Introduction
Pancreatic ductal adenocarcinoma (PDAC) has one of
the worst prognoses of all malignancies worldwide (1). The incidence of PDAC continues to
increase and ranks as fourth cause of cancer related mortality.
Despite radical surgical intervention (R0 resection) and
chemo-radiation, the survival for all stages at 5 years is about 6%
in Europe and the United States (2,3).
The most effective single chemotherapy intervention
against advanced PDAC has been achieved with gemcitabine (GEM). GEM
has significantly improved the median overall survival of PDAC
patients by 1.2 months over patients treated with 5-fluorouracil
and became the reference drug for this disease (4). 5-Fluorouracil has also some clinical
activity against advanced PDACA with a median overall survival of
4.4 months (2).
However, many trials with other drugs in combination
with GEM were investigated in the last decade; a combination of GEM
and oxaliplatin has shown a benefit in a phase II study (5). In addition, a recent randomized trial
with FOLFIRINOX has enhanced the median overall survival, when
compared with GEM (11.1 months vs. 6.8 months respectively)
(4). This combination was even
superior to the recently published combination of gemcitabine with
nab-paclitaxel, which resulted in a median survival time of 8.5
months vs. 6.7 months following gemcitabine alone (6).
The efficacy of chemo-radiation in advanced PDAC has
been evaluated in many randomised trials. It was shown that
chemo-radiation is superior to best supportive care and to
radiotherapy alone, but more toxic and equally effective in
comparison to chemotherapy alone (7).
Altogether, the relatively poor efficacy of
chemotherapy and chemo-radiation against this devastating disease
indicates that the development of new therapeutic strategies is a
crucial step to improve the survival of advanced PDAC. This
includes modification of established therapies and testing of new
substances.
Ribosome inhibiting proteins are a group of highly
potent plant lectins, which can kill tumor cells at nano- to
picomolar concentrations. Certain members of this group like
viscumin, abrin and ricin have been analysed for their anticancer
activities in several clinical and preclinical trials (8,9). More than
4 decades ago, Abrin and Ricin have been found to be more toxic to
tumor than to normal cells (10).
Aviscumin, which was produced in Escherichia coli as a
recombinant lectin, has shown immunomodulatory and cytotoxic
activities. In clinical phase I/II studies, it achieved disease
stabilization in some cases (11).
Rips of type II consist of two protein chains. The A-chain is
cytotoxic due to its de-purinating activity in ribosomes, leading
to a stop in protein synthesis. The B-chain shows affinity for
certain sugar moieties, which confer its lectin binding. The
ribosome inactivating protein (RIP) A-chain has been used to
construct toxic antibody conjugates targeting cancer specific
antigens (9). For example, the
immunotoxin Combotox consists of the ricin A-chain coupled to an
antibody directed against cell surface antigens CD19 and CD22 and
has been investigated in a phase I study as a candidate for
treatment of children with refractory leukaemia (8).
Riproximin is a new plant lectin, which was isolated
from the plant Ximenia americana and identified as the
active component of a powdered plant material used in African
traditional medicine. Riproximin was classified as a RIP of type II
(12,13). In addition to the conventional mode of
action, Rpx was found to induce the unfolded protein response, a
cellular mechanism activated in response to endoplasmic reticulum
stress. Apoptosis was induced by concentrations at which
translation of UPR-related genes occurred, despite concomitant
ribosomal depurination (14). This
study was set up to explore the antineoplastic efficacy of Rpx in
PDAC. The ASML rat PDAC cell line and clones derived from this line
were used as model for in vivo and in vitro
experiments on the activity of Rpx. Here, we report on the
anticancer activity of Rpx against ASML cells in comparison to GEM
as standard compound.
Materials and methods
Cell culture
The pancreatic cancer cell line ASML and its clones
2, 5 and 10 were used. The cells were maintained in an incubator
under standard culture conditions at 37°C in humidified air with 5%
CO2. For keeping the cells in logarithmic growth, they
were propagated 1–3 times per week depending on their growth
rate.
MTT assay
For determining the proliferation rates and the
effect of riproximin on the ASML cells, they were seeded at
densities of 1×103-10×103 cells/ml (100 µl
medium per well) into 96 well-plates (flat bottom; Becton
Dickinson, Heidelberg, Germany). Subsequent experiments on the
sensitivity to Rpx were performed with
2×103-3×103 cells per well, which were
treated with different concentrations of Rpx and thereafter grown
for periods of 24, 48, 72 and 96 h. After these periods, 10 µl MTT
[3-(4.5-dimethylthiazol-2-yl)-2.5-diphenyl tetrazolium bromide;
Serva Electrophoresis GmbH, Heidelberg, Germany] solution (10
mg/ml) was added and following an incubation period of 3 h at 37°C,
the medium was discarded and the cells were lysed by adding 200 µl
per well acidified 2-propanol (0.04 N HCl). After all formazan
crystals had been carefully dissolved, the absorption was measured
at 540 nm (reference filter of 690 nm) in an automated microtiter
plate spectrophotometer (Anthos Mikrosysteme, Krefeld, Germany).
The absorption of exposed cells was given in percent of untreated
control cells.
Reagents and chemicals
Gemcitabine (GEM) was provided by (Eli Lilly, Bad
Homburg, Germany) at a quality suited for clinical use. Before each
experiment, the antimetabolite was dissolved and diluted in sterile
0.9% saline to appropriate concentrations. Riproximin (Rpx) was
purified from Ximenia americana kernels according to a
described method, Bayer et al (15). Rpx was diluted in sterile
physiological saline for the in vitro and in vivo
experiments. The ASML cells were exposed to Rpx at concentrations
ranging from 0.1 to 333 pM. The cell survival was controlled by
MTT-test after 24, 48, 72 and 96 h. Dinaline
[4-amino-N-(2′-aminophenyl) benzamide, Din] was supplied by
Goedecke AG (Freiburg, Germany) in a purity required for clinical
use. For administration to animals, the compound was suspended in
0.8% Methocel (Nordmann-Rasmann, Hamburg, Germany) immediately
before application.
