Introduction
Current evidence demonstrates that cancer stem cells
(CSCs) or cancer stem like cells (CSLCs) are present in tumors
(1). In 1994, Lapidot et al
(2) isolated and identified tumor
stem cells from acute myeloid leukemia for the first time. Since
then, an increasing number of studies have demonstrated that CSLCs
are present in breast cancer and other common tumors (3–5). Only a
small fraction of original cells that are maintained in tumor
progression, metastasis and recurrence are capable of self-renewal
and multi-differentiation into tumor cells (6–9). Stem
cells can proliferate to form suspension sphere cell clones when
cultured in low adhesion culture vessels supplemented with
epidermal growth factor (EGF) and other growth factors (10). Thus, such sphere-formation cells have
been widely used in the isolation and identification of CSCs.
Various therapeutic measures are not effective for CSCs, which may
be the main reason for the failure of clinical treatment and tumor
recurrence.
Matrine, as an alkaloid, has a wide spectrum of
biological activities including immune regulation (11), antifibrosis (12) and antiviral activity (13). Previous studies have shown that
matrine may be used as one type of antitumor drug for leukemia and
lung, liver, gastric, breast and cervical cancers, as well as
numerous other types of malignant tumors with high drug resistant
activity (14,15). The present study confirmed that
certain concentrations of matrine could significantly inhibit the
proliferation and invasion of the human hepatocellular carcinoma
(HCC) cell line (16,17). However, to the best of our knowledge,
the effect and mechanism of matrine on hepatic cancer stem cells
in vitro has not yet been reported.
In the present study, CSLCs were isolated and
purified from a human liver cancer cell line, and the positive
antitumor effects of matrine were observed. Furthermore, the
associated mechanism was studied in vitro.
Materials and methods
Materials
The human liver cancer SMMC-7721 cell line was
purchased from American Type Culture Collection (Manassas, VA,
USA). BALB/c nude mice (40 males, aged between 4 and 6 weeks and
weighing between 16 and 18 g) were purchased from Shanghai
Experimental Animal Center of Chinese Academy of Sciences
(Shanghai, China). The nude mice were caged individually under
specific-pathogen free conditions in the Laboratory Animal Research
Center of Ningbo University at a temperature of 22±2°C, a relative
humidity between 40 and 60% and artificially illuminated on an
~12-h light/dark cycle. The air exchange rate was about 18 times/h.
All of the nude mice were provided with sterilized normal pellet
food and sterile water ad libitum. The Ningbo University
Experimental Animal Care Commission approved the experimental
protocol. Dulbecco's modified Eagle's medium (DMEM), fetal bovine
serum, epidermal growth factor (EGF), basic fibroblast growth
factor (bFGF), noggin, cisplatin, Verso 1-Step RT-PCR kit was
purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
The fluorescence microscope (magnification, ×100) and digital image
photographic system were Leica products (Leica Microsystems, Inc.,
Buffalo Grove, IL, USA).
Hepatoma stem like cell enrichment and
cultivation
The human hepatocellular carcinoma SMMC-7721 cell
line was cultured in DMEM containing 10% fetal bovine serum at 37°C
in a 5% CO2 atmosphere. At the logarithmic growth phase,
cells were harvested, resuspended in tumor stem cell enrichment
medium (DMEM serum-free culture medium + 10 ng/ml EGF + 10 ng/ml
bFGF + 10 ng/ml noggin + 1,000 µ/ml leukemia inhibitory factor) and
then plated into polyhydroxy ehtyl methacrylate pretreatment flasks
for two weeks. Subsequent to the formation of clones, cells were
digested, centrifuged (1,000 × g for 10 min) at 4°C and resuspended
in PBS. The cell suspension (~5×105 cells) was
inoculated into the back of nude mice to form a solid tumor. After
two weeks, 1, 2 and 5 mg/kg of cisplatin was injected into mice to
select the strongest resistance of tumor stem cells. Subsequently,
the tumor tissue within the most notable effects of cisplatin
intervention was irrigated, trimmed, cut broken, repeatedly
digested into single cells and then cultured in tryptose sulfite
cycloserine (TSC) medium at 37°C in a 5% CO2 atmosphere.
During this time period, the adherent cells were continually
discarded. The suspended cells were continuously collected,
repeatedly triturated into a single cell suspension and
reinoculated in TSC medium.
Immunofluorescence microscopy
The expression of CD24 was firstly analyzed using
immunofluorescence microscopy. Liver CSC clones from the SMMC-7721
cell line were digested into single cells with trypsin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and incubated in a
blocking solution consisting of 10% normal fetal bovine serum and
0.1% Triton X-100 (Sigma-Aldrich; Merck KGaA) in 0.1 M PBS for 1 h
at room temperature. Then, rat anti-human CD24 (cat. no. 563545;
dilution, 1:1,000; BD Pharmingen; BD Biosciences, Franklin Lakes,
NJ, USA) were added to the cells and incubated at room temperature
for 1 h. FITC-conjugated anti-rat IgM monoclonal antibody (cat. no.
