.23±2.89%), CD44
(98.17±1.35%) and CD105 (97.62±1.56%), and negative for CD14
(0.62±0.15%), CD11b (0.38±0.18%), CD34 (1.25±0.26%) and CD45
(0.59±0.21%). This major phenotype is consistent with the
identified features (positive for CD90, CD44 and CD105; negative
for CD14, CD11b, CD34 and CD45) of human ASCs (19,20). This
result also indicated that the ASCs used in the present study were
primarily fibroblasts and myofibroblasts (8,9), thereby
validating the usage of WI-38 cells as controls. Data analysis was
conducted using FCS Express 5.0 software (De Novo software,
Glendale, CA, USA).
Cytokine antibody array
An antibody-based cytokine array system was used to
detect the levels of growth factors and cytokines in the
supernatants from co-culture media. In order to minimize the effect
of exogenous cytokines and growth factors present in FBS, the FBS
concentration in the co-culture medium was reduced to ~1% (v/v).
The experiments were performed using the RayBio G-Series Human
Cytokine Antibody Array kit (catalog no., AAH-CYT-G2000;
RayBiotech, Norcross, GA, USA) to detect the expression of 174
cytokines according to the manufacturer's protocol. The cell-free
supernatant was used undiluted. The signal intensity was quantified
by light densitometry. ASC/MCF-7 co-culture media and
ASC/MDA-MB-231 co-culture media were examined once. WI-38/MCF-7
co-culture media and WI-38/MDA-MB-231 co-culture media were used as
positive controls to normalize the results.
Anti-CXCL5 treatment
Co-cultured ASCs and tumor cells at the density of
1×104/cm2 were incubated at 37°C with an
anti-human CXCL5 monoclonal antibody (catalog no., MAB 254, R&D
Systems) diluted to 2.5 µg/ml to neutralize CXCL5 or with a mouse
monoclonal IgG1 isotype control diluted to 2.5 µg/ml (catalog no.,
MAB 002; R&D Systems, Minneapolis, MN, USA) for 4 days, which
were placed in the upper and lower sides of the chamber to inhibit
all possible functions of CXCL5. This CXCL5 neutralization