Animals
All animal experiments were performed in accordance
with the Regierungspräsidium Karlsruhe (Germany), which as
Institutional Animal Care and Use Committee (IACUC) approved the
animal ethics of this study for the German Cancer Research Center
(DKFZ), Heidelberg. According to this permit, the animals were
randomly allocated to treatment and control groups and they were
humanely euthanized after four weeks at the end of the experiment
or when they met certain clinical criteria indicating a moribund
status. The criteria used to determine when the animals should be
humanely euthanized included a weight loss of more than 10%, pale
skin color indicating anemia, icterus, as well as pain or reduced
general conditions as indicated by reluctance to move, abnormal
posturing, and decreased appetite. All animals were daily monitored
for their condition. For sacrifice, a narcosis with isofluorane
followed by CO2 was used. Steps taken to minimize
suffering of the animals, included analgesics (metamizole)
administered post-surgically for up to three days and anesthetics
(isoflurane) for inhalation anesthesia during surgery. As
appropriate, the German guidelines, which are similar to the ARRIVE
guidelines for reporting animal studies were followed.
Male RNU rats were used for the in vivo
experiments. They were obtained from Charles River (Sulzfeld,
Germany) at an age of 5–7 weeks and a corresponding body weight of
120–160 g. They were kept under specific pathogen free (SPF)
conditions in Macrolon-III-cages of a ventilated rack (Ventirock,
UNO Roestvaststaal B.V., Zevenaar, The Netherlands) providing a
50-fold exchange of filtered air per hour as well as positive air
pressure inside the cages. Constant room temperature (22±1°C), air
humidity (50±10%) and dark-light-rhythm (12 h) were maintained
throughout. The animals had free access to autoclaved water and
standard laboratory diet. An acclimatisation period of 7 days was
adhered to before starting any experiments.
Tumor cell transplantation
The procedure used was as described previously
(16): In short, logarithmically
growing ASML GFP-LUC cells were trypsinized and
suspended (2×106 cells) in 0.25 ml PBS
(phosphate-buffered saline without calcium and magnesium ions) and
0.15 ml matrigel (extract of the Engelbreth-Holm-Swarm-mouse tumor;
Biomatrix EHS solution; Serva Electrophoresis GmbH). This
suspension was stored on ice until injection. For tumor cell
transplantation, the rats were anaesthetized with isoflurane at 1.5
vol% together with 0.5 l/min oxygen and 1 l/min nitrous oxide.
After a median laparotomy, the caecum was
exteriorized onto a compress moistened with sterile physiological
saline and a mesocolic vein was isolated from mesenteric fat. Under
microscopic control the tumor cell suspension was injected into
this vessel with a 28-gauge needle.
Thereafter the vessel was compressed with two cotton
swabs for a period of 1–2 min to prevent bleeding; the caecum was
moved back into the abdomen; the musculature was sutured (4-0
vicryl, Ethicon GmbH, Norderstedt, Germany) and the skin was closed
with metal clips.
In vivo imaging
Live animal bioluminescence imaging was performed
using the IVIS100 imaging system (Xenogen Corp., Alamede, CA, USA).
Prior to imaging, the animals were injected intraperitoneally with
the substrate D-Luciferin (Synchem Corp. Elk Grove Village, IL,
USA) at a dose of 10 mg/animal and anesthetized with
isoflurane/oxygen via the XGI-8 Anesthesia System (Xenogen Corp.),
The images obtained by the IVIS 100 camera were subsequently
analyzed using the Living Image v2.5 software provided by Xenogen
Corp.
In vivo experiments
For determining the effect of riproximin in a rat
liver metastasis model, the ASML-rat model was used, as described
before (see ASML model in reference 16). Tumor-bearing rats were
treated per-orally, intra-peritoneally or intravenously with
different concentration of riproximin, gemcitabine, or dinaline. To
observe the tumor development, the animals were imaged according to
the previously described protocol (see in vivo imaging).
Three to four weeks later, the experiment was terminated, the
animals euthanized, the liver of the animals was removed, weighed,
and kept at −80°C until further analysis.
Single and combined drug exposure. The design of
treating ASML cells growing in nude rats is given in Table I. The dosages used for treatment were
based on earlier studies.
 | Table I.Experimental design for
transplantation and treatment of ASML PDAC cells in nude rats. |
Table I.
Experimental design for
transplantation and treatment of ASML PDAC cells in nude rats.
| Trial | Group no. | Animal no. | Treatment | Route | Dose | Regimen | Total dose |
|---|
| 1 | 1a | 3 | Control 1 | – | – | – | – |
|
| 1b | 3 | Rpxa | p.o. | 3.4 µg/kg | Days 1–3 for one
week | 10.2 µg/kg |
|
| 1c | 3 | Rpx | i.v. | 3.4 µg/kg | Days 1–3 for one
week | 10.2 µg/kg |
| 2 | 2a | 8 | Control 2 | – | – | – | – |
|
| 2b | 5 | Rpx | i.v. | 1.7 µg/kg | 3× daily every 2nd
day for 2 weeks | 35.7 µg/kg |
|
| 2c | 5 | Dinaline | p.o. | 10 mg/kg | 1× daily for 10
days | 100 mg/kg |
|
| 2d | 5 | Rpx+ | i.v+ | 1.7 µg/kg+ | 3× daily every 2nd
day for 2 weeks+ | 35.7 µg/kg+ |
|
|
|
| Din | p.o. | 5 mg/kg | 1× daily for 10
days | 100 mg/kg |
| 3 | 3a | 5 | Control 3 | – | – | – | – |
|
| 3b | 5 | Rpx | i.p | 5.1 µg/kg | Every 2nd day for 20
days | 51 µg/kg |
|
| 3c | 5 | Rpx | i.p. | 3.4 µg/kg | Every 2nd day for 20
days | 34 µg/kg |
|
| 3d | 5 | Rpx | i.p | 1.7 µg/kg | Every 2nd day for 20
days | 17 µg/kg |
|
| 3e | 5 | GEMb | i.p. | 50 mg/kg | 1× weekly for 2
weeks | 100 mg/kg |
|
| 3f | 5 | Rpx+ | i.p+ | 1.7 µg/kg+ | Every 2nd day for
20 days+ | 17 µg/kg+ |
|
|
|
| GEM | i.p. | 50 mg/kg | 1× weekly for 2
weeks | 100 mg/kg |
By various application forms (i.v., p.o., i.p.),
riproximin was administered twice weekly at a dose range of 1.7 to
5.1 µg/kg to RNU rats. GEM and DIN were administered to animals
either intravenously at a dose of 50 mg/kg weekly or perorally at a
dose of 10 mg/kg, 5 times weekly.