553887; dilution, 1:500; BD Pharmingen; BD Biosciences) were
applied at 4°C overnight for visualization. Following antibody
staining, cells were fixed with 2% paraformaldehyde at room
temperature for 15 min and mounted with DAPI-fluoromount-G
(SouthernBiotech, Birmingham, AL, USA). Fluorescent micrographs
were obtained using Leica DM IL LED inverted fluorescent microscope
(magnification, ×100; Leica Microsystems, Inc.).
Cell viability assay
MTT (Sigma-Aldrich; Merck KGaA) was applied to
evaluate the effects on proliferation and viability of liver stem
cells. Liver CSC clones were digested into single cells and plated
in triplicate at 5,000 cells per well onto 96-well plates, and
cultured with different concentrations of matrine (0, 5, 25, 50,
100 and 200 µg/ml). Following incubation for 72 h, 10 µl of MTT
solution (5 µg/ml) was added to each well. The plates were then
incubated for 4 h at 37°C. Intracellular formazan crystals were
dissolved by the addition of 100 µl of isopropanol-hydrochloric
acid-sodium dodecyl sulfate solution (10%) to each well. Following
overnight incubation at 37°C, the optical density of the samples
was determined at 570 nm. Rate of inhibition was calculated using
the equation: Cell inhibition rate (%)=(treated group-control
group)/control group ×100.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA of hepatocellular carcinoma SMMC-7721
cells (SMMC-7721), hepatocellular carcinoma stem cell
SMMC-7721-sphere (SMMC-7721-sphere) and hepatocellular carcinoma
stem cell SMMC-7721-sphere with 50 µg/ml matrine treatment
(SMMC-7721-sphere-Matrine) were extracted using the
TRIzol® Reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) Reverse transcription was performed using 2 µg of total RNA,
according to the manufacturer's protocol for Verso 1-Step RT-PCR
kit's (Thermo Fisher Scientific, Inc.). Surface marker expression
of octamer-binding transcription factor-4 (OCT-4), NANOG, Notch,
cluster of differentiation (CD)133, CD24, sex determining region
Y-box-2 (SOX-2) and CD90 in tumor stem-like cells was detected
using RT-qPCR. Furthermore, the association between mRNA levels of
CAR, E-cadherin, laminin and fibronectin and cell invasion and
metastasis was also determined. β-actin was used as an internal
control. The primers used for amplification are shown in Table I.
 | Table I.PCR amplification primers. |
Table I.
PCR amplification primers.
| Genes | Primers |
|---|
| OCT-4 | F:
5′-CTGGGTTGATCCTCGGACCT-3′ |
|
| R:
5′-CCATCGGAGTTGCTCTCCA-3′ |
| NANOG | F:
5′-TTTGTGGGCCTGAAGAAAACT-3′ |
|
| R:
5′-AGGGCTGTCCTGAATAAGCAG-3′ |
| Notch | F:
5′-GCACTTTCTGTGAGGAGGACAT-3′ |
|
| R:
5′-AGCAGGAGCTCTCTGTGCAGT-3′ |
| CD133 | F:
5′-TCGGAAACTGGCAGATAGCAA-3′ |
|
| R:
5′-GTGAACGCCTTGTCCT-3′ |
| CD24 | F:
5′-TTTGACTAGATGATGAATGCCAAT-3′ |
|
| R:
5′-GGATGTTGCCTCTCCTTCAT-3′ |
| SOX-2 | F:
5′-AAGAGAACACCAATCCCATCCA-3′ |
|
| R:
5′-AGTCCCCCAAAAAGAAGTCCA-3′ |
| CD90 | F:
5′-GTTAGGCTGGTCACCTTCTG-3′ |
|
| R:
5′-GAGATCCCAGAACCATGAACC-3′ |
| CAR | F:
5′-TGTTCATGCCGACGCTTGCA-3′ |
|
| R:
5′-TTCCAACTACACAGTTTATT-3′ |
| E-cadherin | F:
5′-GGTCTCCTCATGGCTTTGCC-3′ |
|
| R:
5′-CACAGTTCTCAAAGCACAGCG-3′ |
| Laminin | F:
5′-CAGGCCCGCAAACAAGCAGC-3′ |
|
| R:
5′-TCCAAGCGTGTGGACCCGGA-3′ |
| Fibronectin | F:
5′-GCCGCCACGTGCCAGGATTA-3′ |
|
| R:
5′-ACCAGTTGGGGAAGCTCGTCTG-3′ |
| β-actin | F:
5′-CTGTCTGGCGGCACCACCAT-3′ |
|
| R:
5′-GCAACTAAGTCATAGTCCGC-3′ |
Western blot analysis
Collected cells were lysed immediately in RIPA
buffer (150 mM NaCl, 50 mM Tris, 1% NP-40, 0.5% sodium
deoxycholate, 0.1% SDS, pH 7.4), supplemented with protease
inhibitor cocktail (Roche Diagnostics, Basel, Switzerland), Protein
concentration was determined using the Micro BCA kit (Pierce;
Thermo Fisher Scientific, Inc.). Equal amounts of protein (60 µg)
were boiled for 5 min, separated by SDS-PAGE and electro-blotted to
a nitrocellulose membrane. Following blocking, the blots were
incubated with an appropriate dilution of specific rabbit
antibodies against E-cadherin (catalog no. 3195S; dilution,
1:1,000; Cell Signaling Technology Inc., Dnavers, MA, USA), laminin
(catalog no. ab11575; dilution, 1:1,000; Abcam, Cambridge, UK),
fibronectin (catalog no. ab2413; dilution, 1:1,000; Abcam) for 1 h
at room temperature. The blots were washed by TBST reagent (50 mM
Tris, pH 7.4, 150 mM NaCl, 0.05% Tween-20) three times and then
incubated with a 1:2,000 dilution of horseradish
peroxidase-conjugated secondary antibody (catalog no. sc-2492;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h at room
temperature. The blots were washed three times and then developed
using a chemiluminescence assay. Finally, β-actin (Cell Signaling
Technology, Inc., Danvers, MA, USA) was used as a loading
control.
