Introduction
Hodgkin's lymphoma (HL) is an uncommon malignancy
mainly involving the lymph nodes and the lymphatic system. The
majority of patients commonly present with progressive painless
enlargement of the lymph nodes, particularly around the cervical
and supraclavicular lymph node regions. Extra-nodal forms of HL are
rare, accounting for <1% of all cases (1,2). In
late-stage HL, only 9–35% of cases have been described as
presenting with osseous involvement (3). HL lesions in the bone are mostly located
in the vertebrae and ribs (4), while
an extra-nodal presentation in the sternum is even more rare, with
nine cases clearly reported (4). The
current study presents a case of HL in which sternal involvement
was secondary to extension from the mediastinal lymph nodes. A
major point of interest in this case is the obvious extra-nodal
infiltration of the sternum by the mediastinal lymph node. Another
aspect of interest is that this type of HL is among the most
relatively benign forms, the nodular sclerosing variety, which has
an improved prognosis compared with other types, including mixed
cell type and lymphocyte depletion type. With current
polychemotherapy and involved-field radiation therapy, the
long-term prognosis of patient appears promising.
Case report
A 25-year-old woman presented with intermittent,
needle-like sternal pain on December 8, 2013 and then admitted to
The Cancer Center, Union Hospital of Huazhong University of Science
and Technology (Wuhan, China). There was no evidence of B-symptoms;
the patient was clinically well and apyrexial, with no history of
night sweats or weight loss. There was no history of
breathlessness.
Upon clinical examination, there was a direct
tenderness on the upper middle thorax. A small (1.5 cm in diameter)
mobile lymph node was found in the right supraclavicular region,
and another (3×1.5 cm) in the right axilla. Careful examination did
not reveal any other palpable nodes elsewhere. The surface skin had
a normal appearance and a normal temperature. No other
