Introduction
Glioma, which is the most common intracranial tumor
in adults, is characterized by high vascularization and invasive
capacity, as well as peritumoral brain edema (PTBE) (1–4). In
preoperative magnetic resonance imaging (MRI), the degree and
morphological characteristics of PTBE vary between different types
of glioma (5). PTBE, which
facilitates glioma cell invasion and significantly affects patient
prognosis, is a major cause of the high disability and mortality
rate of glioma (4,6). A previous study has demonstrated that
glioma recurrence patterns are affected by the degree and
morphological characteristics of PTBE (7). In addition, a study has identified that
PTBE is an imaging feature of glioma cell invasion, a complex
process regulated by specific signaling pathways (8). Previous studies have shown that the cell
surface C-X-C receptor 4 (CXCR4), and its protein ligand,
CXC-motif-chemokine 12 (CXCL12), are overexpressed in glioma
tissues (9–11). The CXCR4-CXCL12 signaling pathway
regulates glioma cell differentiation, invasion and metastasis
(12,13). According to the law of tissue
remodeling presented by Lin (14),
tissue morphology is the result of the interactions among molecules
within the tumor microenvironment. It was hypothesized that the
CXCR4-CXCL12 signaling pathway affects the degree and morphological
characteristics of PTBE. To the best of our knowledge, there are
currently no studies describing the association between the
CXCR4-CXCL12 axis and PTBE in glioma patients. Therefore, the
present study investigated the association between CXCR4/CXCL12 and
PTBE in glioma tissues.
Materials and methods
Study samples
The clinicopathological data of patients with
supratentorial glioma (n=58; 41 male, 17 female) treated at the
First Affiliated Hospital of Fujian Medical University (Fujian,
China) between December 2007 and April 2014 were collected
retrospectively. The median age was 44.57±13.90 years (range, 17–75
years). The pathological diagnosis of each patient was confirmed by
neuropathologists based on the brain tumor pathology classification
standard of 2007 (15). All patients
received postoperative chemoradiotherapy. Chemotherapy (at least
four cycles) with temozolomide was administered at a dose of
150–200 mg/m2/day. Radiotherapy (60 Gy in 2 Gy
fractions) was applied to the field of the tumor lesion plus a 2 cm
margin, according to MRI at the time of the second chemotherapy
cycle. The present study was approved by the Ethics Committee of
Fujian Medical University (Fuzhou, China), and all enrolled
patients provided written informed consent for inclusion in the
present study.
Immunohistochemistry
Glioma and adjacent non-tumor tissue acquired during
surgery from the patients described previously were embedded in
paraffin immediately and sectioned to 5 µm using a microtome.
Sections were mounted on glass slides, dew axed and hydrated for
immunohistochemistry. For CXCL12 and CXCR4, heat-induced epitope
retrieval was performed by boiling the tissue sections in 10 mmol/l
citrate buffer (pH 6.0) for 15 min in a microwave oven. Subsequent
to cooling to room temperature, the sections were exposed to
methanol containing 3% hydrogen peroxide for 10 min at room
temperature to block endogenous peroxidase. CXCL12 was detected
with an anti-human CXCL12 monoclonal antibody (5 µg/ml; cat. no.
MAB350; R&D Systems, Inc., Minneapolis, MN, USA) for 1 h at
room temperature. CXCR4 was detected with an anti-human CXCR4
monoclonal antibody (0.5 µg/ml; cat. no. MAB172, R&D Systems,
Inc.) for 1 h at room temperature. The slides were then washed with
PBS and incubated with the secondary detection antibody,
polyperoxidase-anti-rabbit/mouse IgG (dilution, 1:1,000; cat. no.
C0101, ZSGB-BIO, Beijing, China) for 50 min at room temperature.