Statistical processing
Statistical analysis was performed using SPSS 16.0
(SPSS, Inc., Chicago, IL, USA). All measurement data was analyzed
by one-way analysis of variance test, followed by Tukey's test. For
data without a normal distribution, Wilcoxon test were used to
analyze the difference between the same cell groups exposed to
different treatments. P<0.05 was considered to indicate a
statistically significant difference. Dose-dependent associations
were determined by multiple linear regressions. All experiments
were repeated at least twice (with duplicate assays).
Results
CSLC clone formation of the SMMC-7721
cell line is induced by chemotherapeutic drug resistance
screening
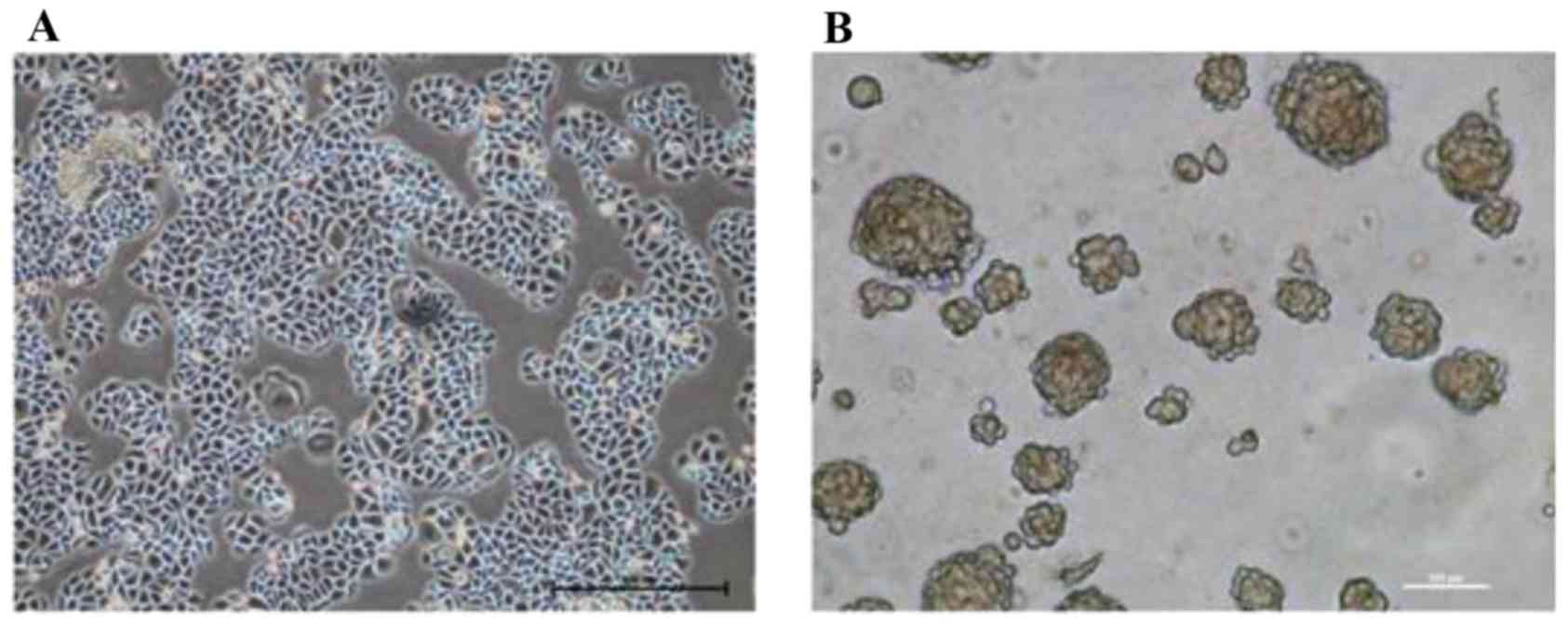
Morphological observation revealed that the cultured
SMMC-7721 cells possessed the typical characteristics of epithelial
cells, and adhered to the bottom of the dish in a monolayer
(Fig. 1A). Following TSC medium
suspension culture for two weeks, SMMC-7721-sphere cell clones were
formed (Fig. 1B) and transplanted
into nude mice to generate xenogeneic tumors. After two weeks,
cisplatin was selected to interfere with the proliferation of the
tumor. The results suggested that cisplatin treatments inhibited
tumor growth in a time and dose-dependent manner; among them, 5
mg/kg of cisplatin treatment exhibited the most drug resistance.