abnormalities were found. There was no other evidence of
lymphadenopathy or bone lesions.
Initial investigations revealed an elevated
erythrocyte sedimentation rate (22 mm/h. reference value is
0.00–20.00 mm/h), with a white a blood cell count of
17.41×109/l (normal range, 4.0–10.0×109/l)
and neutrophilia 14.24×109/l (normal range,
2.00–7.00×109/l). Monocyte count (1.26×109/l)
was slightly increased (normal range, 0.12–0.80×109/l).
The rest of the important laboratory results were within normal
limits, including creatinine, liver enzymes, alkaline phosphatase,
lactate dehydrogenase (LDH) and hemoglobin levels.
A cellular bone marrow aspiration smear showed no
evidence of lymphoma infiltration. Upon performing a computed
tomography (CT) plain scan, the anterior middle mediastinum
presented with multiple nodules of different sizes. Sternal plain
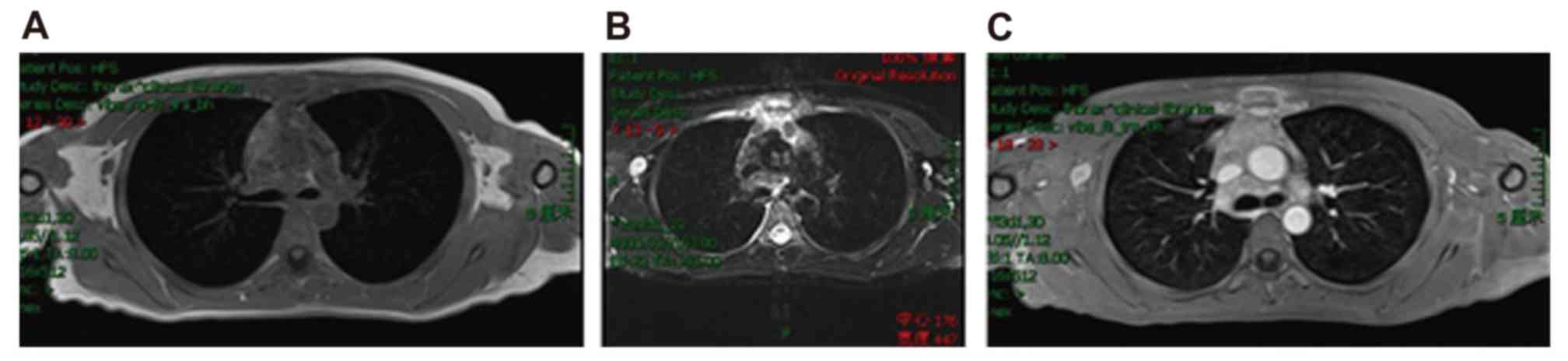
and contrast-enhanced magnetic resonance imaging confirmed
lymphomatous involvement of the sternum with slightly short
T1-weighted images and slightly long T2-weighted images in the
mesosternum and ensisternum. Furthermore, pathological enhancement
was observed of the sternum marrow cavity and the surrounding
soft-tissue shadow (Fig. 1). Positron
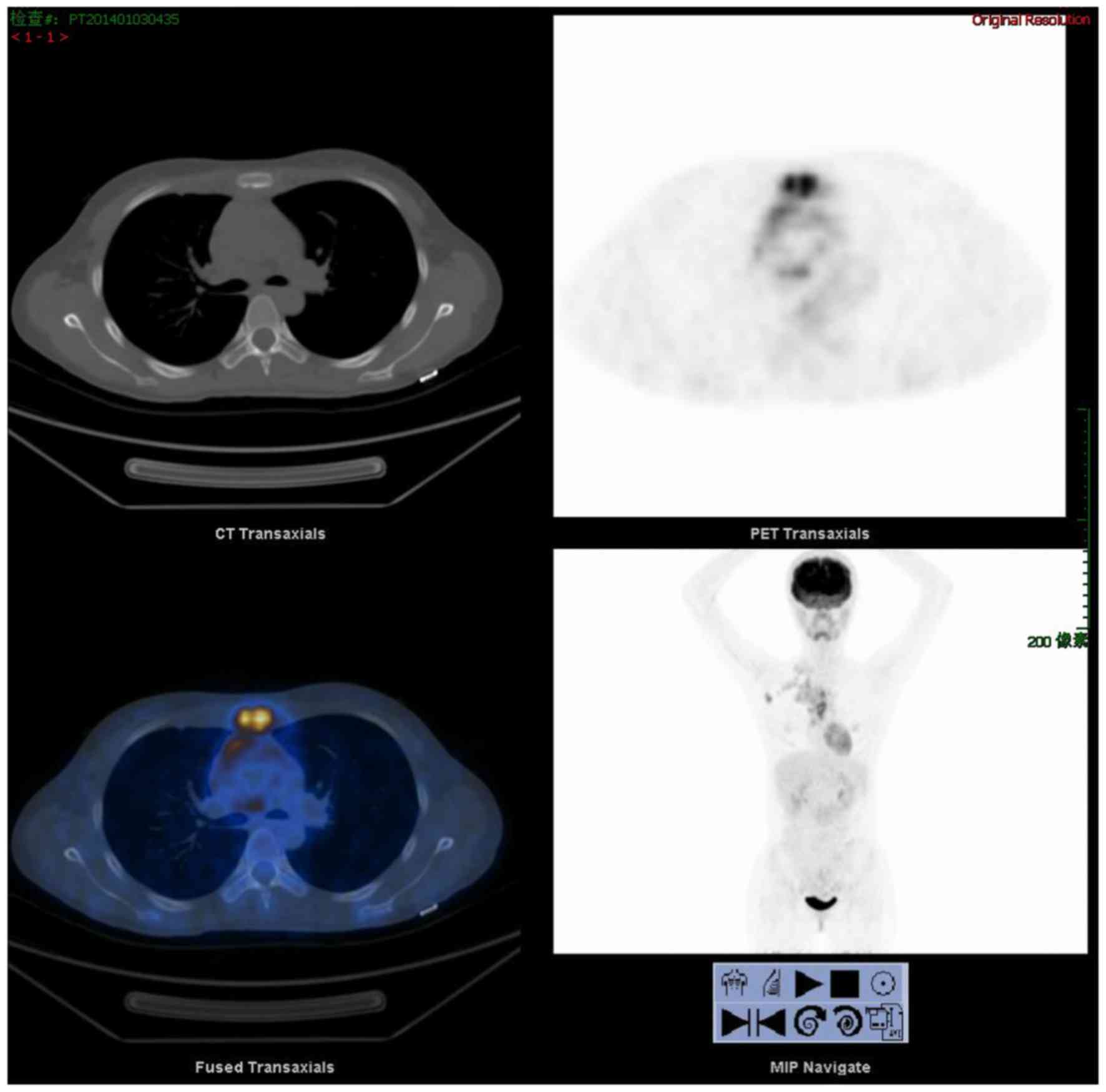
emission tomography/computed tomography (PET/CT) revealed increased
uptake in the sternum and multiple swollen lymph nodes of the right
clavicle region, the right axilla, the mediastinum and the right
side of sternum (Fig. 2). The
fluorodeoxyglucose maximum standardized uptake value (SUVmax) of
the sternum was 7.5. The lymph node SUVmax of the right clavicle
region, the right axilla, the mediastinum and the right sternum
aside was 2.7–3.8, 5.5 and 3.7–6.3 respectively. Clinically, an
SUVmax value of ≥2.5 is often regarded as a criterion for malignant
lesions. These results (SUVmax>2.5) suggested that malignant
lymphoma. Excluding those results, the remainder of the
investigated body regions showed no signs of lymphoma
infiltration.
