Introduction
Cholangiocarcinoma (CCA) is the second most common
primary liver malignancy after hepatocellular carcinoma in 2012
worldwide (1). It is defined as a
rare and fatal tumor, which in previous decades has demonstrated a
steady increase in the incidence and mortality rate worldwide
(2). The early clinical symptoms of
CCA are challenging to identify, resulting in a lack of accurate
diagnosis at the early stage, and inability to control the
incidence rate (3). Patients with
early stage CCA are usually recommended to undergo surgical
resection, which is considered to be the most favorable therapy to
achieve good prognosis and, to a certain extent, prolong the
survival of CCA patients (4,5). While the prognosis of advanced CCA
patients remains poor, metastasis is regarded as the major factor
that contributes to their poor outcome (5–7). Tumor
metastasis is a complex and multistep biological process, wherein
metastasis-promoting genes and inhibition of metastasis suppressors
play critical roles during the entire metastatic process (8). A number of genes can affect the tumor
metastatic process, and therefore, researchers aim to develop novel
therapeutic targets to control CCA metastasis.
Fyn, a member of the Src tyrosine kinase family
(SFK), is known to be involved in several biological activities
associated with cancer. Fyn expression is upregulated in multiple
human cancers including prostate cancer, glioblastoma, chronic
myelogenous leukemia, melanoma and squamous cell carcinoma
(9–13). Fyn expression is also associated with
tumor metastasis. Chen et al (14,15) have
found that Fyn expression is upregulated in pancreatic cancer and
that Fyn requires HnRNPA2B1 and Sam68 to coordinate and regulate
metastasis in pancreatic cancer. In advanced prostate cancer, Fyn
was found to enhance the neuroendocrine phenotype and increase
visceral metastasis (16). In
colorectal cancer, Fyn is induced by cellular prion protein
accelerates colorectal cancer metastasis (17). In breast cancer, Fyn expression is
induced by Ras/PI3K/Akt signaling and is essential for enhanced
breast cancer migration and invasion (18).
The exact function and mechanism of Fyn in CCA
metastasis remains unclear. In the present study, the expression of
Fyn in CCA cell lines and the association with CCA cell migration
and invasion were investigated. The results showed that Fyn is
upregulated in CCA cell lines and that downregulation of Fyn
suppresses CCA cell migration and invasion. Mechanistic studies
revealed that Fyn inhibited AMPK/mTOR signaling, which promoted CCA
cell migration and invasion. The present results confirmed that Fyn
blockade is a potential target for anti-CCA therapy.
Materials and methods
Cell culture
The normal bile duct HIBEC cell line and the human
CCA QBC939, RBE, CCLP1 and HCCC-9810 cell lines were purchased from
the Cell Bank of Chinese Academy of Sciences (Shanghai, China).
Cells were cultured in RPMI-1640 medium with 10% fetal bovine serum
(FBS), penicillin (100 U/ml) and streptomycin (100 µg/ml) (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a
humidified atmosphere containing 5% CO2.
Fyn knockdown
Suppression of Fyn (NM_002037.5) expression was
achieved using short hairpin RNA (shRNA) lentiviral transduction
particles. The short hairpin RNA (shRNA) of Fyn (NM_002037.5;
shFyn) was amplified from normal human genomic DNA using the
following primers (Genewiz, Inc., Suzhou, China): shRNA forward
(BamHI),
5′-GATCCCACAGGTGGCTGCAGGAATGGTCAAGAGCCATTCCTGCAGCCACCTGTGTTTTTTTG-3′
and reverse, (EcoRI),
5′-AATTCCAAAAAAACACAGGTGGCTGCAGGAATGGCTCTTGACCATTCCTGCAGCCACCTGTGG-3′.
The shFyn was cloned into pLVX-shRNA1 vectors (Clontech
Laboratories, Inc., Mountain View, CA, USA), termed pLVX-shFyn.
Recombinant pLVX-shFyn and pHelper 1.0 and 2.0 plasmids (Clontech
Laboratories, Inc.) were generated by the transient transfection of
HEK 293T cells (2×106 cells; Cell Bank of Chinese
Academy of Sciences), using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) following the manufacturer's protocol, and
the lentivirus was packaged in accordance with a previous study
(19). For stable transfection,
QBC939 cells were infected with the virus supernatant fluid along
with 8 µg/ml polybrene and selected in 0.5 µg/ml puromycin for one
week, followed by 0.2 µg/ml puromycin for one month. The stable
cells were harvested 24 or 48 h after transfection for additional
analysis.
Cell groups and treatment
The stable QBC939 cells expressing shFyn were termed
shFyn. The QBC939 cells transfected with lentivirus expressing
negative control shRNA were termed shNC. To validate that Fyn
promotes CCA metastasis through the activated AMPK/mTOR signaling
pathway, AMPK inhibitor compound C (10 µM; Calbiochem, San Diego,
CA, USA) was used to inhibit the AMPK/mTOR signaling and 1%
dimethyl sulphoxide (DMSO) was used as negative control (NC). In
the present study, 2×105 shFyn-transfected QBC939 cells
were cultured at 37°C for 24 h in a humidified atmosphere
containing 5% CO2, and subsequently with 10 µl compound
C or DMSO at 37°C for 24 or 48 h. Then, the shFyn-transfected
QBC939 cells were harvested for additional analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from cultured QBC939 cells
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) according
to the manufacturer's instructions, and the RNA was reverse
transcribed into cDNA at 42°C for 15 min using AffinityScript QPCR
cDNA Synthesis kit (Agilent Technologies, Inc., Santa Clara, CA,
USA). Fyn RNA expression was detected by RT-qPCR using Brilliant II
SYBR Green QPCR Master Mix kit (Agilent Technologies, Inc.).