treatment started immediately following co-culturing, and tumor
cells were observed under microscope (Model, CX22RF; Olympus,
Tokyo, Japan) at magnification, ×10, every 24 h, 5–10 visual fields
were randomly selected, and the cell numbers in every visual field
were counted.
Statistical analysis
Data were analyzed with the paired Student's t-test
for comparisons between two independent groups, and with analysis
of variance (ANOVA) for comparisons between three groups. Data were
expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference. Data
analysis was conducted using SPSS 17.0 software (IBM SPSS, Armonk,
NY, USA).
Results
Effects of co-culturing human ASCs
with cancer cells on the proliferation of breast cancer cells
Human ASCs were isolated from adipose tissues and
evaluated for their ability to induce the proliferation of estrogen
receptor (ER)-positive (MCF-7) and ER-negative (MDA-MB-231) breast
cancer cells. As a comparison, the human WI-38 fibroblast cell
line, which had been previously demonstrated to stimulate tumor
growth, was also included (21). The
negative control was set by adding regular growth medium into the
chambers to monitor potential changes in the number of breast
cancer cells. At day 6 after co-culture, the average number of
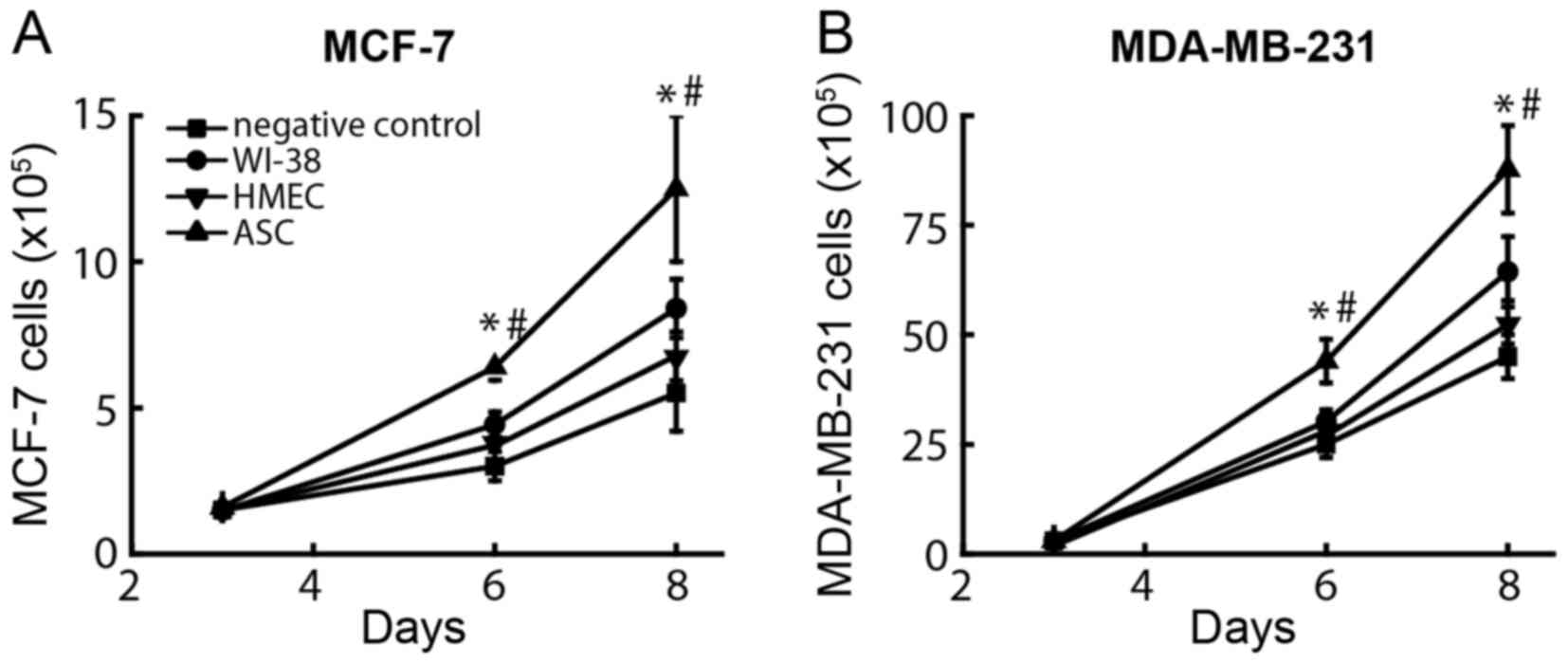
tumor cells was different between different groups (Fig. 1A), namely 6.4±0.7×105,
4.4±0.5×105, 3.8±0.4×105 and
3.0±0.5×105 MCF-7 cells when these were co-cultured with
ASCs, WI-38 cells, HMECs and growth medium, respectively.
Similarly, the numbers of MDA-MB-231 cells (Fig. 1B) co-cultured with ASCs
(44.0±6.1×105) were significantly increased compared
with those observed following co-culture with WI-38 cells
(30.2±3.5×105) and with the negative control
(24.9±2.9×105) (P<0.001). By contrast, the number of
proliferated breast cancer cells only modestly increased in upon