Cell lysis for western blot
analysis
Cell pellets from the ASML cell line were collected
and re-suspended in buffer containing 0.1 M NaCl, 10 mM Tris-HCl
and 1 mM ethylenediaminetetraacetic acid (EDTA). Thereafter, an
equal quantity of lysis buffer (100 mM Tris-HCl, 4% sodium dodecyl
sulfate (SDS), 20% glycerol) was added. Both buffers were
supplemented with Complete TM protease inhibitor mixture (Roche
Molecular Biochemicals, Mannheim, Germany) as recommended by the
manufacturer. The lysis buffer was additionally supplemented with
dithiotreitol (DTT) to a final concentration of 200 mM. Lysates
were boiled for 10 min at 99°C in a Thermomixer (Eppendorf,
Hamburg, Germany) at a mixing frequency of 500 rpm and thereafter
centrifuged at 10,600 × g for 10 min at 4°C. The protein
concentration in a portion of each sample, lysed without DTT, was
determined using the Pierce BCA Protein Assay kit (Pierce
Biotechnology, Rockford, IL, USA) according to the recommendations
of the manufacturer.
Immunoblot detection
Equal amounts of protein (20–50 µg from whole cell
lysates) were loaded onto a 4–12% Bis-Tris gel (Invitrogen,
Karlsruhe, Germany), and separated by electrophoresis under
reducing and denaturing conditions. Proteins were then transferred
to a PVDF membrane using the XCell II blot module (Invitrogen). For
membrane blocking and antibody dilution, milk powder (5% in TBS-T,
0.1% Tween 20) was used. The membrane was blocked for 1 h at RT
followed by incubation with the primary antibody at 4°C overnight.
Antibodies against caspases 3, 8, and 9, ATF3 and ERK2 were
obtained from Santa Cruz (Heidelberg, Germany). After washing with
TBS-T (3 times for 10 min), the blot membrane was incubated with
the appropriate secondary antibody conjugated to horseradish
peroxidase at RT for 1 h, followed by four washing steps with TBS-T
(10 min each). Visualization of the antibody complexes was done by
incubating the membrane with a chemiluminescent peroxidase
substrate and exposing it to X-ray films. Light signals were
quantified by the Quantity One software (Biorad, Munich, Germany).
Intensities of target protein bands were normalized to ERK2 as the
loading control.
Statistical analysis
The cell survival fractions were calculated as the
ratio of MTT-absorptions from treated and control cells. The
results of multiple measurements were given as mean with
corresponding standard deviation. Response was normalized by
dividing by mean control response. The 4-parameter log-logistic
model was fitted to individual normalized response values,
constraining the lower limit to be non-negative. Calculations were
performed using R version 3.0.1 (http://www.R-project.org) and R-Package ‘drc’ version
2.3–96 for dose-response analysis.
Results
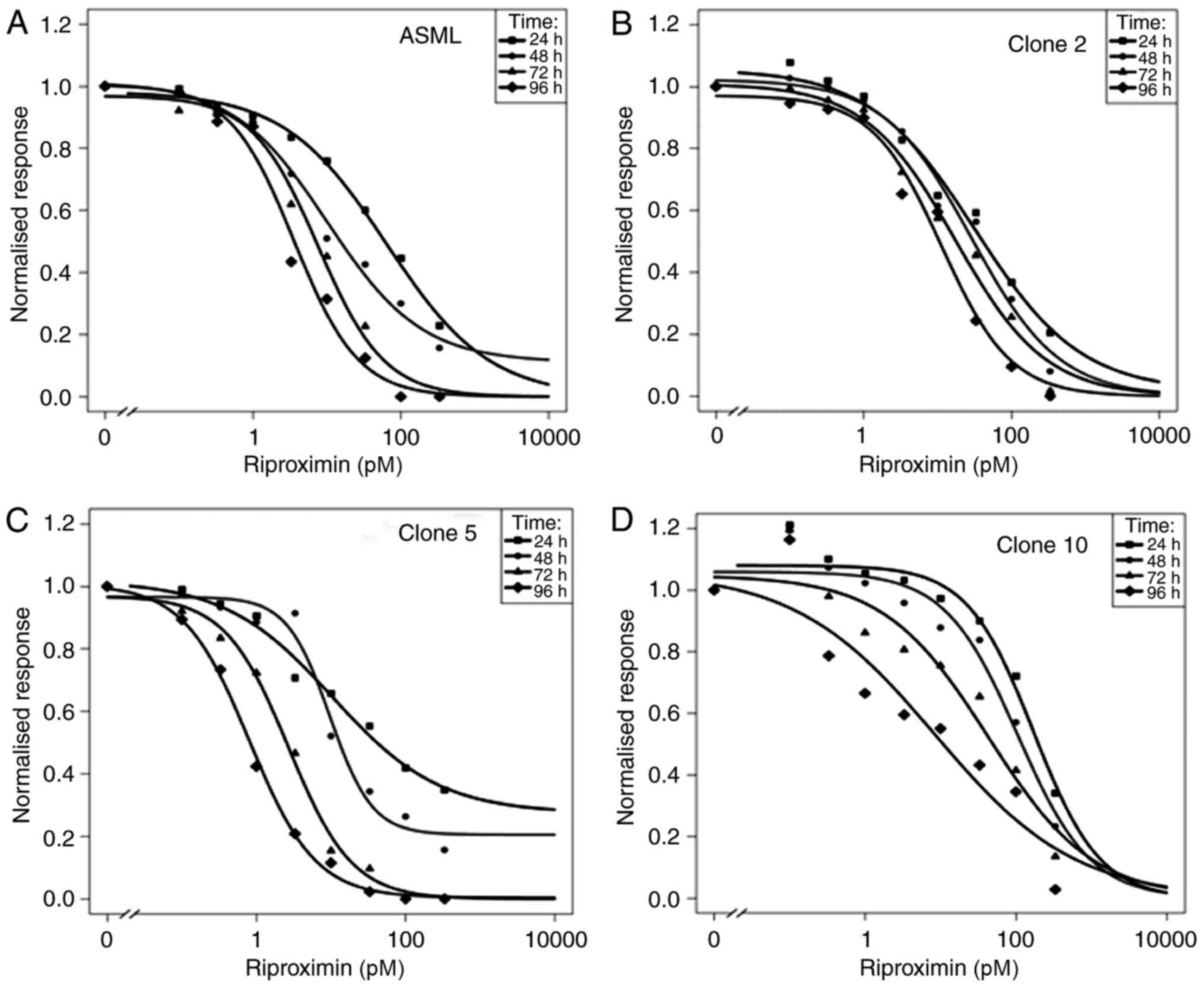
Rpx antineoplastic activity
The cytotoxic activity of Rpx was assessed in ASML
rat PDAC cells as well as in the clones 2, 5, and 10, which are
derived from ASML.