Following intervention for 18 days, the cells of the 5 mg/kg
cisplatin group were isolated, digested and cultured in TSC medium
for another two weeks until the new cell clones were reformed.
These SMMC-7721-sphere-cisplatin cell clones had CSLC
characteristics, including amplification ability, strong
tumorigenesis ability in vivo and resistance to chemotherapy
(Table II).
 | Table II.Tumor size in nude mice inoculated
with different concentrations of cisplatin (mm2). |
Table II.
Tumor size in nude mice inoculated
with different concentrations of cisplatin (mm2).
| Time, days | Control | 1 mg/kg | 2 mg/kg | 5 mg/kg |
|---|
| 3 | 520.20±3.36 | 519.70±5.21 | 517.90±7.08 |
510.30±6.09b |
| 6 | 699.90±5.53 | 650.20±5.71 |
599.90±5.61a |
529.90±5.72b |
| 9 | 1,000.00±6.99 | 971.10±6.67 |
739.80±5.77a |
560.20±5.71b |
| 12 | 1,502.00±10.09 | 1,299.10±7.37 |
799.30±5.30a |
529.80±5.14b |
| 15 | 1,601.00±8.39 | 1,502.30±6.42 |
829.60±4.55a |
520.20±3.35b |
| 18 | 1,700.50±8.24 | 1,600.90±8.53 |
799.50±5.40a |
499.90±6.72b |
Different types of stem cell markers
were expressed in the obtained stem-like cell clones
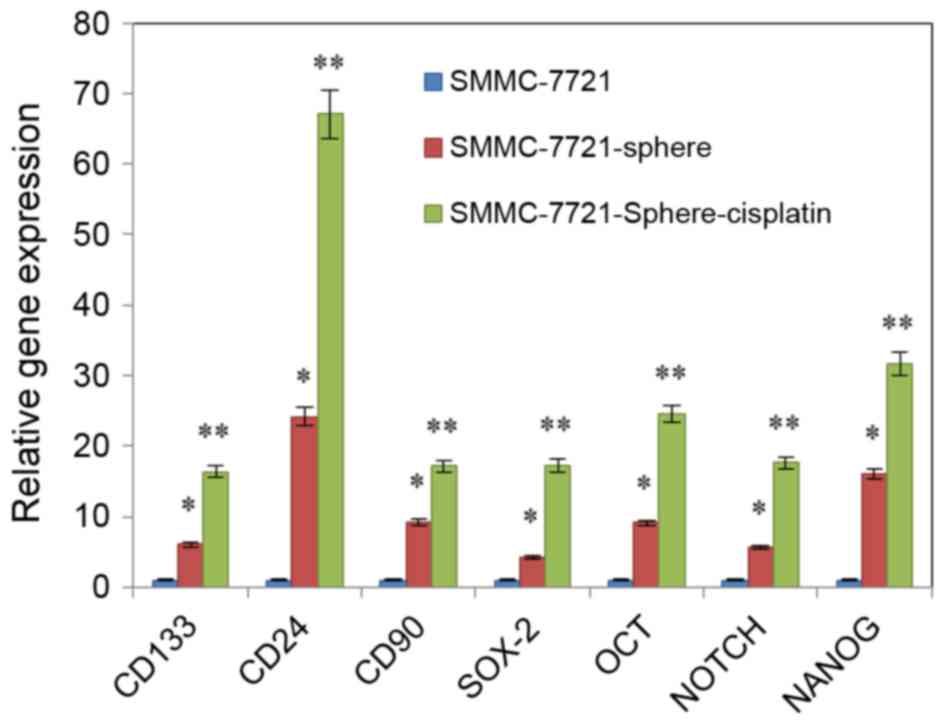
qPCR revealed that the expression levels of
NANOG, SOX-2, OCT-4, Notch,
CD24, CD90 and CD133 were upregulated in
SMMC-7721-sphere-cisplatin as compared with SMMC-7721 and
SMMC-7721-sphere. Among them, expression of CD24 was the most
increased (Fig. 2; P<0.01).