Amplification was performed using the following PCR conditions:
Preheating at 95°C for 10 min, followed by 40 cycles of 95°C for 10
sec, 60°C for 20 sec and 72°C for 10 sec. Fyn and 18srRNA (internal
control) primers were synthesized by Sangon Biotech (Shanghai,
China). The following primer sequences were used: Fyn forward,
5′-GGGTGCTAATGTGGAGACTG-3′ and reverse, 5′-GCTTTGATGCTGACTTGCAG-3′;
and 18srRNA forward, 5′-CCTGGATACCGCAGCTAGGA-3′ and reverse,
5′-GCGGCGCAATACGAATGCCCC-3′. RT-qPCR was performed on an Applied
Biosystems 7500 system (Thermo Fisher Scientific, Inc.) with melt
curve analysis. Gene expression was measured in triplicate. The
data were analyzed using the 2−ΔΔCq method (20). Quantitative analysis was performed
using the ratio of the target gene to 18srRNA, and normalized to
the control.
Western blotting
Protein expression of Fyn, AMPK, phosphorylated AMPK
(p-AMPK), mTOR and phosphorylated mTOR (p-mTOR) was detected by
western blot analysis. The normal bile duct HIBEC cell line and
human CCA QBC939, RBE, CCLP1 and HCCC-9810 cell lines were
harvested for Fyn protein expression analysis. QBC939 cells, stably
expressing shNC or shFyn, were harvested for Fyn, AMPK, p-AMPK,
mTOR and p-mTOR analysis. All harvested cells were lysed using
radioimmunoprecipitation assay buffer (Takara Biotechnology, Co.,
Ltd., Dalian, China), followed by protein quantification using BCA
Protein Assay kit (Beyotime Institute of Biotechnology, Haimen,
China). Approximately 30 µg of protein sample was loaded per lane.
Proteins were separated on 10% SDS-PAGE gel and transferred to a
polyvinylidene difluoride membrane. The membrane was blocked in
Tris-buffered saline with 1% Tween-20 buffer containing 5% skimmed
dried milk and incubated at 4°C overnight. The primary antibodies,
consisting of rabbit monoclonal anti-Fyn (dilution, 1:1,000;
catalog no. ab125016), mouse monoclonal anti-AMPK (dilution, 1:500;
catalog no. ab80039), rabbit monoclonal anti-p-AMPK (dilution,
1:2,000; catalog no. ab133448), rabbit polyclonal anti-mTOR
(dilution, 1:2,000; catalog no. ab2732), rabbit polyclonal
anti-p-mTOR (dilution, 1:1,000; catalog no. ab109268) (all from
Abcam, Cambridge, MA, USA) and anti-GAPDH (dilution, 1:10,000;
catalog no. KC-5G5; Kangchen Biotech Co., Ltd., Shanghai, China),
were added and the mixture was incubated overnight at 4°C on a
rocking platform. This was followed by incubation of the membrane
with goat anti-mouse or goat anti-rabbit IgG (H+L) secondary
antibody conjugated to horseradish peroxidase (1:5,000; catalog no.
1034-05 or 4050-05, respectively; Southern Biotech, Birmingham, AL,
USA) for 2 h. The proteins were visualized using Pierce™ ECL
Western Blotting Substrate (Pierce; Thermo Fisher Scientific, Inc.)
and were exposed on X-ray film. Gray scale images were then
analyzed using ImageJ software (National Institutes of Health,
Bethesda, MD, USA). The expression levels of proteins of interest
were normalized to the expression of GAPDH.
Transwell migration and invasion
assays
Cell migration and invasion were assessed using a
Transwell migration assay. For the migration assay, the suspension
(200 µl) containing 1×105 QBC939 cells, stably
expressing shNC or shFyn, was dispensed into the upper chamber (8
µm pore size; BD Biosciences, San Diego, CA, USA) and RPMI-1640
medium containing 10% FBS was added to the lower chamber of the
Transwell. The chamber was incubated at 37°C for 48 h in a
humidified atmosphere containing 5% CO2. Following,
cells were fixed with 4% paraformaldehyde for 15 min at 25°C and
stained with 0.1% crystal violet in 20% ethanol for 10 min at 25°C.
Images were captured using a LEICA light microscope (magnification,
×200). The number of migrating cells in the center and five
surrounding independent fields were counted, and average counts
were calculated as the migrating cell numbers. For the invasion
assay, the artificial substrate Matrigel (BD Biosciences) was
layered in the Transwell chamber and 1×105 QBC939 cells
from each group were dispensed into the upper chamber followed by
incubation at 37°C for 48 h in a humidified atmosphere containing
5% CO2. The subsequent treatment procedures followed
those used for the migration assays. Each experiment was performed
with three wells and the same experiment was measured in
triplicate.