Excisional biopsy of the cervical lymph node under
local anesthesia revealed the histological features of classical
HL, nodular sclerosis type (Fig. 3).
Fig. 3 presents the hematoxylin and
eosin stain, as well as immunohistochemistry. Tissues were fixed
with 4% formol at room temperature for 50 min, and embedded in
paraffin. Sections were 4 µm thick and were studied histologically
with hematoxylin and eosin staining and immunohistochemistry. In
brief, the process for hematoxylin and eosin staining included
dewaxing with xylene for 10 min at 60°C (repeat twice), staining
(hematoxylin, 2 min and eosin, 2 min; at room temperature),
dehydrating using graded ethanol (70% ethanol for 2 min, 80%
ethanol for 2 min and 95% ethanol for 2 min), transparentizing, and
mounting with neutral gum. For immunohistochemistry, tissue
sections were deparaffinized in xylene and rehydrated in serial
ethanol dilutions. For deparaffinization, tissue sections were
placed in a 60°C thermostat for 1 h first and subsequently in
xylene solution for 20 min. For rehydration, sections were placed
in 100% absolute ethanol for 5 min, 95% ethanol for 2 min, 90%
ethanol for 2 min, 80% ethanol for 2 min, distilled water for 2 min
and then washed using PBS for 5 mins two times. Antigen retrieval
was performed through heating the tissue sections in 10 mM sodium
citrate buffer for 10 min. Following blocking of endogenous
nonspecific peroxidase activity in 3% H2O2
for 15 min, sections were then incubated in 5% normal goat serum
(Beijing ComWin Biotech Co., Ltd., Beijing, China) at room
temperature for 30 min. Tissue sections were subsequently incubated
with primary antibodies (1:1 dilution), including the following
antibodies for cluster of differentiation (CD)30 (cat no.
MAB-0023), paired box 5 (cat no. MAB-0706), CD1a (cat no.
MAB-0336), CD68 (cat no. Kit-0026), marker of proliferation Ki-67
(cat no. Kit-0005), CD15 (cat no. MAB-0015), CD21 (cat no.
MAB-0339), CD20 (cat no. MAB-0669), CD3 (cat no. Kit-0003), CD23
(cat no. RMA-0504), CD34 (cat no. Kit-0004), latent membrane
protein 1 (cat no. MAB-0063), ALK receptor tyrosine kinase (cat no.
MAB-0281), epithelial membrane antigen (cat no. Kit-0011), smooth
muscle actin (cat no. Kit-0006), D2-40 (cat no. MAB-0567) and S-100
(cat no. Kit-0007) at 4°C overnight followed by incubation with
horseradish-peroxidase (HRP)-conjugated secondary antibody kits
(Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China): HRP-polymer