The mother cell line and its clones differ by
morphologic and proliferative properties as indicated by tumor cell
number doubling times of 34 h for ASML cells, 18 h for clone 2, 12
h for clone 5, and 36 h for clone 10. With respect to morphologic
criteria, cells of the mother cell line ASML have a mostly rounded
form. They show coherent and homogenous growth. The cells of clone
2 are relatively large with a central small nucleus. They grow
coherent and homogenous in an epithelial mode with formation of
ductal like structures. The cells of clone 5 are either large and
partly polygonal with a polar nucleus, or small and round. They are
mostly coherent to each other. Clone 10 cells are mainly small and
round. During the log phase of their growth, a part of these cells
show a spindle-cell like form. Spontaneous apoptosis is observed
during this phase.
In the original cell line, Rpx caused 50% growth
inhibition (IC 50) at a concentration of 65 pM after 24 h. The IC50
decreased 18 fold over the subsequent three days of observation,
from 10 pM (48 h) to 8 pm (72 h) and 4 pM (96 h), Fig. 1A and Table
II. The ASML clone 2 was more sensitive than the ASML-mother
cells after 24 h (IC50 33 pM), but showed a distinctly lower
reduction in IC 50 values during the subsequent three days of
observation. These dropped only 3 fold to 29 pM (48 h), 17 pM (72
h), and 11 pM (96 h, Fig. 1B). In
addition, clone 2 cells were stimulated in growth by maximally 8%
in response to the lowest dose of Rpx (0.1 pM).
 | Table II.Efficacy of riproximin in ASML cell
lines: IC50 values and 95% confidence limits. |
Table II.
Efficacy of riproximin in ASML cell
lines: IC50 values and 95% confidence limits.
| Cell line | Time | IC 50a | Lower 95%
confidence limita | Upper 95%
confidence limita |
|---|
| ASML | 24 h | 64,5 | 38,7 | 107,4 |
|
| 48 h | 10,1 | 7,0 | 14,8 |
|
| 72 h | 7,6 | 6,6 | 8,8 |
|
| 96 h | 3,6 | 3,0 | 4,3 |
| Clone 2 | 24 h | 32,5 | 17,0 | 62,1 |
|
| 48 h | 28,7 | 21,2 | 38,8 |
|
| 72 h | 17,3 | 13,5 | 22,3 |
|
| 96 h | 11,3 | 9,4 | 13,6 |
| Clone 5 | 24 h | 10,4 | 6,3 | 17,2 |
|
| 48 h | 9,6 | 7,6 | 12,2 |
|
| 72 h | 2,7 | 2,5 | 2,9 |
|
| 96 h | 0,8 | 0,8 | 0,9 |
| Clone 10 | 24 h | 171,5 | 91,9 | 320,0 |
|
| 48 h | 105,6 | 65,8 | 169,6 |
|
| 72 h | 42,9 | 22,9 | 80,4 |
|
| 96 h | 8,5 | 2,6 | 28,2 |
Clone 5 was most sensitive to Rpx as shown by IC50
values ranging from 10 pM (24 h) to 1 pM (96 h), with a 13 fold
reduction during the observation time (Fig. 1C).
In addition, there was an initial slight stimulation
of ASML-clone 2 cells at the low concentrations of Rpx
(<1pM).
In comparison to the other clones, the ASML-clone 10
showed a clear and prolonged stimulation (up to 23%) at low Rpx
concentrations (0.1–3.3 pM). In addition to this observation, the
high IC 50 values, ranging from 172 pM (24 h) to 9 pM (96 h)
indicate a low sensitivity of clone 10 to Rpx, Fig. 1D. Nevertheless, there was a 20 fold
difference between the highest and lowest IC50 values.