Statistical analysis
Statistical analyses were performed using SPSS
version 19.0 (IBM Corp., Armonk, NY, USA). Quantitative data are
presented as the mean ± standard deviation. Fisher's least
significant difference test was used to control for the multiple
comparisons of comparing all CCA cell lines to the same HIBEC
control. The differences between shNC and shFyn groups were
analyzed by the Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
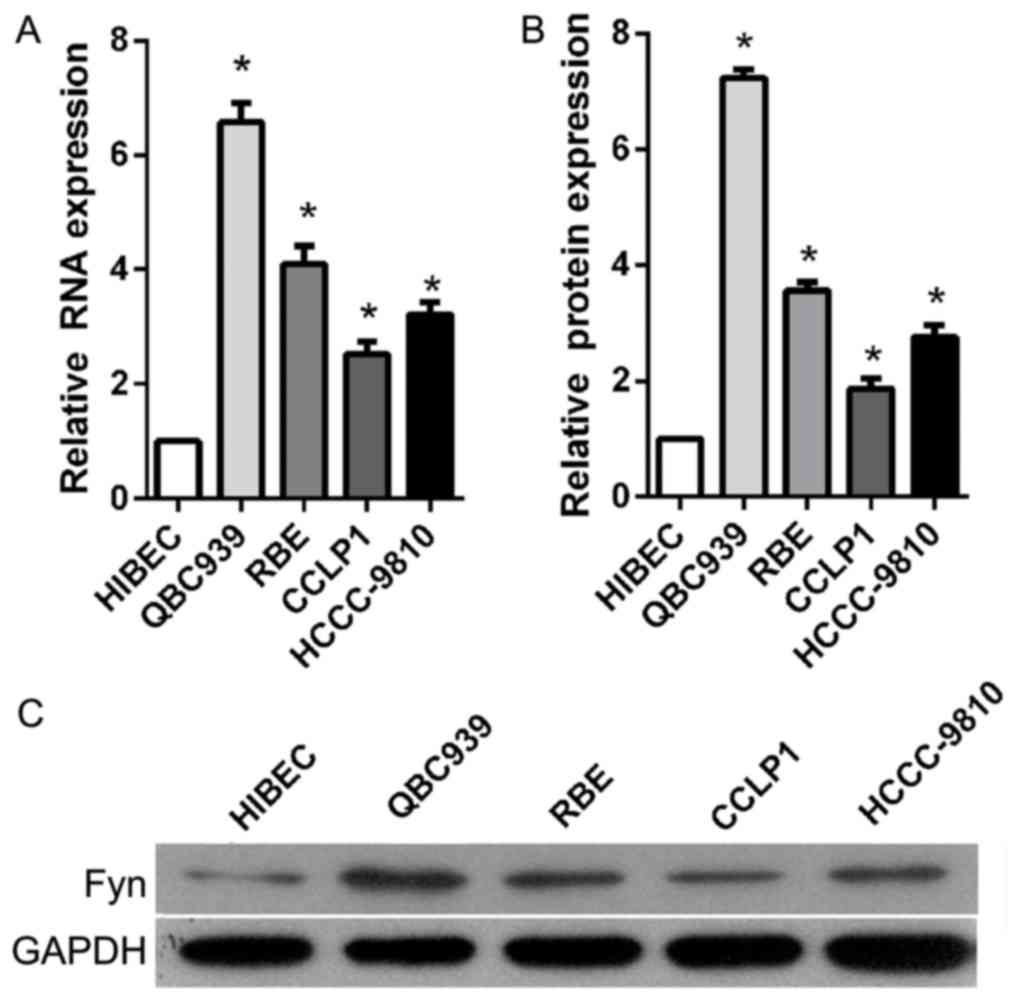
Fyn was upregulated in CCA tissues and
cell lines
To investigate the role of Fyn in the regulation of
CCA cell migration and invasion, the present study determined Fyn
expression levels in the normal bile duct HIBEC cell line and in
human CCA QBC939, RBE, CCLP1, and HCCC-9810 cell lines using
RT-qPCR and western blot analysis. The results showed that Fyn
expression was significantly decreased in CCA cell lines compared
with HIBEC cells, particularly QBC939 cells (Fig. 1). Based on these results, QBC939 cells
were used for subsequent experiments.