co-culturing with HMECs or WI-38 cells, while the negative controls
exerted no significant effect on breast cancer cell proliferation.
These results suggest that the effect of ASCs was independent of
estrogen, since both ER-positive and ER-negative tumor cells
increased their cell number significantly following co-culture with
ASCs.
Effects of co-culture medium of ASCs
on the proliferation of breast cancer cells
The chambers with ASCs and cancer cells were
separated by a filter, and the pore size of the filter was smaller
than the diameter of either ASCs or cancer cells, therefore, there
was almost no direct contact between breast cancer cells and ASCs
under the current experimental conditions. The ASC-secreted factors
were most likely responsible for the stimulatory effect on tumor
cell proliferation. To evaluate this hypothesis, conditioned
co-culture medium was used instead of ASCs to stimulate breast
cancer cell proliferation. ASCs and breast cancer cells were first
co-cultured for 6 days, and then the supernatant was collected and
used as growth medium to stimulate the proliferation of MCF-7
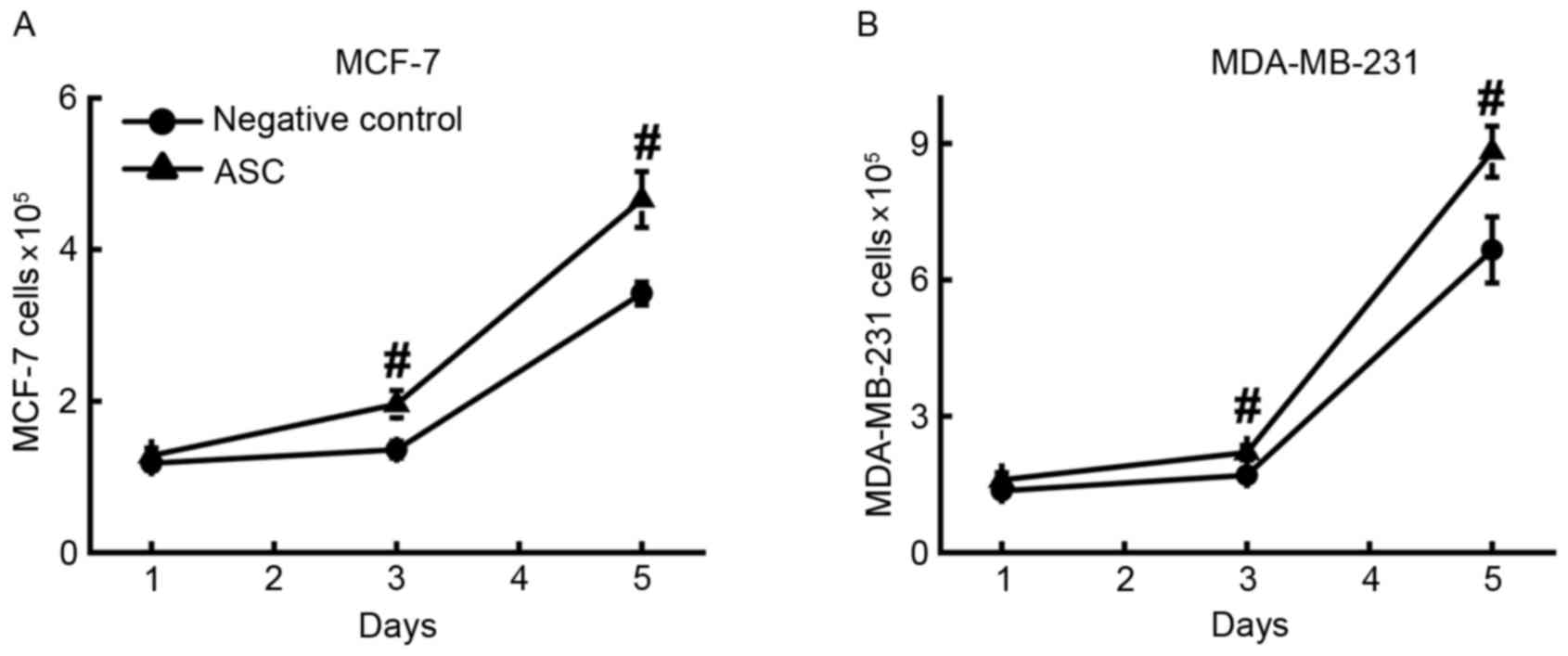
(Fig. 2A) and MDA-MB-231 (Fig. 2B) cells. The results demonstrated that
the numbers of MCF-7 (4.7±0.4×105) and MDA-MB-231 cells
(8.9±0.6×105) in ASC-conditioned medium were
significantly (P<0.001) higher compared with those in their
corresponding controls of regular culture medium. This result
supports the aforementioned hypothesis. It is also notable that the
cancer cells cultured in this growth medium proliferated at a
slower rate than the cancer cells co-cultured with ASCs.
Chemokines in ASC-conditioned
medium
In order to identify the factors that promoted the
proliferation of breast cancer cell lines, an antibody-based
cytokine array that is capable of detecting 174 different growth
factors and cytokines was used to analyze the differences in
factors between control medium and ASC-conditioned medium.