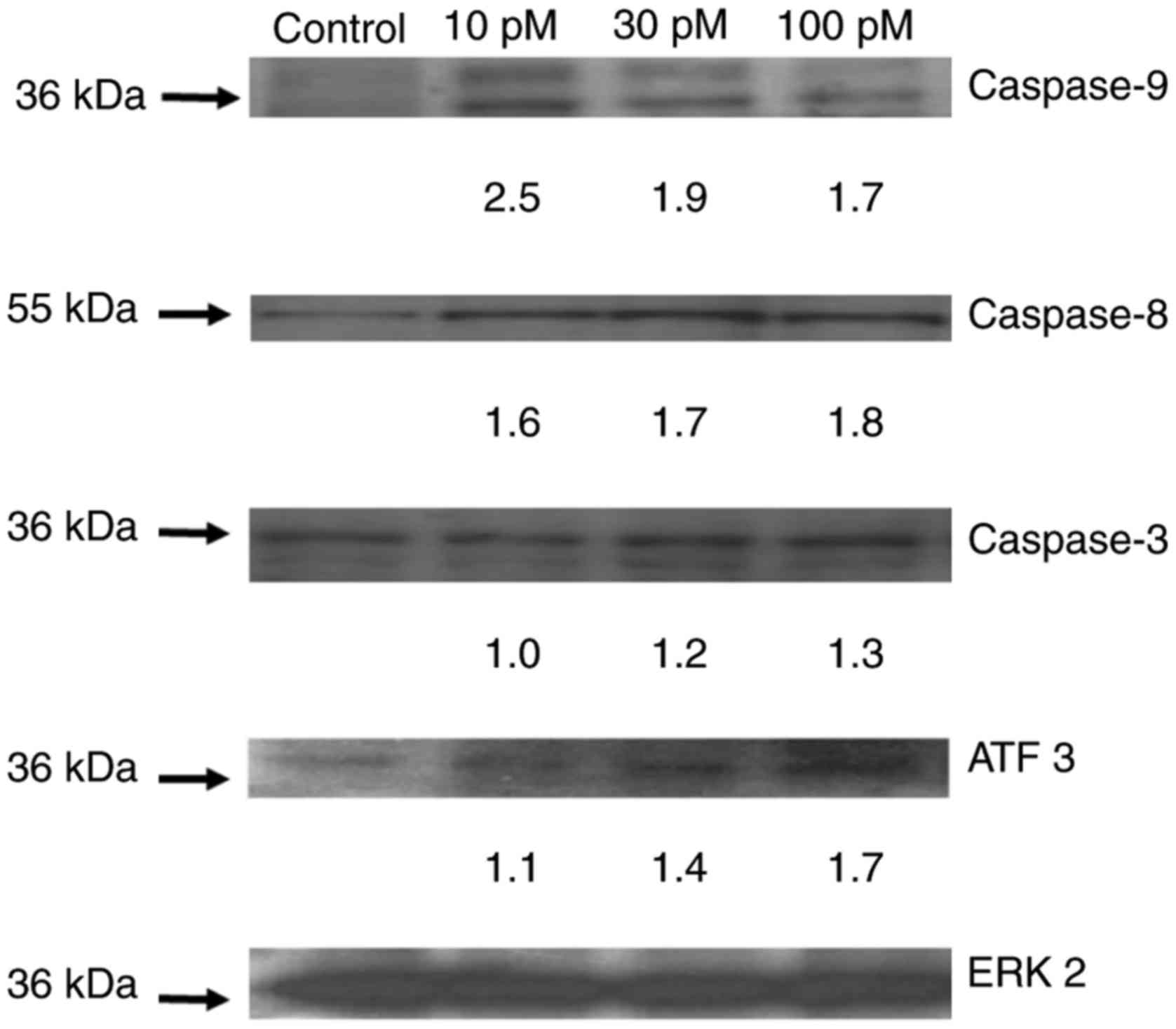
Western blot analysis
After exposing ASML cells to various concentrations
of riproximin, selected apoptosis related proteins were
investigated by western blot analysis (Fig. 2). After 48 h, caspases 8, 9 and 3 were
upregulated and activated at concentrations ranging from 10–100 pM.
The upregulation varied concentration dependently from 0 to 1.3
fold (caspase-3), 1.6 to 1.8 fold (caspase-8), and 2.5 to 1.7 fold
(caspase-9). In addition, the activation of caspases was shown by
the fragmentation of pro-caspases to the active caspase.
Furthermore, ATF3 was upupregulated to a slightly increased plateau
following all concentrations used (1.1 to 1.7 fold).