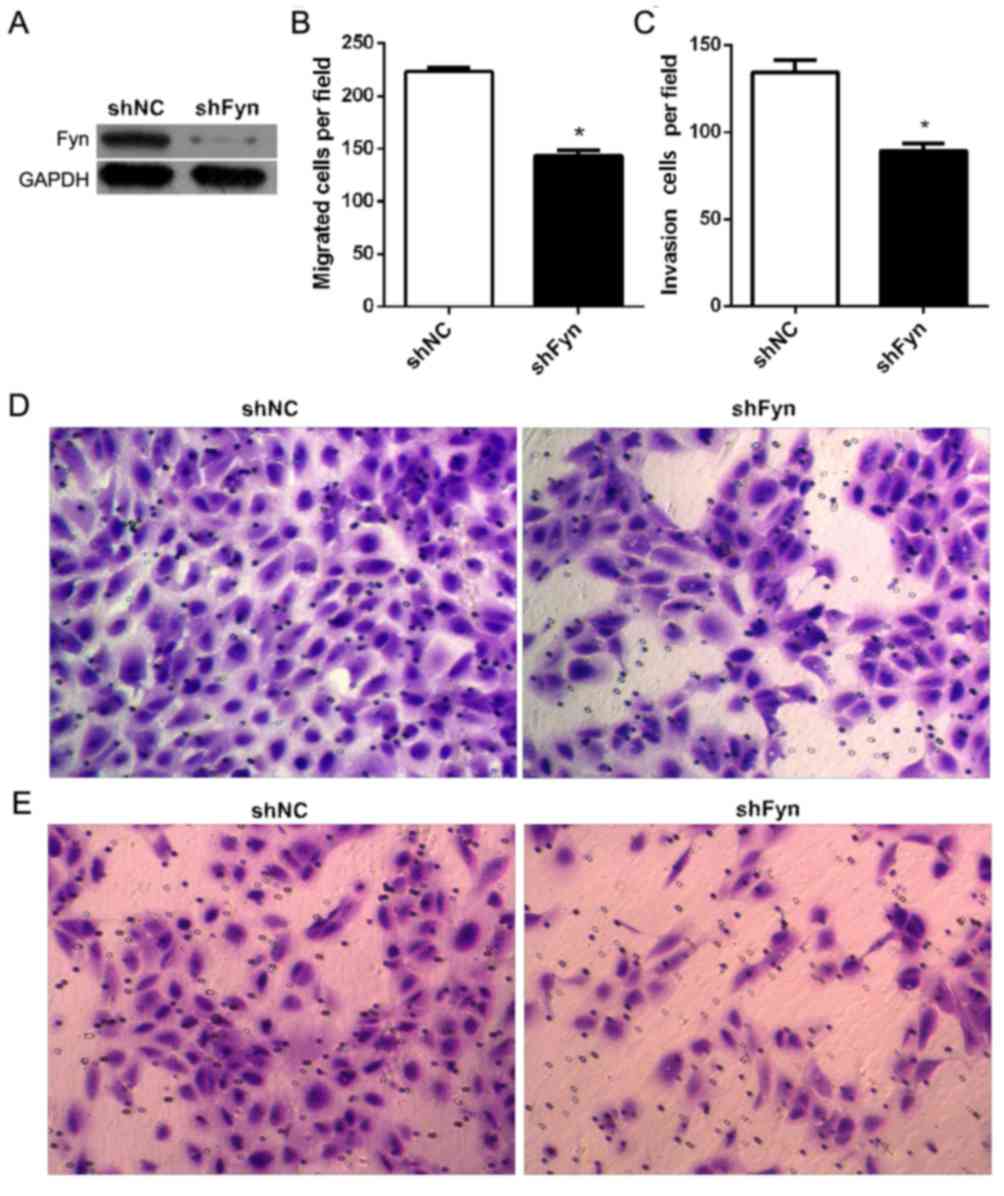
Fyn knockdown inhibited CCA cell
migration and invasion
To investigate the role of Fyn in the regulation of
CCA cell migration and invasion, shFyn was transfected into QBC939
cells. After 48 h of transfection, QBC939 cells were harvested for
western blot analysis. The results showed that transfection of
QBC939 cells with shFyn effectively inhibited Fyn expression
(Fig. 2A). Furthermore, in order to
measure QBC939 cell migration and invasion, Transwell assays were
used subsequent to transfection of cells with shFyn or shNC for 48
h. The number of migrating cells after transfection with shNC or
shFyn was 223±9 and 144±13, respectively (P<0.05; Fig. 2B), and the number of invading cells
was 134±17 and 89±10, respectively (P<0.05) (Fig. 2C). The Transwell migration assay
showed that the ability of migration through the membrane into the
lower chamber was significantly inhibited in cells transfected with
shFyn compared with shNC-transfected cells (P<0.05; Fig. 2D). The Transwell invasion assay showed
that the ability of cells to invade a Matrigel-coated membrane and
migrate into the lower chamber was significantly decreased in cells
transfected with shFyn compared with shNC-transfected cells
(P<0.05; Fig. 2E).