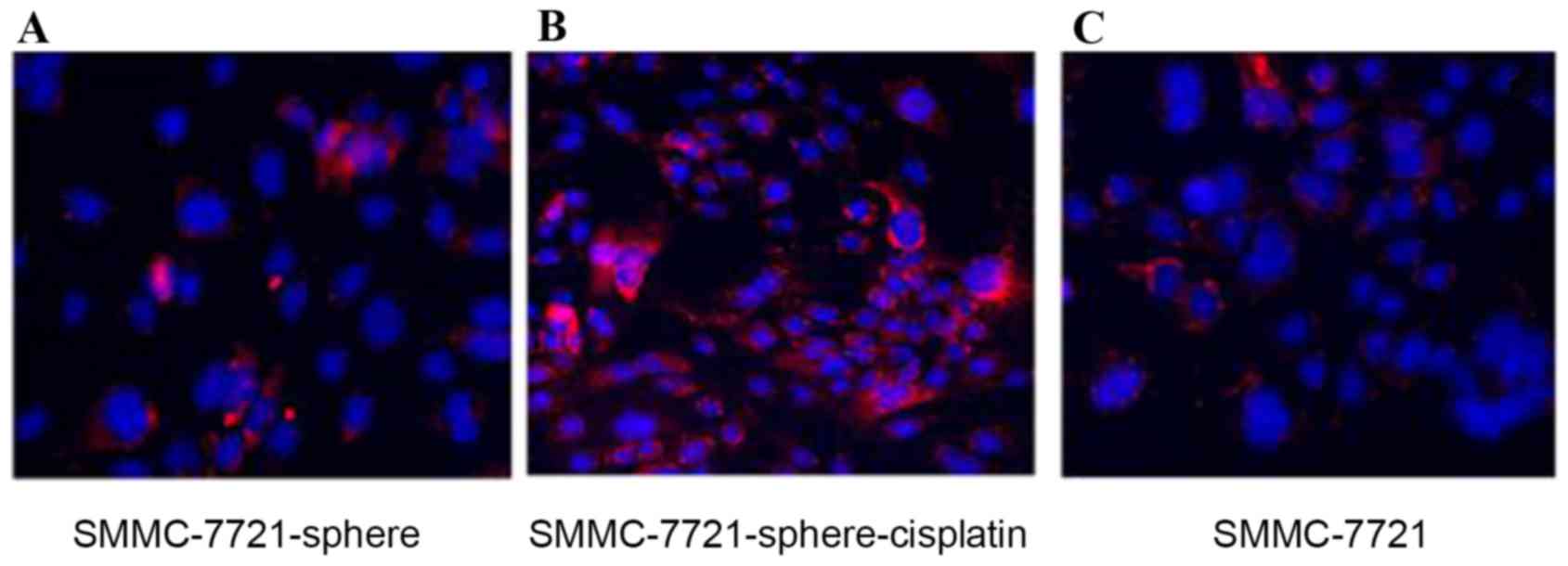
Fig. 3 also showed that CD24 was
highly expressed in SMMC-7721-sphere-cisplatin compared with
SMMC-7721 and SMMC-7721-sphere (Fig.
3).
Matrine inhibits the proliferation of
liver CSLCs in a dose-dependent manner
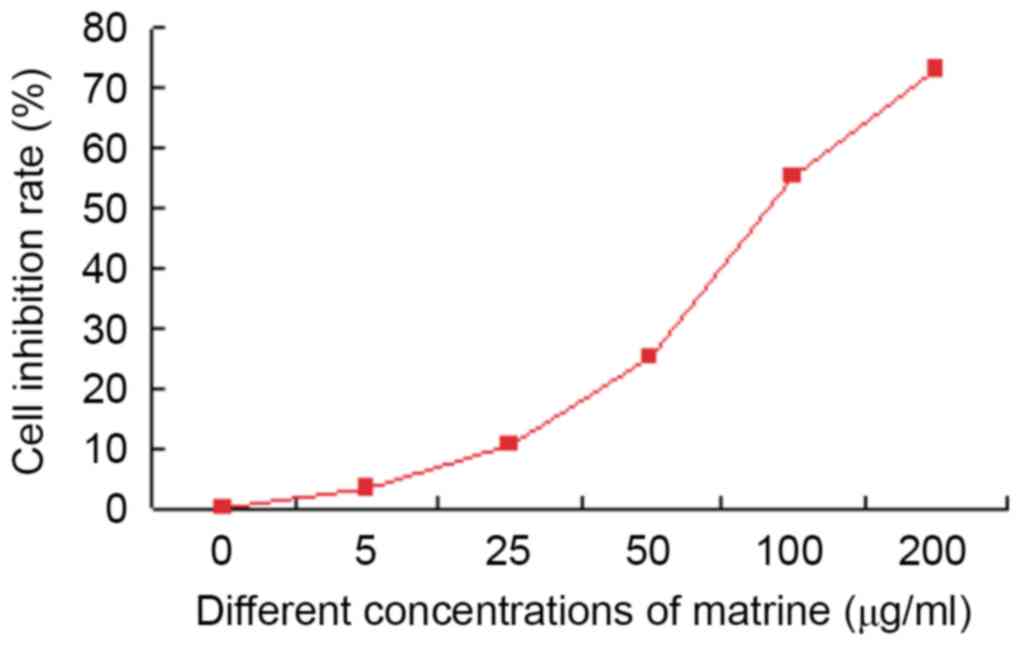
MTT results revealed that matrine inhibited the
growth of liver cancer stem cells in vitro. Matrine (5
µg/ml) showed little effect (inhibition rate only 3.0%±0.3%). The
inhibition rate of 100 µg/ml matrine was >50% (55.1%±0.6%). In
addition, the cytotoxic effect of 200 µg/ml matrine on liver cancer
stem cells was observed at 72 h with an inhibition rate of
77.4%±0.8%. On the basis of the aforementioned results, 50 µg/ml of
matrine was selected for the subsequent experiments (Fig. 4).