anti-mouse IHC kit (cat no. KIT-5003) and HRP-polymer anti-rabbit
IHC kit (dilution, 1:1; cat no. KIT-5006). at room temperature for
1 h. 0.025% hematoxylin was then applied for nuclear
counterstaining for 1 min at room temperature. Primary antibodies
of IHC listed in table I were all
purchased from Fuzhou Maixin Biotech Co., Ltd. (Fuzhou, China). As
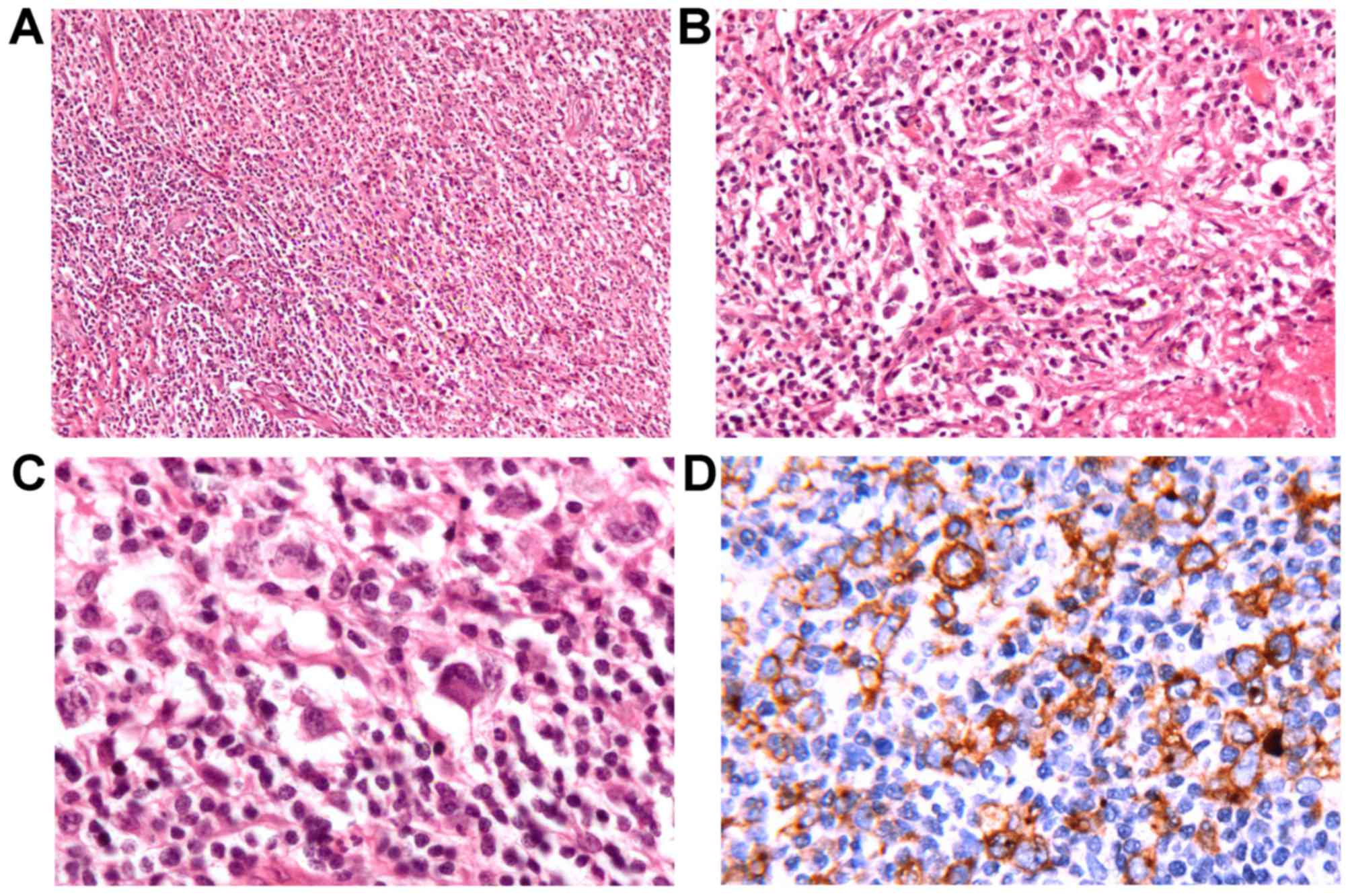
presented in Fig. 3A, multiple cell
components could be observed in eosinophils, plasmacytes,
lymphocytes and atypical cells. Typical Sternberg-Reed cells and
lacunar cells are presented in Fig. 3B
and C respectively. Furthermore, the large cells were positive
for CD30 identified on the surface of R-S cells in Fig. 3D. Immunohistochemically, the diagnosis
of HL was confirmed (Table I). The
EBER-in situ hybridisation (EBER-ISH) detection was
negative. EBER-ISH was performed using a commercially available kit
purchased from Zhongshan Goldenbridge Biotech Co., Ltd. (Beijing,
China) according to the manufacturer protocol. Briefly, tissue
sections were dewaxed, endogenous peroxidase activity was blocked
by incubating in 3% H2O2 in methanol for 5
min and tissues digested with 100 µg/ml proteinase K for 5 min at
37°C. Subsequently sections were dehydrated and hybridised
overnight at 42°C using a mixture of digoxigenin-labeled EBER-1/2
anti-sense oligonucleotide probes. The hybridized probes were
detected using the HRP-anti-digoxin monoclonal antibody and
diaminobenzidine tetrahydrochloride (DAB) was used as a chromogen.
All results were in accordance with HL and infiltration of the
sternum.
 | Table I.Final results of IHC and ISH
assessment. |
Table I.
Final results of IHC and ISH
assessment.
| Assessment | Result |
|---|
| IHC |
|
| CD30 | (+) |
| Pax5 | (+) |
| CD1a | (histocyte+) |
| CD68 | (histocyte+) |
|
Ki-67 | (oncocyte+) |
| CD15 | (−) |
|
CD21 | (−) |
|
CD20 | (−) |
|
CD3 | (−) |
|
CD23 | (−) |
|
CD34 | (−) |
|
LMP1 | (−) |
|
ALK | (−) |
|
EMA | (−) |
|
SMA | (−) |
|
D2-40 | (−) |
|
S-100 | (−) |
| ISH |
|
|
EBER | (−) |
Therefore, the clinical diagnosis was of classical
HL (nodular sclerosis type) involving infiltration of the sternum,
stage IIE, A category (localized involvement of an extra-lymphatic
organ or site and of one or more lymph node regions on the same
side of the diaphragm, with no systemic symptoms of fever, night
sweats or weight loss). As the imaging studies and excisional lymph
node biopsy showed HL and infiltration of the sternum, a biopsy of
the sternal lesion was not performed.