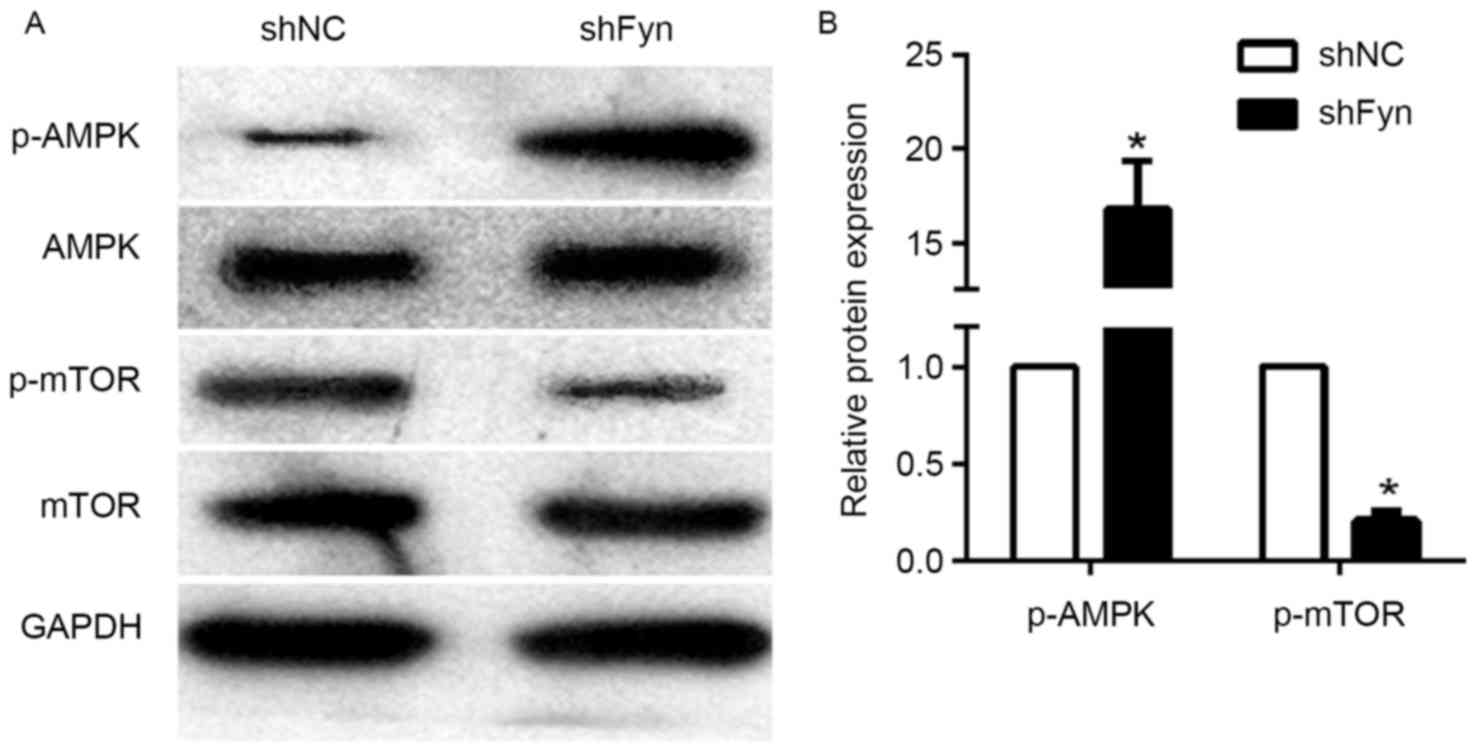
Effects of Fyn inhibition on AMPK/mTOR
signaling pathway
The AMPK/mTOR signaling pathway has an important
role in tumor development and metastasis. To investigate the
potential mechanism of Fyn in regulation of CCA cell migration and
invasion, the present study examined the effects of Fyn on
AMPK/mTOR signaling. The expression of p-AMPK, AMPK, p-mTOR and
mTOR in the QBC939 cells transfected with shFyn and shNC was
analyzed by western blotting (Fig.
3A). The results showed that phosphorylated AMPK levels in
QBC939 cells transfected with shFyn were significantly increased
compared with QBC939 cells transfected with shNC. By contrast, the
expression of phosphorylated mTOR was decreased, but the expression
of AMPK and mTOR were not significantly different in both groups
(Fig. 3B). Thus, Fyn knockdown can
activate the AMPK/mTOR signaling pathway.
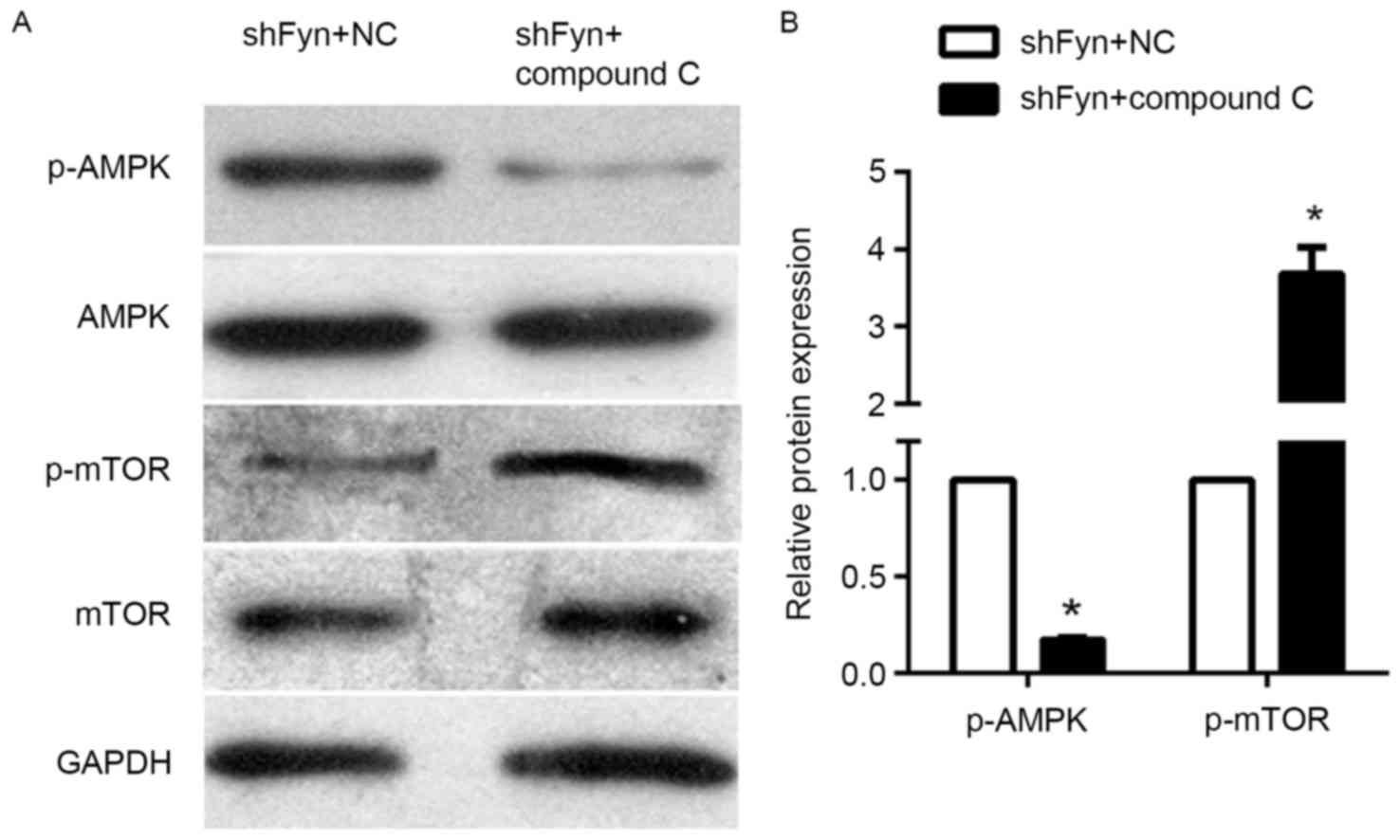
Compound C can reverse the effect of
AMPK/mTOR signaling
The expression of p-AMPK, AMPK, p-mTOR and mTOR in
the CCA QBC939 cell line treated with shFyn plus compound C or NC
were analyzed by western blotting (Fig.
4A). The results of western blotting experiments showed that
levels of phosphorylated AMPK in QBC939 cells treated with shFyn
plus compound C were significantly decreased compared with QBC939
cells treated with shFyn plus NC. By contrast, the levels of
phosphorylated mTOR were increased, whereas the expression of AMPK
and mTOR were not significantly different between both the groups
(Fig. 4B). Thus, compound C can
reverse the effect the effect of Fyn shRNA on AMPK/mTOR signaling
pathway in QBC939 cells.
Inhibition of AMPK/mTOR promoted CCA
cell migration and invasion
In order to investigate the role of Fyn and
AMPK/mTOR signaling in the regulation of CCA cell migration and
invasion, the present study cultured QBC939 cells treated with