Considering high concentration of FBS would generate high
background noise, the FBS concentration in the culture medium was
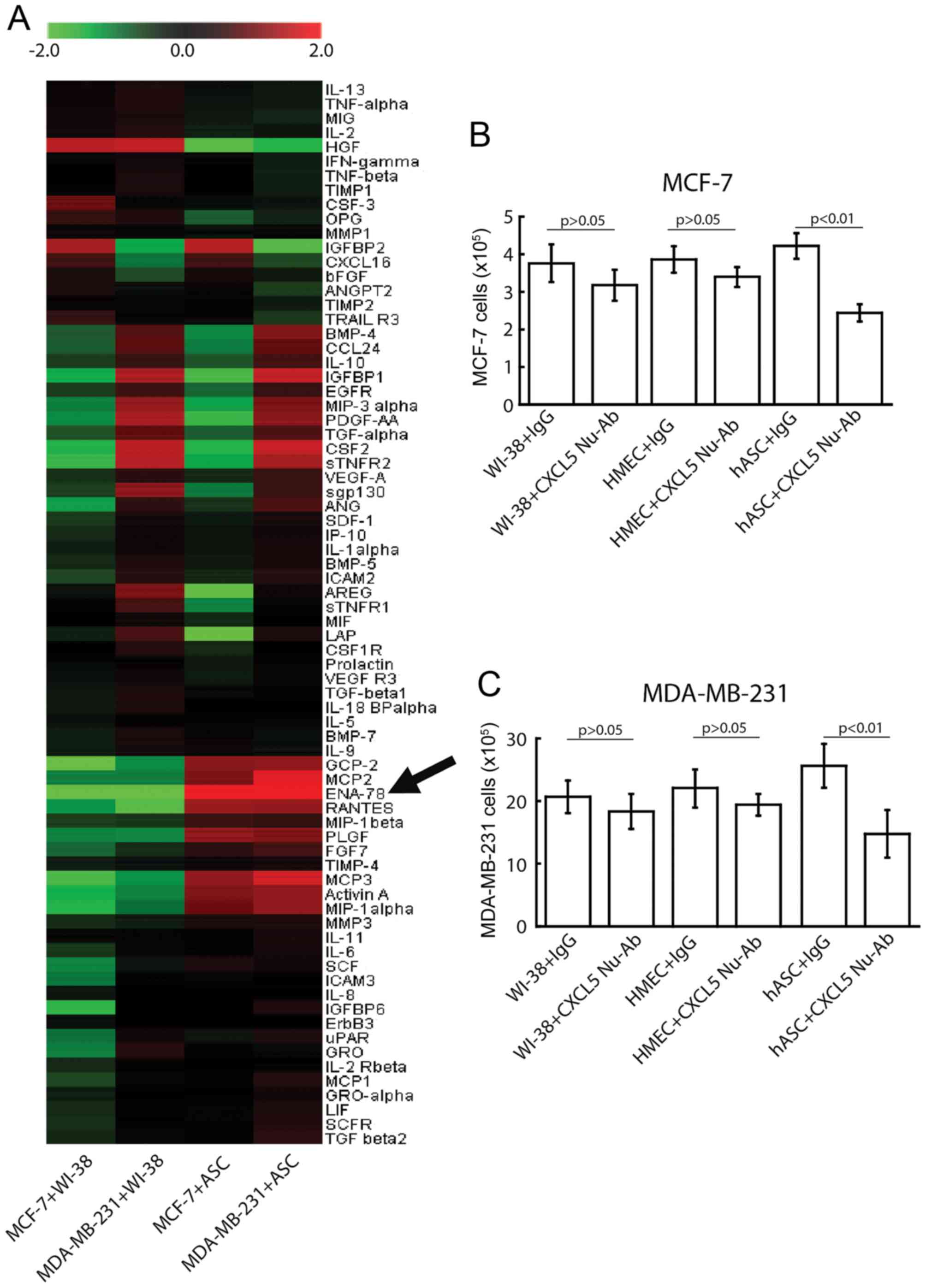
reduced to 1%. As demonstrated in Fig.
3A, a number of typical cytokines and growth factors, including
CXCL5, also known as epithelial cell-derived neutrophil-activating
peptide-78 (ENA-78), monocyte chemotactic protein (MCP) 2, MCP3 and
regulated on activation, normal T cell expression and secreted
(RANTES), were significantly upregulated, at least ≥2-fold vs.
control levels, in ASC-conditioned medium. Among all the detected
factors, increases in the protein level of CXCL5 were most
significant in ASC-conditioned medium. By contrast, the levels of
CXCL5, MCP2, MCP3, ENA-78 and RANTES were not changed significantly
in WI-38/breast cancer cell co-cultures, thus supporting a specific
expression of CXCL5 in ASCs.
Consequences of CXCL5 neutralization
on the stimulatory effect of ASCs
CXCL5 is a chemokine that has been implicated in the
chemotaxis of inflammatory cells (22). CXCL5 may contribute to tumor
metastasis and recurrence of intrahepatic cholangiocarcinoma
(23), and may serve critical roles
in bladder tumor growth and progression (24). To explore its potential on promoting
breast cancer cell proliferation in a paracrine manner, CXCL5 was
neutralized with a CXCL5-specific antibody, and then the effect of
co-culture of WI-38 cells, HMECs or ASCs on the number of breast
cancer cells was observed. In the present study, non-specific
monoclonal IgG1 was used as a control antibody, and WI-38 cells and
HMECs were used as additional controls to highlight the specific
effect of ASC-secreted CXCL5. All experiments were repeated 5
times. The results demonstrated that at day 4 after co-culture
(Fig. 3), CXCL5 depletion by the
above CXCL5-specific antibody blocked the proliferation-promoting
activity of ASCs significantly (P<0.001 by ANOVA) in MCF-7
(4.2±0.6×105 vs. 2.6±0.5×105) and MDA-MB-231
(25.6±4.3×105 vs. 15.8±4.9×105) cells.