The patient's International Prognostic Index
(5) score was zero; the patient was
aged <60 years, with an Eastern Cooperative Oncology Group score
(6) of 1, stage II disease, one
extra-nodal site (sternum) and an LDH level within the normal
range. The risk assessment was classified as low level.
According to the National Comprehensive Cancer
Network (7) guidelines (Version 2016,
Hodgkin lymphoma), for stage I to II patients with unfavorable
disease, chemotherapy is recommended [4–6 cycles of an adriamycin,
bleomycin, vinblastine and dacarbazine (ABVD) or Stanford V
regimen] followed by involved-field radiation therapy (IFRT). The
patient was recommended to undergo 6 cycles of standard-dose
chemotherapy with ABVD, which consisted of adriamycin 25
mg/m2 [days 1 and 15, intravenous injection (i.v.)],
bleomycin 10 mg/m2 (days 1 and 15, i.v.), vinblastine 6
mg/m2 (days 1 and 15, i.v.) and dacarbazine 375
mg/m2 (days 1 and 15, i.v.). One cycle of ABVD
chemotherapy was given over 4 weeks. Subsequent to the first cycle
of chemotherapy (January 7 and 21, 2014), the symptom of sternal
pain improved immediately and markedly. PET-CT after the second
cycle was negative, showing no evidence of hypermetabolic nodules
or regions. The curative effect evaluation was of complete
remission. Finally, the patient refused the sixth chemotherapy
cycle due to personal reasons. Following completion of the
chemotherapy, consolidative IFRT of the mediastinum and sternum was
performed.
Follow-up PET-CT subsequent to all treatment once
again returned negative results and the patient achieved complete
remission. Almost 6 months after therapy, the patient was found to
be well without any signs of local recurrence or any further lymph
node metastasis. At present (42 months until November 2017), the
patient remains disease-free. Written consent was obtained from the
patient for publication of this case report.
Literature review
A literature review of the osseous involvement of HL
indicates that the majority of cases have been associated with
local or distant lymph node involvement. Osseous involvement of HL
presenting as a sternal lesion is quite unusual. In 1999, Ostrowski
et al (8) reported that only 3
cases among 25 patients with osseous involvement of HL showed
sternal involvement. Toussirot et al (9) reported that in 10 cases of anterior
chest wall malignancies, 2 were due to osseous HL. In another
retrospective review reported by Franczyk et al (10), 3 out of 42 patients with HL (2 with
mixed cellularity type and 1 with nodular sclerosis type) had
sternal lesions, all from direct extension from mediastinal lymph
nodes. Another case of HL with unusual sternal presentation has
also been reported in India (11).
Table II summarizes
the clinical characteristics of the patients with osseous
involvement of HL in recent years (4,12–19). The bones most frequently involved were
the vertebrae, ribs, femur and sternum. Through chemotherapy and
radiation, most patients could achieve complete remission. The
outcome of the patients was usually a good prognosis.
 | Table II.Cases in the literature that
presented with osseous involvement of Hodgkin's lymphoma. |
Table II.
Cases in the literature that
presented with osseous involvement of Hodgkin's lymphoma.
| First author,
year | Age, years | Gender | Site(s) | Therapy | Outcome | (Refs.) |
|---|
| Eustace et
al, 1995 | 63 | M | Left proximal
femur, right iliac wing | Chemo | NED (6 months) | (12) |
| Fried et al,
1995 | 21 | F | Left lateral
clavicle | Chemo | NED (36
months) | (13) |
| Citow, et
al, 2001 | 54 | F | T4, T5 | Surgery + chemo +
IRF | AWD (36
months) | (14) |
| Gebert et
al, 2005 | 21 | M | Right proximal
femur, right proximal tibia | Curettage + chemo +
IRF | NED (4 years) | (15) |
| Langley et
al, 2008 | 7 | M | Sternum, L1
vertebra, the left sacro-iliac joint and the right acetabulum | Chemo | AWD | (16) |
| Chandra et
al, 2006 | 51 | F | Left ileum | Chemo + IRF | Alive | (17) |
| Biswas et
al, 2008 | 21 | M | Sternum | Chemo + IRF | After PD gave
salvage chemo | (4) |
| Li et al,
2012 | 38 | F | Right second
rib | Chemo + IRF | Alive | (18) |
| Binesh et
al, 2012 | 63 | F | L2 to L5
vertebra | Chemo | Alive | (19) |
| Present study | 25 | F | Sternum | Chemo + IRF | NED (42 months,
until Nov. 2017) |
|
Radiotherapy alone was a standard treatment option
for patients with favorable early-stage HL for a number of decades
(20). However, the potential
long-term toxicities of a high-dose and large-field irradiation
include an increased risk for heart disease, pulmonary dysfunction
and secondary cancer (21). For this
reason, many investigators have been contemplating the use of
chemotherapy alone as a treatment modality.