Then the slides were treated with with diaminobenzidine (dilution,
1:1,000; cat. no. 070004; Cell Chip Biotechnology Co., Beijing,
China) as per the manufacturer's protocol. The sections were
counterstained with hematoxylin and mounted in gelatin. Gastric
cancer and tonsil tissues were used as positive controls for CXCR4
and CXCL12 expression, respectively. Gastric cancer tissues were
acquired from a patient with postoperative pathological diagnosis
of gastric carcinoma. Tonsil tissues were acquired from a normal
control. PBS was substituted for the primary antibodies as a
negative control.
Histological assessment
Immunohistochemical staining of five randomly
selected fields for each tumor specimen was evaluated under a light
microscope (magnification, ×200) and classified according to the
method proposed by Zagzag et al (16): Low expression, staining <5% of
structures; high expression, staining of >5% of structures. The
results were evaluated independently by two pathologists. In cases
of discordance, a third pathologist reviewed the results to achieve
a consensus.
Imaging methodology and analyses
Preoperative MRI scans [T1 weighted image (T1WI), T2
weighted image (T2WI), axes, arrows and coronal enhanced images]
were performed at the First Affiliated Hospital of Fujian Medical
University. The boundaries of the contrast enhancement area on T1
weighted images were drawn. These maps were then compared with
their respective T2 weighted images. According to Schoenegger et
al (2), PTBE was classified
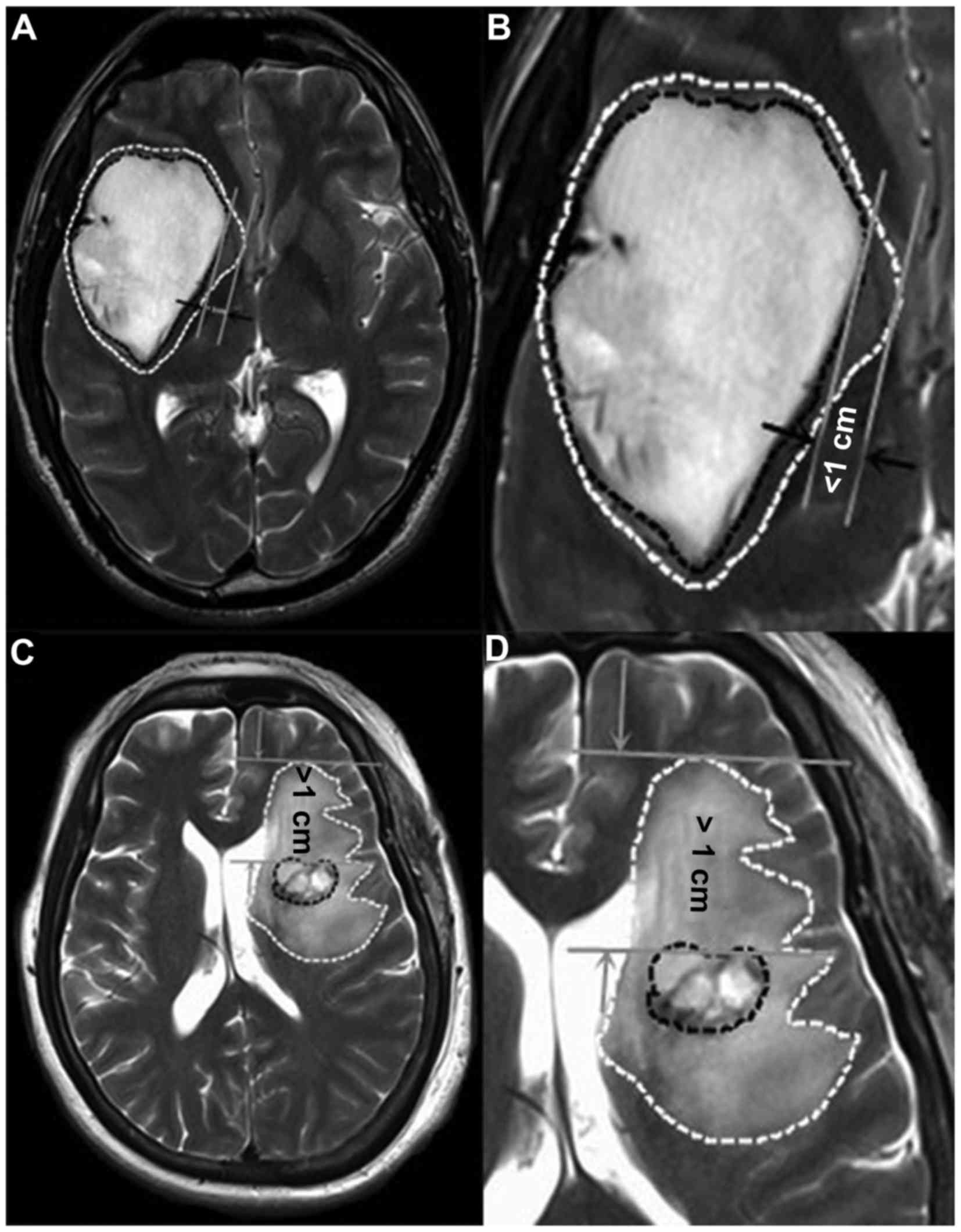
according to degree as follows: Minor, edema extending ≤1 cm from
the tumor margin (Fig. 1A and B) and
major, edema extending >1 cm from the tumor margin (Fig. 1C and D). According to Hartmann et
al (7), the PTBE morphology types
were classified as: Ring form, circular region of increased T2
signal intensity (Fig. 1A and B); and
irregular form, irregular regions of increased T2 signal intensity
(Fig. 1C and D). Based on these
definitions, PTBE types and degrees on preoperative MR images were
classified by two experienced imageologists. Discrepancies in the
classification were reviewed by a third imageologist until a
consensus was achieved.
Analysis
Each patient was followed-up in outpatient visits or