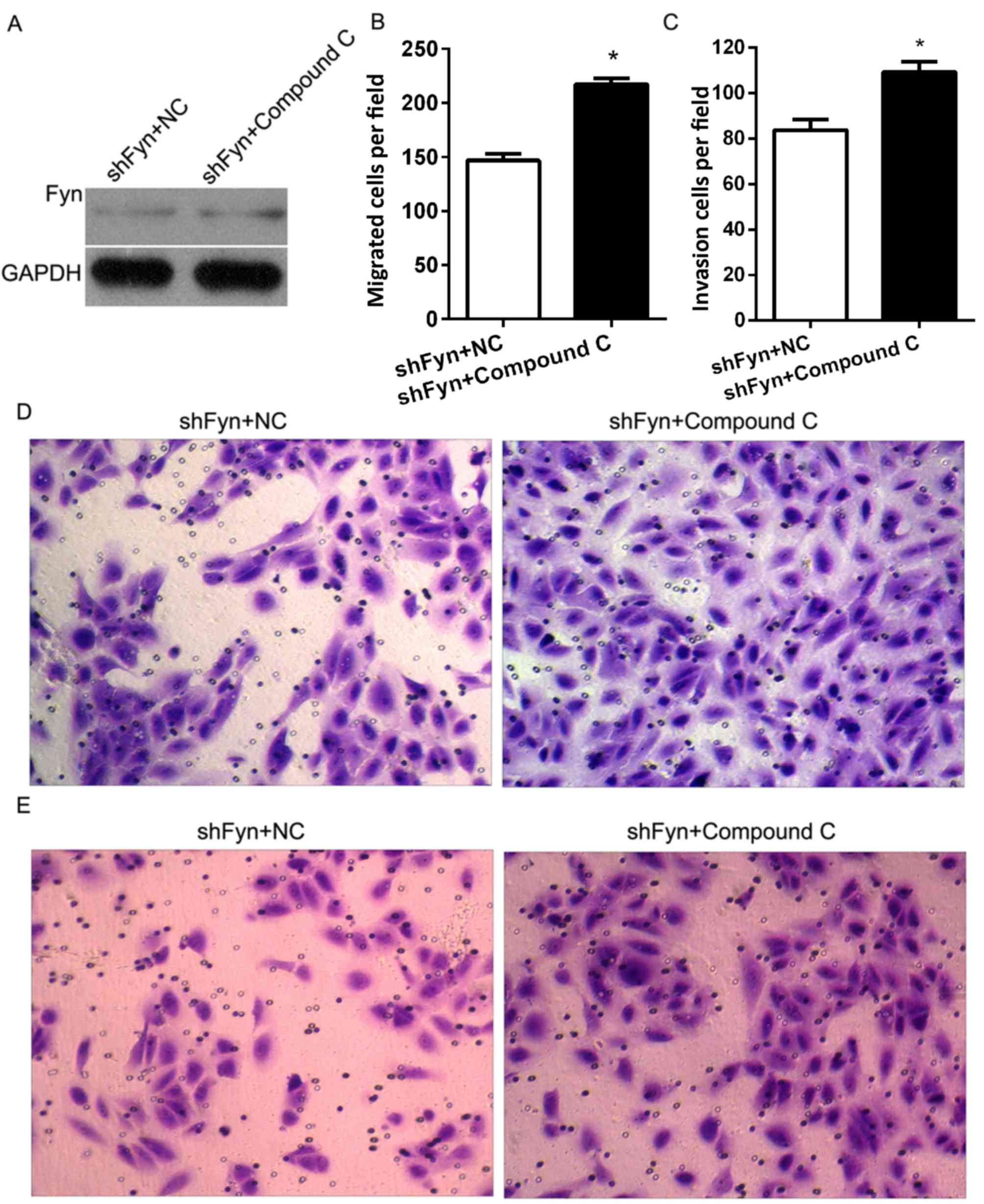
shFyn plus compound C or NC. After 48 h of culture, Fyn expression
was detected by western blotting. The results showed no differences
in Fyn expression between the QBC939 cells treated with shFyn plus
compound C and the QBC939 cells treated with shFyn plus NC
(Fig. 5A). In addition, Transwell
assays were used to estimate the migration and invasion of QBC939
cells treated with shFyn plus compound C or NC. After 48 h of
culture, the migration numbers of QBC939 cells treated with shFyn
plus compound C or NC were 147±16 and 217±15, respectively
(P<0.05; Fig. 5B), and the
invasion numbers of QBC939 cells treated with shFyn plus compound C
or NC were 83±11 and 109±11, respectively (P<0.05; Fig. 5C). The Transwell migration assay
showed that the ability of the cells to migrate through the
membrane into the lower chamber was significantly inhibited in the
shFyn plus compound C group compared to the shFyn plus NC group
(P<0.05; Fig. 5D). The Transwell
invasion assay showed that the ability of the cells to pass through
a Matrigel-coated membrane into the lower chamber was significantly
decreased in shFyn plus compound C group compared to shFyn plus NC
group (P<0.05; Fig. 5E).
Discussion
CCA is a rare and fatal tumor with steadily
increasing incidence and mortality rates worldwide over previous
decades (1). Metastasis is regarded
as the major factor that contributes to the poor prognosis of CCA
patients (5). Therefore, researchers
aim to develop novel therapeutic targets to control CCA metastasis.
Fyn, a member of the SFK, has been found to enhance expression and
promote tumor metastasis in various cancers, including pancreatic,
prostate and colorectal cancers (15–17). In
the present study, the function of Fyn in the regulation of CCA
cell migration and invasion was investigated. The present results