Now, in comparison to a single treatment type, the
use of combined modality therapy (IFRT plus chemotherapy) has
considerably improved the patient outcome and is the current gold
standard of treatment (22–24). The past few years have witnessed
significant progress in the management of HL; it is now curable in
at least 80% of patients (25).
Newcomer et al (22) reported
that of the 18 patients of osseous HL treated with combined
modality therapy, only 3 were induction failures and the condition
of 1 patient was subsequently salvaged with additional therapy.
According to Mendenhall et al (26), radiotherapy should be delayed until
the completion of chemotherapy to reduce the dose and volume of
radiation, which can of course reduce the number of adverse events
(26).
Discussion
HL is an uncommon malignancy mainly involving the
lymph nodes and the lymphatic system. An extranodal presentation of
HL is unusual and the most common sites of presentation for
extranodal extension are the spleen, liver, lungs, bones and
marrow. However, such instances are seldom observed in the clinic.
The bones frequently involved are the vertebrae, pelvis, ribs and
femur (4,8,22,27,28).
Involvement of the sternum has occasionally been reported (4,8,27).
Osseous involvement may be either due to primary
lymphoma of the bone or may be secondary to hematogenous or direct
contiguous spread from the lymph nodes and a soft-tissue mass
(22,28). Primary osseous HL is a heterogeneous
group of disorders. There should not be any signs or symptoms of
systemic disease at the time of presentation or at the time of
staging. Primary osseous HL is defined as malignant lymphoid
infiltration within the bones, with or without cortical invasion or
extension into contiguous soft tissues, and without involvement of
regional lymph nodes or distant viscera within 6 months of
presentation (23,29). The case of sternal involvement in the
present study was secondary to extension, reflecting direct
extension from the mediastinal lymph nodes into the sternum.
The clinical stage has a critical role in the
selection of treatment. According to Ann Arbor staging (30), stage IV adult HL is characterized by
disseminated (multifocal) involvement of one or more
extra-lymphatic organs, with or without associated lymph node
involvement, or isolated extra-lymphatic organ involvement with
distant (non-regional) nodal involvement.
In the past, scholars believed that bone involvement
heralds disease progression. Osseous infiltration usually indicates
widespread disease (16). Therefore,
cases with osseous involvement should be classified as stage IV.
However, certain individuals now consider the bone as just an
extra-lymphatic organ (31). A
solitary lymph node tumor may cause changes in the adjacent bone by
pressure or direct invasion (29).
Direct extension from a contiguous lymph node does not constitute
stage IV disease, but rather an ‘E’ classification with a better
prognosis (31,32). Certain clinicians have reached the
consensus that osseous involvement per se is not an adverse
prognostic factor (8,22,24,27).
Taking the aforementioned into consideration, the
sternal infiltration of HL in the present study was diagnosed as
stage IIE disease. A major point of interest in this case is the
marked extra-nodal infiltration of the sternum by the mediastinal
lymph node. Another point of interest is that this type of HL is
among the most relatively benign forms, the nodular sclerosing
variety, which has a better prognosis than the other type.
It is worth mentioning that in the era of biological
imaging, whole-body PET-CT is gaining increasing attention.
Compared with other methods, the advantage of PET-CT is the early
detection of disease, as biochemical changes in the tumor
microenvironment occur long before gross morphological alteration
(28,33). PET-CT scanning has been used for the
initial staging, restaging and follow-up of patients with lymphoma
(34). In a previous meta-analysis,
PET-CT demonstrated high positivity and specificity when used to
stage and restage patients with lymphoma (35). PET-CT is widely used during and after
therapy for the assessment of response (36). Early interim PET-CT scans after 2–4
cycles of standard-dose chemotherapy have also been shown to be a
sensitive prognostic indicator in patients with advanced stage and
extra-nodal disease (37–39). The 2-year progression-free survival
rate has been recorded to be significantly higher for patients with
negative PET-CT results after 2 cycles of ABVD compared with that
for patients with positive PET-CT results (95 vs. 13%) (40).