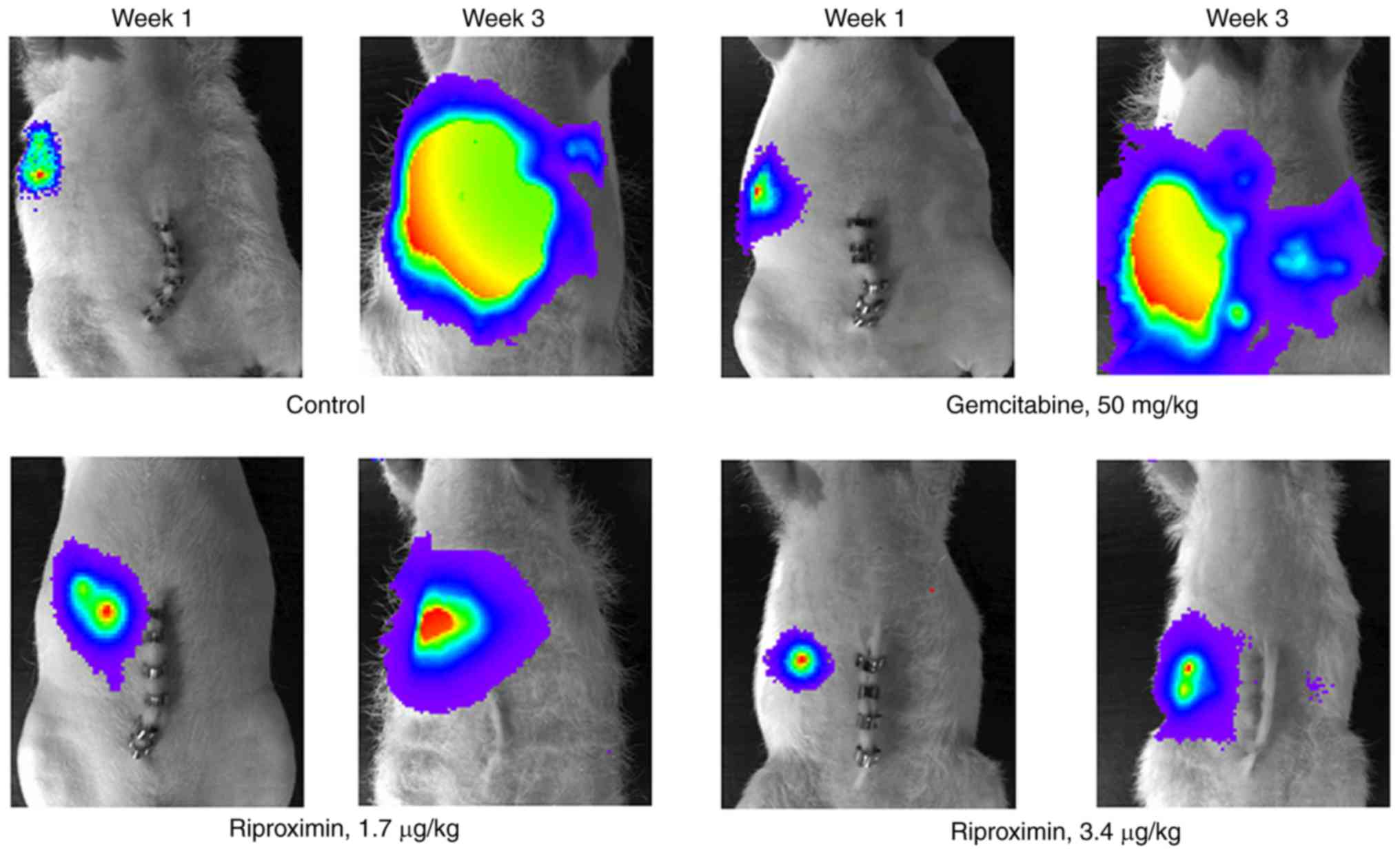
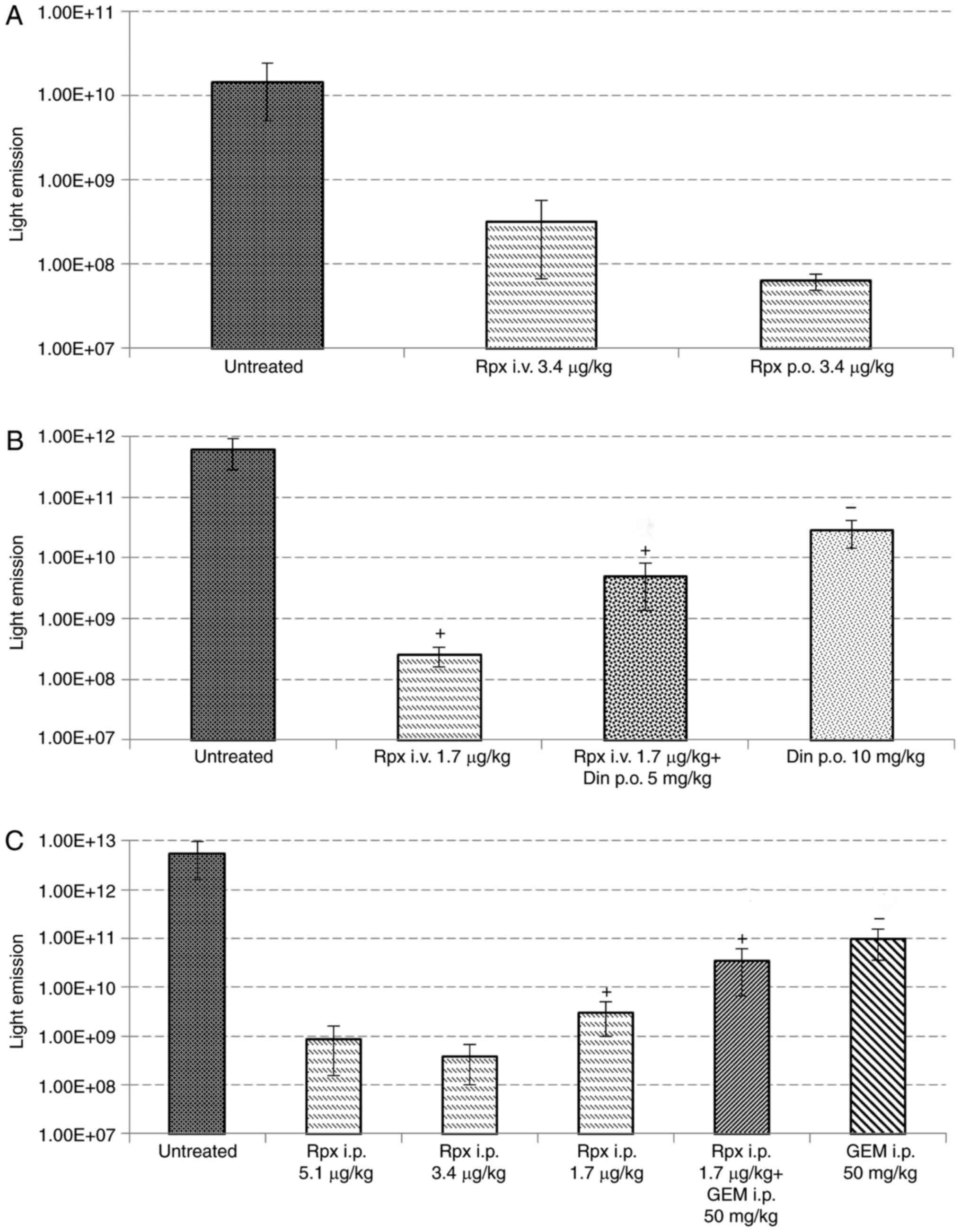
Response of tumor bearing nude rats to
treatment
Control nude rats showed a mean survival time of 17
days (Table II). At necropsy, a mean
liver weight of 22 g was found, as well as a positive body weight
development. Per-oral administration of riproximin (3.4 µg/kg,
group 1b, Table II) was not
associated with a positive therapy effect; i.v. administration of
this dose, however, was highly effective, but caused toxicity
leading to premature death of the animals. Therefore, a reduced
dose of riproximin was used subsequently via the intravenous route
(1.7 µg/kg, group 2b), which prolonged the live span of tumor
bearing animals by 18% and reduced the liver weight to 10.2 g
(P<0.01, respectively).