Conversely, 4 days after co-culture, the anti-CXCL5 antibody did
not significantly (P>0.05) affect breast cancer cell
proliferation in the WI-38 cells co-cultured with MCF-7
(3.8±0.7×105 with control IgG vs. 3.2±0.6×105
with CXCL5 neutralizing antibody) or MDA-MB-231
(20.7±3.6×105 with control IgG vs.
18.3±3.8×105 with CXCL5 neutralizing antibody) cells.
Similar results were also observed when cancer cells were
co-cultured with HMECs. These data are illustrated in Fig. 3B for MCF-7 cells and Fig. 3C for MDA-MB-231 cells. These results
support the hypothesis that the neutralizing effect of the
anti-CXCL5 antibody was specific to the ASC-conditioned medium.
Discussion
In the present study, it was revealed that ASCs may
promote breast tumor proliferation by releasing specific cytokines.
Among the numerous cytokines secreted by ASCs, the increase in
CXCL5 levels was the most marked, and its neutralization reversed
the proliferation effect of ASCs on breast tumor cells. To the best
of our knowledge, the present study indicates for the first time
that CXCL5 is a key factor of the promotion of breast tumor cell
proliferation by ASC secretion through paracrine and endocrine
effects.
Within adipose tissue, ASC is increasingly
recognized as one of the most promising cell types responsible for
a number of important functions (25), including the secretion of chemokines
(8) and the formation of a
microenvironment that tightly controls the proliferation of cells
(9). However, it remains unknown how
ASC facilitates breast tumor proliferation. The results of the
present study suggest that ASCs provide a potent stimulus for tumor
cell proliferation in vitro. As the ASCs used in the present
study were obtained from cancer-free individuals and had never been
exposed to any tumor milieu, the present data suggest that ASCs
possess an inherent ability to enhance breast tumor
proliferation.
Using cytokine array analysis, it was demonstrated
that ASC secreted a number of factors known to promote tumor cell
proliferation. As the ASCs were from breast and abdominal tissues,
their promotion of breast tumorigenesis is attributable to
paracrine and endocrine effects, a conclusion consistent with
previous studies (3,4). In fact, adipose tissue has been known to
actively participate in endocrine processes by secreting numerous
cytokines and growth factors (26).
ASCs release high levels of epidermal growth factor, bFGF,
platelet-derived growth factor, hepatocyte growth factor, vascular
endothelial growth factor, transforming growth factor-β,
insulin-like growth factor and brain-derived neurotrophic factor
(26–29). It has also been indicated that ASCs
may secrete cytokines such as granulocyte colony-stimulating
factor, macrophage colony-stimulating factor, tumor necrosis
factor-α, IL-6, IL-7, IL-8, IL-11, IL-12 and leukemia inhibitory
factor (26,27). It is considered that these growth
factors and cytokines are released in bioactive levels by ASCs, and
that their secretion increases significantly under certain
conditions such as hypoxia or tumorigenesis (27,30). In
the present study, it was additionally identified that the release
of CXCL5 from ASCs was the most significant incidence in the
co-culture medium, and that CXCL5 is a key factor in the ASC
promotion of tumor cell proliferation.
The anti-CXCL5 antibody used in the present study
was a monoclonal antibody that had been used by numerous other
studies (31,32). As a monoclonal antibody, it will bind
CXCL5 directly without interaction with other molecules such as
C-X-C chemokine receptor 2 (CXCR2), which is the receptor of CXCL5
(33). Thus, according to the present
ASC-breast cancer cell co-culture data, it can be proposed that
CXCL5 directly acts on breast cancer cells to promote cancer
proliferation. It is notable that CXCR2 is also the receptor for
CXCL2, CXCL3 and IL8 (34). Among
them, IL-8 derived from local tissue-resident stromal cells is
suggested to promote breast cancer cell proliferation (14). Accordingly, results from
CXCR2-blocking experiments may be due to the dysfunction of
IL-8/CXCR2 instead of CXCL5/CXCR2. Based on the suppressive effect
of the anti-CXCL5 antibody, it can be hypothesized that the effect
of the other cancer-promoting factors from ASCs is either very weak
or dependent on the action of CXCL5 on ASC-cancer cell
interactions.
It has been well established that cancer cells
exhibit the ability to recruit stem cells into the vicinity of
tumors, and that this recruitment is important for the generation
of a microenvironment that promotes cancer growth (35,36). In
addition, evidence suggests that the chemokines produced by bone
marrow-derived mesenchymal stem cells (BM-MSCs) serve an important
role in tumorigenesis and tumor progression (37). Halpern et al (38) have demonstrated that BM-MSCs express
chemokines that enhance the migration of CXCR2-positive cancer
cells via the secretion of chemokine ligands such as CXCL1 and
CXCL5. In this regard, it is notable that the cytokine profiles
released from the ASCs (as shown in Fig.