In conclusion, the present case study describes an
unusual extra-nodal presentation of HL. The patient responded well
to IFRT combined with ABVD chemotherapy, resulting in apparently
complete remission of the tumor without further evidence of local
recurrence or spread of the disease. The requirement for rapid
histological and immunocytochemical examination in patients should
be stressed in order to prevent systemic dissemination. With
current polychemotherapy, the long-term prognosis of affected
patients appears good.
Acknowledgements
The present study was financially supported by the
National Natural Science Foundation of China (grant no.
81202962).
References
|
1
|
Guermazi A, Brice P, de Kerviler EE, Fermé
C, Hennequin C, Meignin V and Frija J: Extranodal Hodgkin disease:
Spectrum of disease. Radiographics. 21:161–179. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zucca E: Extranodal lymphoma: A
reappraisal. Ann Oncol. 19 Suppl 4:iv77–iv80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chan KW, Rosen G, Miller DR and Tan CT:
Hodgkin's diseases in adolescents presenting as a primary bone
lesion. A report of four cases and review of literature. Am J
Pediatr Hematol Oncol. 4:11–17. 1982.PubMed/NCBI
|
|
4
|
Biswas A, Puri T, Goyal S, Haresh KP,
Gupta R, Julka PK and Rath GK: Osseous Hodgkin's lymphoma-review of
literature and report of an unusual case presenting as a large
ulcerofungating sternal mass. Bone. 43:636–640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
The International Non-Hodgkin's Lymphoma
Prognostic Factors Project: A predictive model for aggressive
non-Hodgkin's lymphoma. N Engl J Med. 329:987–994. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
National Comprehensive Cancer and Network
(NCCN), . NCCN Guidelines for Patients®Version 2016
Hodgkin Lymphoma. NCCN; Fort Washington, PA: 2016
|
|
8
|
Ostrowski ML, Inwards CY, Strickler JG,
Witzig TE, Wenger DE and Unni KK: Osseous Hodgkin disease. Cancer.
85:1166–1178. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Toussirot E, Gallinet E, Augé B, Voillat L
and Wendling D: Anterior chest wall malignancies. A review of ten
cases. Rev Rhum Engl Ed. 65:397–405. 1998.PubMed/NCBI
|
|
10
|
Franczyk J, Samuels T, Rubenstein J,
Srigley J and Morava-Protzner I: Skeletal lymphoma. Can Assoc
Radiol J. 40:75–79. 1989.PubMed/NCBI
|
|
11
|
Meher-Homji DR, De Souza LJ, Mohanty B and
Culcuttawalla TF: Unusual sternal mass in Hodgkin's disease. A case
report. J Bone Joint Surg Am. 54:402–404. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eustace S, O'Regan R, Graham D and Carney
D: Primary multifocal skeletal Hodgkin's disease confined to bone.
Skeletal Radiol. 24:61–63. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fried G, Ben Arieh Y, Haim N, Dale J and
Stein M: Primary Hodgkin's disease of the bone. Med Pediatr Oncol.
24:204–207. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Citow JS, Rini B, Wollmann R and Macdonald
RL: Isolated, primary extranodal Hodgkin's disease of the spine:
Case report. Neurosurgery. 49:453–457. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gebert C, Hardes J, Ahrens H, Buerger H,
Winkelmann W and Gosheger G: Primary multifocal osseous Hodgkin
disease: A case report and review of the literature. J Cancer Res
Clin Oncol. 131:163–168. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Langley CR, Garrett SJ, Urand J, Kohler J
and Clarke NM: Primary multifocal osseous Hodgkin's lymphoma. World
J Surg Oncol. 6:342008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chandra D, Ewton A and Baker K: Hodgkin's
disease presenting with osseous involvement. Am J Hematol.