Treatment with dinaline, alone or in addition with
riproximin was less effective. Surprisingly, the antineoplastic
effect of riproximin, as shown by the mean liver weight, was
reduced by co-treatment with dinaline. The significant
antineoplastic effect of riproximin against ASML cells growing in
the liver of nude rats was confirmed in the 3rd
experiment. Treatment with 1.7 µg/kg Rpx administered
intra-peritoneally prolonged the lifespan of tumor bearing animals
and reduced the tumor weight by 90% to an almost normal liver
weight (P<0.01, respectively). Treatment with gemcitabine was
almost equally effective as treatment with riproximin (groups 3d
and e, Table III and Fig. 3), but combination of these drugs was
only minimally more active than the single agents, as shown by a
mean liver weight of 7,9 g (combination) vs. 9,4 g (riproximin) and
9.9 g (gemcitabine).
 | Table III.Response of ASML-PDAC tumor bearing
nude rats to treatment. |
Table III.
Response of ASML-PDAC tumor bearing
nude rats to treatment.
| Trial | Group no. | Mean survival
(days) | P-value | Body weight start
of treatment (g) | Body weight end of
treatment (g) | Mean Liver weight
(g) | P-value | Mortality (3
weeks) | Light emission |
|---|
| 1 | 1a | 16.5 | − | 219.8 | 248.6 | 22 | − | 100 |
|
|
| 1b | 16 | − | 221.7 | 241 | 20 | − | 67 |
|
|
| 1c | 12 | tox.a | 220 | 223.4 | 7.67 | tox.a | 100 |
|
| 2 | 2a | 17 | − | 222 | 254.2 | 20.98 | − | 87.5 |
|
|
| 2b | 20 | −/++ b | 221 | 249.7 | 10.18 | +/++ b | 20 | + |
|
| 2c | 14.8 | − | 219 | 234.2 | 18.16 | − | 60 | − |
|
| 2d | 15 | − | 201.4 | 220 | 16.88 | − | 40 | + |
| 3 | 3a | 17.6 | | 203.5 | 233 | 21.78 | | 100 |
|
|
| 3b | 10.2 | tox. | 210.4 | 211 | 5.7 | tox. | 100 |
|
|
| 3c | 12 | tox. | 212.4 | 220 | 7.58 | tox. | 60 |
|
|
| 3d | 21.8 | +/+ b | 215.4 | 243 | 9.36 | − | 0 | + |
|
| 3e | 20 | − | 225.2 | 232 | 9.92 | − | 20 | − |
|
| 3f | 19.4 | − | 200 | 215 | 7.88 | + | 20 | − |
Light emission recorded at the terminal stage of the
animals, paralleled the tumor mass growing in the rat livers and
the animals' survival time. However, treatment with Gem, although
effective in reducing the liver weight significantly and extending
the life span of treated rats, was related to a distinctly higher
light emission than that observed following treatment with
riproximin or the combination of Rpx with Gem (Fig. 4).
Discussion
This is the first report on the activity of Rpx in
rat ASML pancreatic cancer cells in vitro and in
vivo. PDAC is a malignancy with low sensitivity to current
antineoplastic drugs (2). As a
consequence, the investigation of new drugs with activity in this
disease is of high potential interest. Rpx has fulfilled this
criteria as it showed high antiproliferative activity in ASML
mother cells as well as in three cell clones derived thereof.
Interestingly, there was a relatively high variation in sensitivity
to Rpx between the three ASML clones as the IC50 values obtained
after 24 h ranged from 17fold from 172 to 10 pM. An even larger
variation in sensitivity had been observed before in cell lines
derived from different tissues of origin (13), with the respective IC50 values
differing by a factor of 100. It is plausible to speculate that
these differences may depend on the cells' affinity to Rpx, which
affects its binding and uptake. Recently, the affinity of
riproximin to certain sugar moieties has been investigated
(15). Riproximin was shown to bind
with a high affinity to certain types of glyco-structures in a
carbohydrate microarray, namely to bi- and tri-antennary complex
N-glycan structures (NA2/NA3) and to repetitive
N-acetyl-D-galactosamine (GalNAc), the so-called clustered Tn
antigen, a cancer-specific O-glycan on mucins (15). Future experiments will be related to
the presence of these structures on ASML cells to possibly relate
their sensitivity to the respective presence of these
glyco-structures.
Another reason for variances in sensitivity could be
differences in proliferation rates between the ASML cell clones. It
is generally assumed that cancer cell sensitivity against
cytostatics is correlated with their proliferation rate (17). In line with this notion the ASML clone
5, having the shortest tumor cell doubling time, was most sensitive
to Rpx. This clone showed the lowest IC50 values after 24 to 96 h,
respectively, and did not show any stimulation at low
concentrations of Rpx. The mother cell line and clone 10, on the
other hand, were less sensitive, but showed an about 20-fold
reduction of initial IC50 values after four days of continuous
incubation. This indicates that the exposure to Rpx can cause not
only early but also late events leading to cell death.