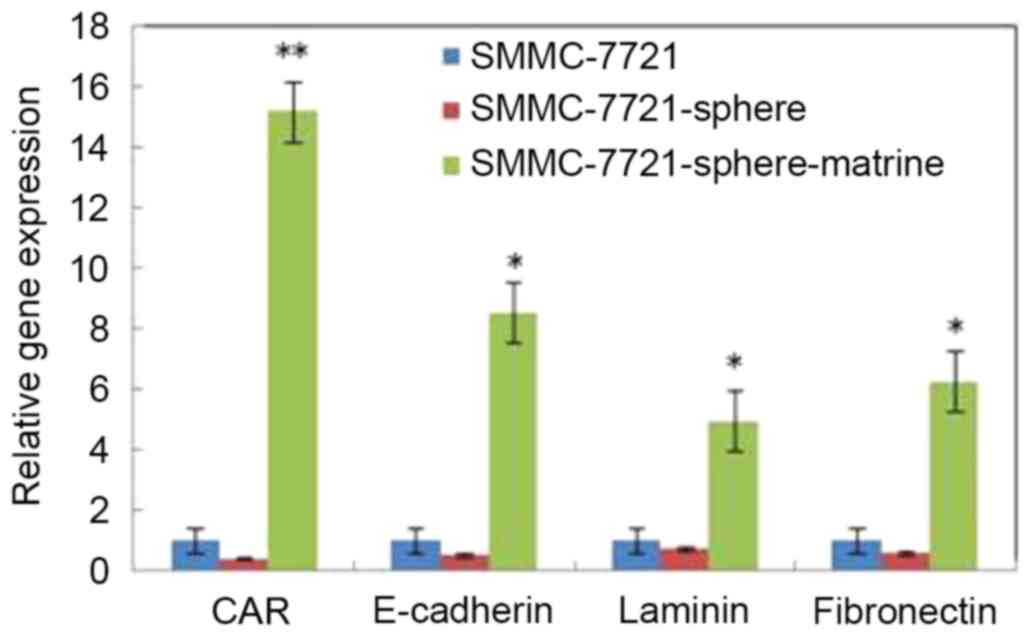
Matrine induces the expression of CAR,
E-cadherin, laminin and fibronectin in liver CSLCs
To explore the mechanisms underlying matrine-induced
apoptosis, the expression of CAR, E-cadherin, laminin and
fibronectin was detected by RT-qPCR. As shown in Fig. 5, the mRNA levels of CAR, laminin,
E-cadherin and fibronectin in SMMC-7721-sphere cells were lower
than SMMC-7721 cells. However, following matrine treatment, the
mRNA levels of these genes were significantly increased by 15.2,
8.5, 4.9 and 6.3 times, respectively (P<0.05).
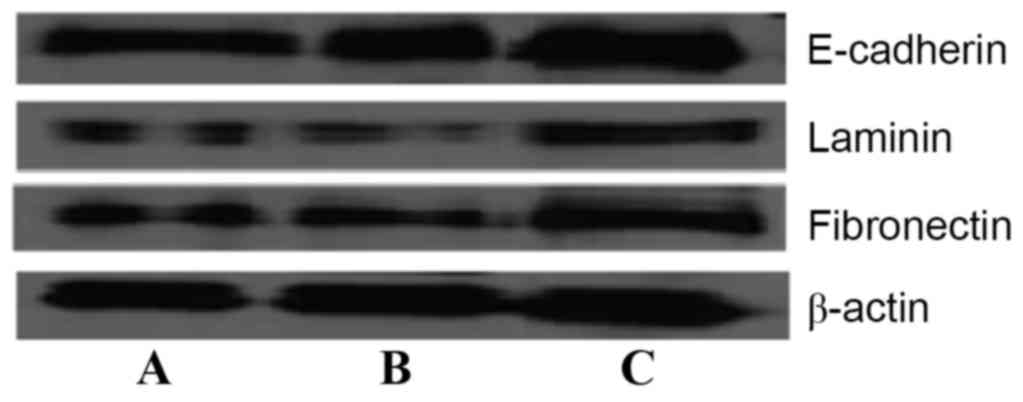
Furthermore, western blot analysis results (Fig. 6) also demonstrated that the expression
of laminin, fibronectin and E-cadherin protein in SMMC-7721-sphere
cells was lower than in SMMC-7721 cells after 72 h. Matrine (50
µg/ml) significantly induced the expression of these proteins,
which was consistent with the aforementioned results
(P<0.05).