3A) are similar to those displayed by MSCs (39). The present in vitro study
clearly indicates the role of ASC-secreted CXCL5 in promoting
breast cancer cell proliferation in ER-positive and ER-negative
cell lines. This result is in accordance with a previous study
demonstrating the growth-promoting effect of CXCL5 in the tunica
intima and tunica adventitia of adipose tissue blood vessels
(32). Additionally, high level of
CXCL5 is a biomarker for poor prognosis in pancreatic cancer
(40) and cholangiocarcinoma
(41). Thus, it is conceivable that
high CXCL5 level provides a microenvironment that is favorable to
tumor growth and progression, which offers an explanation for the
poor survival of patients with breast cancer who are obese
(4).
The results of the present study do not completely
exclude an additional effect of ASCs on guiding cancer cell
proliferation through direct physical contact with the tumor cells
in vivo. It was previously indicated that fibroblasts were
capable of generating tracks and guide the movement of carcinoma
cells when the two types of cells were in contact physically
(42). Considering the highly
migratory characteristics of ASCs, it is possible that the
CXCL5-secreting and track-generating capabilities of ASCs
contribute to their cancer proliferation-promoting effects in
vivo.
It must be noted that there are differences in the
mechanisms of promotion of breast cancer cell proliferation in
fibroblasts (WI-38 cells) and ASCs. In the present study, CXCL5 did
not significantly affect WI-38 cell- or HMEC-mediated breast cancer
cell proliferation, thereby suggesting the existence of multiple
mechanisms responsible for the induction of cancer proliferation.
The present study primarily focused on the biological
characteristics of cancer cells. The data demonstrated that CXCL5
may markedly affect cell proliferation independently of its
expression levels. Certainly, the determination of the expression
of the CXCL5 cytokine and its receptor in MDA-MB-231 and MCF-7
cells will also support the hypothesis of the present study.
The present study included ER-positive and
ER-negative cells, in addition to WI-38 cells HMECs as controls.
However, normal breast-associated fibroblast were not used as a
control based on the following reason: The WI-38 cell line, which
is a diploid human cell line composed of fibroblasts derived from
lung tissue of an aborted Caucasian female fetus in the 1960s
(43), has been widely used as a
control to study breast cancer (17,44). In
addition, normal breast-associated fibroblasts could inhibit
epithelial growth (45). As a result,
to the best of our knowledge, there are limited studies using
normal breast-associated fibroblasts as controls. Therefore, in the
present study, both WI-38 cells as HMECs were used as controls
instead of normal breast-associated fibroblasts, and the same
conclusion was obtained, i.e., ASC-secreted CXCL5 is a key factor
in promoting breast tumor cell proliferation.
In conclusion, CXCL5 is an important factor for the
interactions between ASCs and breast cancer cells. The interactions
between tumors and adipose tissues enhance CXCL5 expression, which
is a key factor in breast tumorigenesis. CXCL5 may be a potential
therapeutic target in breast cancer, and should be more extensively
studied, in addition to other cytokines.
Acknowledgements
The authors would like to thank Dr Haipeng Yang (The
Fourth Affiliated Hospital of Harbin Medical University, Harbin,
China) for his editorial assistance. The present study was
supported by grants from the National Natural Science Foundation of
China (grant nos. 81172181, 81172181H1612 and 81372839H1622).
Glossary
Abbreviations
Abbreviations:
|
ASC
|
adipose tissue-derived stem cell
|
|
bFGF
|
basic fibroblast growth factor
|
|
BM-MSC
|
bone marrow-derived mesenchymal stem
cell
|
|
CXCL5
|
C-X-C motif chemokine ligand 5
|
|
ENA-78
|
epithelial cell-derived
neutrophil-activating peptide-78
|
|
ER
|
estrogen receptor
|
|
FBS
|
fetal bovine serum
|
|
IL
|
interleukin
|
References
|
1
|
Zalesin KC, Franklin BA, Miller WM,
Peterson ED and McCullough PA: Impact of obesity on cardiovascular
disease. Endocrinol Metab Clin North Am. 37:663–684, ix. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gilbert CA and Slingerland JM: Cytokines,
obesity, and cancer: New insights on mechanisms linking obesity to
cancer risk and progression. Annu Rev Med. 64:45–57. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bergström A, Pisani P, Tenet V, Wolk A and
Adami HO: Overweight as an avoidable cause of cancer in Europe. Int
J Cancer. 91:421–430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carmichael AR: Obesity as a risk factor
for development and poor prognosis of breast cancer. BJOG.