The activity of Rpx in vivo against
pancreatic cancer liver metastasis was a main finding of the
present study and is related to the high anti-proliferative
activity against ASML cells in vitro. Regarding the liver as
metastasis site, it is of interest that Rpx showed also high
antineoplastic activity in a rat colorectal cancer liver metastasis
model in vivo (13). The
efficacy observed in this study was only slightly better than that
of gemcitabine, when the liver weight was considered. However, the
liver weight as indicator of remnant tumor mass differed
significantly from control, whereas that following exposure to
gemcitabine did not. In addition, the light emission following both
treatment groups showed that distinctly less tumor cells survived
after exposure to Rpx than after Gem. This indicates that Rpx
killed the ASML tumor cells more specifically than Gem. The
combination of Rpx with GEM did not improve the activity of Rpx,
the combination with DIN even thwarted the activity of Rpx. The
reason(s) why the activity of Rpx is reduced by an HDAC-inhibitor
like DIN remain unclear.
Rpx is a new plant lectin, which was classified as a
RIP of type II that was isolated from the plant Ximenia
americana. Its antineoplastic activity was recently
demonstrated in several studies from our group (13,14). It is
noteworthy that the combination of Rpx with either GEM or DIN did
not result in a further benefit for the survival of liver
metastases bearing animals, in spite of a further reduction in mean
tumor weight as compared to the respective monotherapy. Thus, the
search for suited combination partners will be continued in future
experiments.
As for other RIPs of type II, the antineoplastic
effect of Rpx is based on its ribosome inactivating property
(14). From the immunoblot analyses
of this study, clearly the apoptotic effect of Rpx was one of the
reasons for cell death in ASML pancreatic cancer cells and the
observed antineoplastic activity. In a previous study, we have
shown that the apoptotic property of Rpx was based on the induction
of endoplasmic reticulum stress (14). These results were confirmed in this
study as demonstrated by the reduction of ATF3 levels after
exposing ASML cells to Rpx. In addition, downstream of this
pathway, apoptosis was induced by activation of caspases 3, 8 and
9.
In summary, Rpx inhibited ASML cell proliferation at
low, pM concentrations, caused apoptosis and reduced tumor growth
significantly by 90% (P<0.05). Concomitantly, the survival rate
of rats was significantly increased (P=0.05). Higher doses of Rpx
caused no further reduction in tumor size when compared to the low
dose of Rpx or a combination of Rpx with GEM or DIN. The results
suggest that Rpx has a clear potential for use in pancreatic cancer
treatment. Further experiments are required for elucidating its
affinity for certain cancer cells and optimizing the combination
therapy with other antineoplastic agents.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burris HA IIIrd, Moore MJ, Andersen J,
Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK,
Storniolo AM, Tarassoff P, et al: Improvements in survival and
clinical benefit with gemcitabine as first-line therapy for
patients with advanced pancreas cancer: A randomized trial. J Clin
Oncol. 15:2403–2413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sant M, Allemani C, Santaquilani M, Knijn
A, Marchesi F and Capocaccia R; EUROCARE Working Group, :
EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999.
Results and commentary. Eur J Cancer. 45:931–991. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Demols A, Peeters M, Polus M, Marechal R,
Gay F, Monsaert E, Hendlisz A and Van Laethem JL: Gemcitabine and
oxaliplatin (GEMOX) in gemcitabine refractory advanced pancreatic
adenocarcinoma: A phase II study. Br J Cancer. 94:481–485. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim SK, Kim H, Lee DH, Kim TS, Kim T,
Chung C, Koh GY, Kim H and Lim DS: Reversing the intractable nature
of pancreatic cancer by selectively targeting aldh-high,
therapy-resistant cancer cells. PLoS One. 8:e781302013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Herrera L, Bostrom B, Gore L, Sandler E,
Lew G, Schlegel PG, Aquino V, Ghetie V, Vitetta ES and Schindler J:
A phase 1 study of Combotox in pediatric patients with refractory
B-lineage acute lymphoblastic leukemia. J Pediatr Hematol Oncol.
31:936–941. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stirpe F and Battelli MG:
Ribosome-inactivating proteins: Progress and problems. Cell Mol
Life Sci. 63:1850–1866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin JY, Tserng KY, Chen CC, Lin LT and
Tung TC: Abrin and ricin: New anti-tumour substances. Nature.
227:292–293. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zwierzina H, Bergmann L, Fiebig H, Aamdal
S, Schöffski P, Witthohn K and Lentzen H: The preclinical and
clinical activity of aviscumine: A potential anticancer drug. Eur J
Cancer. 47:1450–1457. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Voss C, Eyol E and Berger MR:
Identification of potent anticancer activity in Ximenia americana
aqueous extracts used by African traditional medicine. Toxicol Appl
Pharmacol. 211:177–187. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Voss C, Eyol E, Frank M, von der Lieth CW
and Berger MR: Identification and characterization of riproximin, a
new type II ribosome-inactivating protein with antineoplastic
activity from Ximenia americana. FASEB J. 20:1194–1196. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Horrix C, Raviv Z, Flescher E, Voss C and
Berger MR: Plant ribosome-inactivating proteins type II induce the
unfolded protein response in human cancer cells. Cell Mol Life Sci.
68:1269–1281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bayer H, Essig K, Stanzel S, Frank M,
Gildersleeve JC, Berger MR and Voss C: Evaluation of riproximin
binding properties reveals a novel mechanism for cellular
targeting. J Biol Chem. 287:35873–35886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eyol E, Murtaga A, Zhivkova-Galunska M,
Georges R, Zepp M, Djandji D, Kleeff J, Berger MR and Adwan H: Few
genes are associated with the capability of pancreatic ductal
adenocarcinoma cells to grow in the liver of nude rats. Once Rep.
28:2177–2187. 2012. View Article : Google Scholar
|
|
17
|
Corrie PG: Cytotoxic chemotherapy:
Clinical aspects. Medicine. 36:24–28. 2008. View Article : Google Scholar
|