provide in vitro evidence that Fyn is overexpressed in CCA
cell lines and that silencing Fyn inhibits CCA cell migration and
invasion. These results reveal the oncogenic potential of Fyn in
CCA in a manner similar to other cancers.
Little is known about the mechanisms by which Fyn
induces migration and invasion of CCA cell lines. AMPK is a
ubiquitous serine/threonine protein kinase that regulates tumor
occurrence, development and chemoresistance through negative
regulation of mTOR. Li et al (21) showed that vitamin D3 potentiates the
growth inhibitory effects of metformin in human prostate DU145
cancer cells through activation of p-AMPK with subsequent
inhibition of downstream mTOR signaling. Wu et al (22) demonstrated that activation of
AMPK/mTOR signaling pathway is involved in autophagy-mediated
cisplatin resistance in lung adenocarcinoma. AMPK/mTOR signaling
also has an important role in tumor metastasis. In human non-small
cell lung carcinoma (NSCLC), activated AMPK/mTOR signaling pathway
suppressed the invasion and migration of NSCLC cells (23). In the present study, the effects of
Fyn on AMPK/mTOR signaling were investigated. These findings
demonstrate that Fyn knockdown can activate the phosphorylation of
AMPK, inhibit downstream phosphorylation of mTOR, and activate the
AMPK/mTOR signaling pathway.
Furthermore, the present study cultured CCA cells
treated with shFyn plus compound C or NC to investigate the role of
Fyn and AMPK/mTOR signaling in the regulation of CCA cell migration
and invasion. As expected, the AMPK inhibitor compound C suppressed
the AMPK phosphorylation and increased mTOR phosphorylation,
without significantly affecting Fyn expression. In addition, shFyn
plus compound C promoted the migration and invasion of CCA cells
compared with shFyn plus NC. Thus, compound C could reverse the
effect of Fyn shRNA on migration and invasion of CCA cells.