Discussion
For decades, matrine, as a traditional Chinese
herbal medicine, has proved to possess cytoprotective effects and
biological safety, which has been used for a number of treatments,
including hepatic fibrosis, atherosclerosis, arrhythmias and
infectious diseases (18,19). Previous studies have suggested that
matrine may eliminate tumor cells by apoptosis induction (20,21). Ma
et al (22) demonstrated that
matrine significantly inhibited the invasion of human pancreatic
cancer cells by downregulating the expression of membrane type
1-matrix metalloproteinase. However, previous studies mainly
focused on the antitumor effects of matrine on common tumor cells.
Based on the theory of CSCs, the malignant degree of tumors is
mainly determined by the proportion of CSCs. Common cancer cells
may be eliminated through various treatments. Due to possessing a
strong drug tolerance, CSCs are not easily destroyed, which leads
to the failure of treatment. Therefore, a key goal for tumor
therapy is to effectively induce the apoptosis of tumor stem cells
(23).
In the present study, tumor stem-like cells were
isolated and purified from the human HCC SMMC-7721 cell line, which
overexpresses the tumor stem cell specific marker CD24 (24–26). MTT
results suggested that different concentrations (25, 50, 100 and
200 µg/ml) of matrine may inhibit the proliferation of stem like
cells in vitro. In addition, the malignancy of the tumor is
not only determined by the ability of proliferation, but is also
associated with the invasion and metastasis of tumor cells.
Therefore, it is necessary to evaluate the effect of matrine on
tumor cell invasion and metastasis. Previous studies demonstrated
that the high expression of E-cadherin, laminin and fibronectin
also proved to be positively associated with tumor recurrence and
metastasis (27–30). Therefore, laminin, fibronectin and
E-cadherin were selected to evaluate the mechanism of matrine on
the invasion and metastasis in the SMMC-7721 cell line. The results
demonstrated that the expression of laminin, fibronectin and
E-cadherin in CSLCs was decreased compared with common liver cancer
cells. However, 50 µg/ml matrine could significantly induce the
expression of laminin (P<0.05), fibronectin (P<0.05) and
E-cadherin (P<0.05), which indicated that matrine could reduce
the adhesion ability of HCC cells, thereby inducing the apoptosis
of liver CSLCs in vitro.
CAR performs a role in the process of tumor
recurrence and metastasis. Yamamoto et al detected 30
patients with primary hepatic carcinoma and identified that CAR
mRNA level was significantly depressed in HCC tissues compared with
the adjacent tissue (31). The
present study also showed that the mRNA level of CAR was markedly
increased by matrine treatment, which suggested that matrine
exerted antitumor effects through the regulation of the CAR signal
transduction pathway. The results of the present study demonstrated
that matrine exhibited preferential anti-tumor effects against
hepatocellular carcinoma stem cells. Therefore, it may be a
therapeutic agent for HCC treatment in the future.
Acknowledgements
The present study was supported by Ningbo Medical
Project Foundation (grant no. 2011B05) and the Major Science and
Technology Planning Program of Ningbo (grant no. 2012C5013).
References
|
1
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature. 367:645–648. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 100:pp.
3983–3988. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh SK, Clarke ID, Hide T and Dirks PB:
Cancer stem cells in nervous system tumors. Oncogene. 23:7267–7273.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Kruithof-de Julio M, Economides
KD, Walker D, Yu H, Halili MV, Hu YP, Price SM, Abate-Shen C and
Shen MM: A luminal epithelial stem cell is a cell of origin for
prostate cancer. Nature. 461:495–500. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells-perspectives on current status and future directions:
AACR workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang YM and Chang JW: Current status and
issues in cancer stem cell study. Cancer Invest. 26:741–755. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vermeulen L, Todaro M, de Sousa Mello F,
Sprick MR, Kemper K, Alea M Perez, Richel DJ, Stassi G and Medema
JP: Single-cell cloning of colon cancer stem cells reveals a
multi-lineage differentiation capacity. Proc Natl Acad Sci USA.
105:pp. 13427–13432. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jordan CT: Cancer stem cell biology: From
leukemia to solid tumors. Curr Opin Cell Biol. 16:708–712. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Uchida S, Yokoo S, Yanagi Y, Usui T,
Yokota C, Mimura T, Araie M, Yamagami S and Amano S: Sphere
formation and expression of neural proteins by human corneal stoma
cells in vitro. Invest Ophthalmol Vis Sci. 46:1620–1625. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu L, Pan QX, Zhang XJ, Xu YM, Chu YJ,
Liu N, Lv P, Zhang GX and Kan QC: Protective effects of matrine on
experimental autoimmune encephalomyelitis via regulation of ProNGF
and NGF signaling. Exp Mol Pathol. 100:337–343. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen JX, Shen HH, Niu M, Guo YM, Liu XQ,
Han YZ, Zhang YM, Zhao YL, Bai BK, Zhou WJ and Xiao XH:
Anti-hepatitis B virus effect of matrine-type alkaloid and
involvement of p38 mitogen-activated protein kinase and tumor
necrosis factor receptor-associated factor 6. Virus Res.
215:104–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang JP, Zhang M, Zhou JP, Liu FT, Zhou
B, Xie WF and Guo C: Antifibrotic effects of matrine on in vitro
and in vivo models of liver fibrosis in rats. Acta Pharmacol Sin.