113:1160–1166. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Kruijsdijk RC, van der Wall E and
Visseren FL: Obesity and cancer: The role of dysfunctional adipose
tissue. Cancer Epidemiol Biomarkers Prev. 18:2569–2578. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Patterson RE, Cadmus LA, Emond JA and
Pierce JP: Physical activity, diet, adiposity and female breast
cancer prognosis: A review of the epidemiologic literature.
Maturitas. 66:5–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: Implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bissell MJ, Radisky DC, Rizki A, Weaver VM
and Petersen OW: The organizing principle: Microenvironmental
influences in the normal and malignant breast. Differentiation.
70:537–546. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Campbell MJ, Tonlaar NY, Garwood ER, Huo
D, Moore DH, Khramtsov AI, Au A, Baehner F, Chen Y, Malaka DO, et
al: Proliferating macrophages associated with high grade, hormone
receptor negative breast cancer and poor clinical outcome. Breast
Cancer Res Treat. 128:703–711. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Knüpfer H and Preiss R: Significance of
interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Res
Treat. 102:129–135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Walter M, Liang S, Ghosh S, Hornsby PJ and
Li R: Interleukin 6 secreted from adipose stromal cells promotes
migration and invasion of breast cancer cells. Oncogene.
28:2745–2755. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Waugh DJ and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Welte G, Alt E, Devarajan E, Krishnappa S,
Jotzu C and Song YH: Interleukin-8 derived from local
tissue-resident stromal cells promotes tumor cell invasion. Mol
Carcinog. 51:861–868. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soria G and Ben-Baruch A: The inflammatory
chemokines CCL2 and CCL5 in breast cancer. Cancer Lett.
267:271–285. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
General assembly of the world medical
association: World medical association declaration of Helsinki:
Ethical priniciples for medical research involving human subjects.
J Am College Dentists. 81:14–18. 2014.
|
|
17
|
Pinilla S, Alt E, Khalek FJ Abdul, Jotzu
C, Muehlberg F, Beckmann C and Song YH: Tissue resident stem cells
produce CCL5 under the influence of cancer cells and thereby
promote breast cancer cell invasion. Cancer Lett. 284:80–85. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Katz AJ, Tholpady A, Tholpady SS, Shang H
and Ogle RC: Cell surface and transcriptional characterization of
human adipose-derived adherent stromal (hADAS) cells. Stem Cells.
23:412–423. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gronthos S, Franklin DM, Leddy HA, Robey
PG, Storms RW and Gimble JM: Surface protein characterization of
human adipose tissue-derived stromal cells. J Cell Physiol.
189:54–63. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wagner W, Wein F, Seckinger A, Frankhauser
M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W
and Ho AD: Comparative characteristics of mesenchymal stem cells
from human bone marrow, adipose tissue, and umbilical cord blood.
Exp Hematol. 33:1402–1416. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krtolica A, Parrinello S, Lockett S,
Desprez PY and Campisi J: Senescent fibroblasts promote epithelial
cell growth and tumorigenesis: A link between cancer and aging.
Proc Natl Acad Sci USA. 98:pp. 12072–12077. 2001; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Walz A, Schmutz P, Mueller C and
Schnyder-Candrian S: Regulation and function of the CXC chemokine
ENA-78 in monocytes and its role in disease. J Leukoc Biol.
62:604–611. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou SL, Dai Z, Zhou ZJ, Chen Q, Wang Z,
Xiao YS, Hu ZQ, Huang XY, Yang GH, Shi YH, et al: CXCL5 contributes
to tumor metastasis and recurrence of intrahepatic
cholangiocarcinoma by recruiting infiltrative intratumoral
neutrophils. Carcinogenesis. 35:597–605. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng J, Zhu X and Zhang J: CXCL5
knockdown expression inhibits human bladder cancer T24 cells
proliferation and migration. Biochem Biophys Res Commun. 446:18–24.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zuk PA: The adipose-derived stem cell:
Looking back and looking ahead. Mol Biol Cell. 21:1783–1787. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kilroy GE, Foster SJ, Wu X, Ruiz J,
Sherwood S, Heifetz A, Ludlow JW, Stricker DM, Potiny S, Green P,
et al: Cytokine profile of human adipose-derived stem cells:
Expression of angiogenic, hematopoietic, and pro-inflammatory
factors. J Cell Physiol. 212:702–709. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rehman J, Traktuev D, Li J, Merfeld-Clauss
S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV
and March KL: Secretion of angiogenic and antiapoptotic factors by
human adipose stromal cells. Circulation. 109:1292–1298. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen CW, Montelatici E, Crisan M, Corselli
M, Huard J, Lazzari L and Péault B: Perivascular multi-lineage
progenitor cells in human organs: Regenerative units, cytokine
sources or both? Cytokine Growth Factor Rev. 20:429–434. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wei X, Du Z, Zhao L, Feng D, Wei G, He Y,
Tan J, Lee WH, Hampel H, Dodel R, et al: IFATS collection: The
conditioned media of adipose stromal cells protect against
hypoxia-ischemia-induced brain damage in neonatal rats. Stem Cells.