In conclusion, Fyn knockdown inhibits cell migration
and invasion by activating the AMPK/mTOR signaling pathway in CCA
cells. The present data only provide in vitro evidence
regarding the role of Fyn in CCA cells. In vivo studies are
required to further confirm the oncogenic role of Fyn and to help
establish a therapeutic strategy based on Fyn targeting.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tyson GL, Ilyas JA, Duan Z, Green LK,
Younes M, El-Serag HB and Davila JA: Secular trends in the
incidence of cholangiocarcinoma in the USA and the impact of
misclassification. Dig Dis Sci. 59:3103–3110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chinese Chapter of International
Hepato-Pancreato-Biliary Association, ; Liver Surgery Group, ;
Surgical Branch of the Chinese Medical Association, ; Cai JQ, Cai
SW, Cong WM, Chen MS, Chen P, Chen XP, Chen YL, Chen YF, Dai CL,
Huang Q, et al: Diagnosis and treatment of cholangiocarcinoma: A
consensus from surgical specialists of China. J Huazhong Univ Sci
Technolog Med Sci. 34:469–475. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luo X, Yuan L, Wang Y, Ge R, Sun Y and Wei
G: Survival outcomes and prognostic factors of surgical therapy for
all potentially resectable intrahepatic cholangiocarcinoma: A large
single-center cohort study. J Gastrointest Surg. 18:562–572. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Yang H, Shen C and Luo J:
Cholangiocarcinoma: Prognostic factors after surgical resection in
China. Int J Clin Exp Med. 8:5506–5512. 2015.PubMed/NCBI
|
|
6
|
Yubin L, Chihua F, Zhixiang J, Jinrui O,
Zixian L, Jianghua Z, Ye L, Haosheng J and Chaomin L: Surgical
management and prognostic factors of hilar cholangiocarcinoma:
Experience with 115 cases in China. Ann Surg Oncol. 15:2113–2119.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng-Rong L, Hai-Bo Y, Xin C, Chuan-Xin
W, Zuo-Jin L, Bing T, Jian-Ping G and Sheng-Wei L: Resection and
drainage of hilar cholangiocarcinoma: An 11-year experience of a
single center in mainland China. Am Surg. 77:627–633.
2011.PubMed/NCBI
|
|
8
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Posadas EM, Al-Ahmadie H, Robinson VL,
Jagadeeswaran R, Otto K, Kasza KE, Tretiakov M, Siddiqui J, Pienta
KJ, Stadler WM, et al: FYN is overexpressed in human prostate
cancer. BJU Int. 103:171–177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu KV, Zhu S, Cvrljevic A, Huang TT,
Sarkaria S, Ahkavan D, Dang J, Dinca EB, Plaisier SB, Oderberg I,
et al: Fyn and SRC are effectors of oncogenic epidermal growth
factor receptor signaling in glioblastoma patients. Cancer Res.
69:6889–6898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ban K, Gao Y, Amin HM, Howard A, Miller C,
Lin Q, Leng X, Munsell M, Bar-Eli M, Arlinghaus RB and Chandra J:
BCR-ABL1 mediates up-regulation of Fyn in chronic myelogenous
leukemia. Blood. 111:2904–2908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang J, Asawa T, Takato T and Sakai R:
Cooperative roles of Fyn and cortactin in cell migration of
metastatic murine melanoma. J Biol Chem. 278:48367–48376. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JE, Roh E, Lee MH, Yu DH, Kim DJ, Lim
TG, Jung SK, Peng C, Cho YY, Dickinson S, et al: Fyn is a redox
sensor involved in solar ultraviolet light-induced signal
transduction in skin carcinogenesis. Oncogene. 35:4091–4101. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen ZY, Cai L, Bie P, Wang SG, Jiang Y,
Dong JH and Li XW: Roles of Fyn in pancreatic cancer metastasis. J
Gastroenterol Hepatol. 25:293–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen ZY, Cai L, Zhu J, Chen M, Chen J, Li
ZH, Liu XD, Wang SG, Bie P, Jiang P, et al: Fyn requires HnRNPA2B1
and Sam68 to synergistically regulate apoptosis in pancreatic
cancer. Carcinogenesis. 32:1419–1426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gururajan M, Cavassani KA, Sievert M, Duan
P, Lichterman J, Huang JM, Smith B, You S, Nandana S, Chu GC, et
al: SRC family kinase FYN promotes the neuroendocrine phenotype and
visceral metastasis in advanced prostate cancer. Oncotarget.
6:44072–44083. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Q, Qian J, Wang F and Ma Z: Cellular
prion protein accelerates colorectal cancer metastasis via the
Fyn-SP1-SATB1 axis. Oncol Rep. 28:2029–2034. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yadav V and Denning MF: Fyn is induced by
Ras/PI3K/Akt signaling and is required for enhanced
invasion/migration. Mol Carcinog. 50:346–352. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song JL, Zheng W, Chen W, Qian Y, Ouyang
YM and Fan CY: Lentivirus-mediated microRNA-124 gene-modified bone
marrow mesenchymal stem cell transplantation promotes the repair of
spinal cord injury in rats. Exp Mol Med. 49:e3322017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li HX, Gao JM, Liang JQ, Xi JM, Fu M and
Wu YJ: Vitamin D3 potentiates the growth inhibitory effects of
metformin in DU145 human prostate cancer cells mediated by
AMPK/mTOR signalling pathway. Clin Exp Pharmacol Physiol.
42:711–717. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu T, Wang MC, Jing L, Liu ZY, Guo H, Liu
Y, Bai YY, Cheng YZ, Nan KJ and Liang X: Autophagy facilitates lung
adenocarcinoma resistance to cisplatin treatment by activation of
AMPK/mTOR signaling pathway. Drug Des Devel Ther. 9:6421–6431.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang JI, Hong JY, Lee HJ, Bae SY, Jung C,
Park HJ and Lee SK: Anti-tumor activity of yuanhuacine by
regulating AMPK/mTOR signaling pathway and actin cytoskeleton
organization in non-small cell lung cancer cells. PLoS One.
10:e01443682015. View Article : Google Scholar : PubMed/NCBI
|