22:183–186. 2001.PubMed/NCBI
|
|
14
|
Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q and
Su C: Anti-tumor activities of matrine and oxymatrine: Literature
review. Tumor Biol. 35:5111–5119. 2014. View Article : Google Scholar
|
|
15
|
Liao H, Zhao X, Qu J, Zhang J and Cai H:
Matrine suppresses invasion and metastasis of NCI-H1299 cells by
enhancing microRNA-133a expression. Int J Clin Exp Med.
8:10714–10722. 2015.PubMed/NCBI
|
|
16
|
Qian L, Liu Y, Xu Y, Ji W, Wu Q, Liu Y,
Gao Q and Su C: Matrine derivative WM130 inhibits hepatocellular
carcinoma by suppressing EGFR/ERK/MMP-2 and PTEN/AKT signaling
pathways. Cancer Lett. 368:126–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang N, Han F, Cui H, Huang J, Wang T,
Zhou Y and Zhou J: Matrine suppresses proliferation and induces
apoptosis in human cholangiocarcinoma cells through suppression of
JAK2/STAT3 signaling. Pharmacol Rep. 67:388–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao HY, Li GY, Lou MM, Li XY, Wei XY and
Wang JH: Hepatoprotective effect of Matrine salvianolic acid B salt
on carbon tetrachloride-induced hepatic fibrosis. J Inflamm.
9:162012. View Article : Google Scholar
|
|
19
|
Xin HB and Liu SF: Effects of matrine on
myocardial contraction and arrhythmia in isolated heart atria.
Zhongguo Yao Li Xue Bao. 8:501–505. 1987.(In Chinese). PubMed/NCBI
|
|
20
|
Wang HQ, Jin JJ and Wang J: Matrine
induces mitochondrial apoptosis in cisplatin-resistant non-small
cell lung cancer cells via suppression of β-catenin/survivin
signaling. Oncol Rep. 33:2561–2566. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang N, Han F, Cui H, Huang J, Wang T,
Zhou Y and Zhou J: Matrine suppresses proliferation and induces
apoptosis in human cholangiocarcinoma cells through suppression of
JAK2/STAT3 signaling. Pharmacol Rep. 67:388–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma Y, Zou F, Xiong J, Wan W, Yin L, Li X,
Bei Z, Yuan L, Meng S, Wang J and Song G: Effect of Matrine on HPAC
cell migration by down-regulating the expression of MT1-MMP via Wnt
signaling. Cancer Cell Int. 15:592015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gilbert CA and Ross AH: Cancer stem cells:
Cell culture, markers and targets for new therapies. J Cell
Biochem. 108:1031–1038. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ke J, Wu X, Wu X, He X, Lian L, Zou Y, He
X, Wang H, Luo Y, Wang L and Lan P: A subpopulation of CD24+ cells
in colon cancer cell lines posse stem cell characteristics.
Neoplasma. 59:282–288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Smith SC, Oxford G, Wu Z, Nitz MD, Conaway
M, Frierson HF, Hampton G and Theodorescu D: The
metastasis-associated gene CD24 is regulated by Ral GTPase and is a
mediator of cell proliferation and survival in human cancer. Cancer
Res. 66:1917–1922. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Wei J, Wang H, Xue X, An Y, Tang
D, Yuan Z, Wang F, Wu J, Zhang J and Miao Y: Epithelial mesenchymal
transition correlates with CD24+CD44+ and CD133+ cells in
pancreatic cancer. Oncology Rep. 27:1599–1605. 2012.
|
|
27
|
Inayoshi J, Iehida T, Sugitani S, Tsuboi
Y, Genda T, Honma N and Asakura H: Gross appearance of
hepatocellular careinoma reflects E-cadherin expression and risk of
early recurrence after surgical treatment. J Gastroen Hepatol.
18:673–677. 2003. View Article : Google Scholar
|
|
28
|
Masaki T, Sugiyama M, Matsuoka H, Abe N,
Izumisato Y, Sakamoto A and Atomi Y: Coexpression of matrilysin and
Laminin-5 gamma2 chain may contribute to tumor cell migration in
colorectal carcinomas. Dig Dis Sci. 48:1262–1267. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mohammadizadeh F, Ghasemibasir H, Rajabi
P, Naimi A, Eftekhari A and Mesbah A: Correlation of E-cadherin
expression and routine immunohistochemistry panel in breast
invasive ductal carcinoma. Cancer Biomark. 5:1–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kowalski PJ, Rubin MA and Kleer CG:
E-cadherin expression in primary carcinomas of the breast and its
distant metastases. Breast Cancer Res. 5:R217–R222. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamamoto H, Itoh F, Sakamoto H, Nakajima
Y, Une Y, Hinoda Y and Imai K: Association of reduced cell adhesion
regulator messenger RNA expression with tumor progression in human
hepatocellular carcinoma. Int J Cancer. 74:251–254. 1997.
View Article : Google Scholar : PubMed/NCBI
|