27:478–488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cai L, Johnstone BH, Cook TG, Liang Z,
Traktuev D, Cornetta K, Ingram DA, Rosen ED and March KL:
Suppression of hepatocyte growth factor production impairs the
ability of adipose-derived stem cells to promote ischemic tissue
revascularization. Stem Cells. 25:3234–3243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Põld M, Zhu LX, Sharma S, Burdick MD, Lin
Y, Lee PP, Põld A, Luo J, Krysan K, Dohadwala M, et al:
Cyclooxygenase-2-dependent expression of angiogenic CXC chemokines
ENA-78/CXC Ligand (CXCL) 5 and interleukin-8/CXCL8 in human
non-small cell lung cancer. Cancer Res. 64:1853–1860. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang H, Ning H, Banie L, Wang G, Lin G,
Lue TF and Lin CS: Adipose tissue-derived stem cells secrete CXCL5
cytokine with chemoattractant and angiogenic properties. Biochem
Biophys Res Commun. 402:560–564. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koltsova EK and Ley K: The mysterious ways
of the chemokine CXCL5. Immunity. 33:7–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Veenstra M and Ransohoff RM: Chemokine
receptor CXCR2: Physiology regulator and neuroinflammation
controller? J Neuroimmunol. 246:1–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bhowmick NA, Neilson EG and Moses HL:
Stromal fibroblasts in cancer initiation and progression. Nature.
432:332–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Karnoub AE, Dash AB, Vo AP, Sullivan A,
Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R and Weinberg
RA: Mesenchymal stem cells within tumour stroma promote breast
cancer metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Raman D, Baugher PJ, Thu YM and Richmond
A: Role of chemokines in tumor growth. Cancer Lett. 256:137–165.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Halpern JL, Kilbarger A and Lynch CC:
Mesenchymal stem cells promote mammary cancer cell migration in
vitro via the CXCR2 receptor. Cancer Lett. 308:91–99. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim DH, Yoo KH, Choi KS, Choi J, Choi SY,
Yang SE, Yang YS, Im HJ, Kim KH, Jung HL, et al: Gene expression
profile of cytokine and growth factor during differentiation of
bone marrow-derived mesenchymal stem cell. Cytokine. 31:119–126.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li A, King J, Moro A, Sugi MD, Dawson DW,
Kaplan J, Li G, Lu X, Strieter RM, Burdick M, et al: Overexpression
of CXCL5 is associated with poor survival in patients with
pancreatic cancer. Am J Pathol. 178:1340–1349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Okabe H, Beppu T, Ueda M, Hayashi H,
Ishiko T, Masuda T, Otao R, Horlad H, Mima K, Miyake K, et al:
Identification of CXCL5/ENA-78 as a factor involved in the
interaction between cholangiocarcinoma cells and cancer-associated
fibroblasts. Int J Cancer. 131:2234–2241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gaggioli C, Hooper S, Hidalgo-Carcedo C,
Grosse R, Marshall JF, Harrington K and Sahai E: Fibroblast-led
collective invasion of carcinoma cells with differing roles for
RhoGTPases in leading and following cells. Nat Cell Biol.
9:1392–1400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hayflick L: The limited in vitro lifetime
of human diploid cell strains. Exp Cell Res. 37:614–636. 1965.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Stuelten CH, DaCosta Byfield S, Arany PR,
Karpova TS, Stetler-Stevenson WG and Roberts AB: Breast cancer
cells induce stromal fibroblasts to express MMP-9 via secretion of
TNF-alpha and TGF-beta. J Cell Sci. 118:2043–2153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sadlonova A, Bowe DB, Novak Z, Mukherjee
S, Duncan VE, Page GP and Frost AR: Identification of molecular
distinctions between normal breast-associated fibroblasts and
breast cancer-associated fibroblast. Cancer Microenviron. 2:9–21.
2009. View Article : Google Scholar : PubMed/NCBI
|