81:550–551. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Wang XB, Tian XY, Li B and Li Z:
Unusual primary osseous Hodgkin lymphoma in rib with associated
soft tissue mass: A case report and review of literature. Diagn
Pathol. 7:642012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Binesh F, Mirjalili MR, Akhavan A and
Navabii H: Primary bony Hodgkin's lymphoma. BMJ Case Rep. 2012:pii:
bcr01201257142012. View Article : Google Scholar
|
|
20
|
Dühmke E, Franklin J, Pfreundschuh M,
Sehlen S, Willich N, Rühl U, Müller RP, Lukas P, Atzinger A, Paulus
U, et al: Low-dose radiation is sufficient for the noninvolved
extended-field treatment in favorable early-stage Hodgkin's
disease: Long-term results of a randomized trial of radiotherapy
alone. J Clin Oncol. 19:2905–2914. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gustavsson A, Osterman B and
Cavallin-Ståhl E: A systematic overview of radiation therapy
effects in Hodgkin's lymphoma. Acta Oncol. 42:589–604. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Newcomer LN, Silverstein MB, Cadman EC,
Farber LR, Bertino JR and Prosnitz LR: Bone involvement in
Hodgkin's disease. Cancer. 49:338–342. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dürr HR, Müller PE, Hiller E, Maier M,
Baur A, Jansson V and Refior HJ: Malignant lymphoma of bone. Arch
Orthop Trauma Surg. 122:10–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Borg MF, Chowdhury AD, Bhoopal S and
Benjamin CS: Bone involvement in Hodgkin's disease. Australas
Radiol. 37:63–66. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gustavsson A, Osterman B and
Cavallin-Ståhl E: A systematic overview of radiation therapy
effects in non-Hodgkin's lymphoma. Acta Oncol. 42:605–619. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mendenhall NP, Jones JJ, Kramer BS, Hudson
TM, Carter RL, Enneking WF, Marcus RB Jr and Million RR: The
management of primary lymphoma of bone. Radiother Oncol. 9:137–145.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feltl D, Marková J and Kozák T: Skeletal
involvement in Hodgkin's lymphoma-personal experience. Vnitr Lek.
50:134–138. 2004.(In Czech). PubMed/NCBI
|
|
28
|
Israel O, Mekel M, Bar-Shalom R, Epelbaum
R, Hermony N, Haim N, Dann EJ, Frenkel A, Ben-Arush M and Gaitini
D: Bone lymphoma: 67Ga scintigraphy and CT for prediction of
outcome after treatment. J Nucl Med. 43:1295–1303. 2002.PubMed/NCBI
|
|
29
|
Dosoretz DE, Raymond AK, Murphy GF, Doppke
KP, Schiller AL, Wang CC and Suit HD: Primary lymphoma of bone: The
relationship of morphologic diversity to clinical behavior. Cancer.
50:1009–1014. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carbone PP, Kaplan HS, Musshoff K,
Smithers DW and Tubiana M: Report of the committee on Hodgkin's
disease staging classification. Cancer Res. 31:1860–1861.
1971.PubMed/NCBI
|
|
31
|
Parker BR, Marglin S and Castellino RA:
Skeletal manifestations of leukemia, Hodgkin disease, and
non-Hodgkin lymphoma. Semin Roentgenol. 15:302–315. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pear BL: Skeletal manifestations of the
lymphomas and leukemias. Semin Roentgenol. 9:229–240. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moog F, Kotzerke J and Reske SN: FDG PET
can replace bone scintigraphy in primary staging of malignant
lymphoma. J Nucl Med. 40:1407–1413. 1999.PubMed/NCBI
|
|
34
|
Seam P, Juweid ME and Cheson BD: The role
of FDG-PET scans in patients with lymphoma. Blood. 110:3507–3516.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Isasi CR, Lu P and Blaufox MD: A
metaanalysis of 18F-2-deoxy-2-fluoro-D-glucose positron emission
tomography in the staging and restaging of patients with lymphoma.
Cancer. 104:1066–1074. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Juweid ME: Utility of positron emission
tomography (PET) scanning in managing patients with Hodgkin
lymphoma. Hematology Am Soc Hematol Educ Program 259–265. 510–511.
2006.
|
|
37
|
Gallamini A, Rigacci L, Merli F, Nassi L,
Bosi A, Capodanno I, Luminari S, Vitolo U, Sancetta R, Iannitto E,
et al: The predictive value of positron emission tomography
scanning performed after two courses of standard therapy on
treatment outcome in advanced stage Hodgkin's disease.
Haematologica. 91:475–481. 2006.PubMed/NCBI
|
|
38
|
Gallamini A, Hutchings M, Avigdor A and
Polliack A: Early interim PET scan in Hodgkin lymphoma: Where do we
stand? Leuk Lymphoma. 49:659–662. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Terasawa T, Lau J, Bardet S, Couturier O,
Hotta T, Hutchings M, Nihashi T and Nagai H:
Fluorine-18-fluorodeoxyglucose positron emission tomography for
interim response assessment of advanced-stage Hodgkin's lymphoma
and diffuse large B-cell lymphoma: A systematic review. J Clin
Oncol. 27:1906–1914. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gallamini A, Hutchings M, Rigacci L,
Specht L, Merli F, Hansen M, Patti C, Loft A, Di Raimondo F,
D'Amore F, et al: Early interim 2-[18F]fluoro-2-deoxy-D-glucose
positron emission tomography is prognostically superior to
international prognostic score in advanced-stage Hodgkin's
lymphoma: A report from a joint Italian-Danish study. J Clin Oncol.
25:3746–3752. 2007. View Article : Google Scholar : PubMed/NCBI
|