telephone conversations. The median progression-free survival time
(PFS, months) was calculated from the date of surgery to the date
of first tumor progression (or mortality or the latest follow-up).
The overall survival (OS, months) time was defined as the period
from the time of surgical resection to the latest follow-up (or
mortality). PFS and OS times for patients without tumor progression
or mortality ended with the latest follow-up.
Statistical analysis
Statistical analysis was performed with SPSS 19.0
for Windows (IBM SPSS, Armonk, NY, USA). The associations between
age, sex, pathological grade, morphological types of PTBE, degrees
of PTBE and CXCR4/CXCL12 expression levels were initially examined
by χ2 analysis or Fisher's exact test. Non-parametric
tests were used to analyze the association between pathological
grades and expression levels of CXCL12 or CXCR4. The associations
between PFS and OS and CXCL12/CXCR4 expression levels were
determined by log-rank tests and presented as Kaplan-Meier curves.
In multivariate analyses, the COX proportional hazards model was
adopted to assess the effects of CXCL12/CXCR4 expression levels on
PFS and OS. In addition, hazard ratios as well as the corresponding
95% confidence intervals were calculated. P<0.05 was considered
to indicate a statistically significant difference.
Results
CXCL12 and CXCR4 expression in glioma
tissues
Immunohistochemical staining demonstrated that
CXCL12 and CXCR4 were located in the cell membrane and cytoplasm.
Widespread staining of CXCL12, but not CXCR4, was also observed in
adjacent normal brain tissues. In the 58 glioma tissue samples,
CXCL12 expression was observed mainly in the vascular endothelial
cells, with low levels of expression also observed in tumor cells.
CXCL12 positive staining was detected in 52/58 glioma specimens. In
vascular endothelial cells, CXCL12 expression was detected in
glomeruloid vessels (Fig. 2A) and
non-glomeruloid vessels (Fig. 2B),
with low expression identified in 26 and high expression in 32
glioma tissue samples. In addition, CXCL12-positive endothelial
cells were particularly high expression in areas of tumor adjacent
to necrosis (Fig. 2C). In tumor cells
(Fig. 2D), low CXCL12 expression was
detected in 49 samples and high expression in 9 samples.
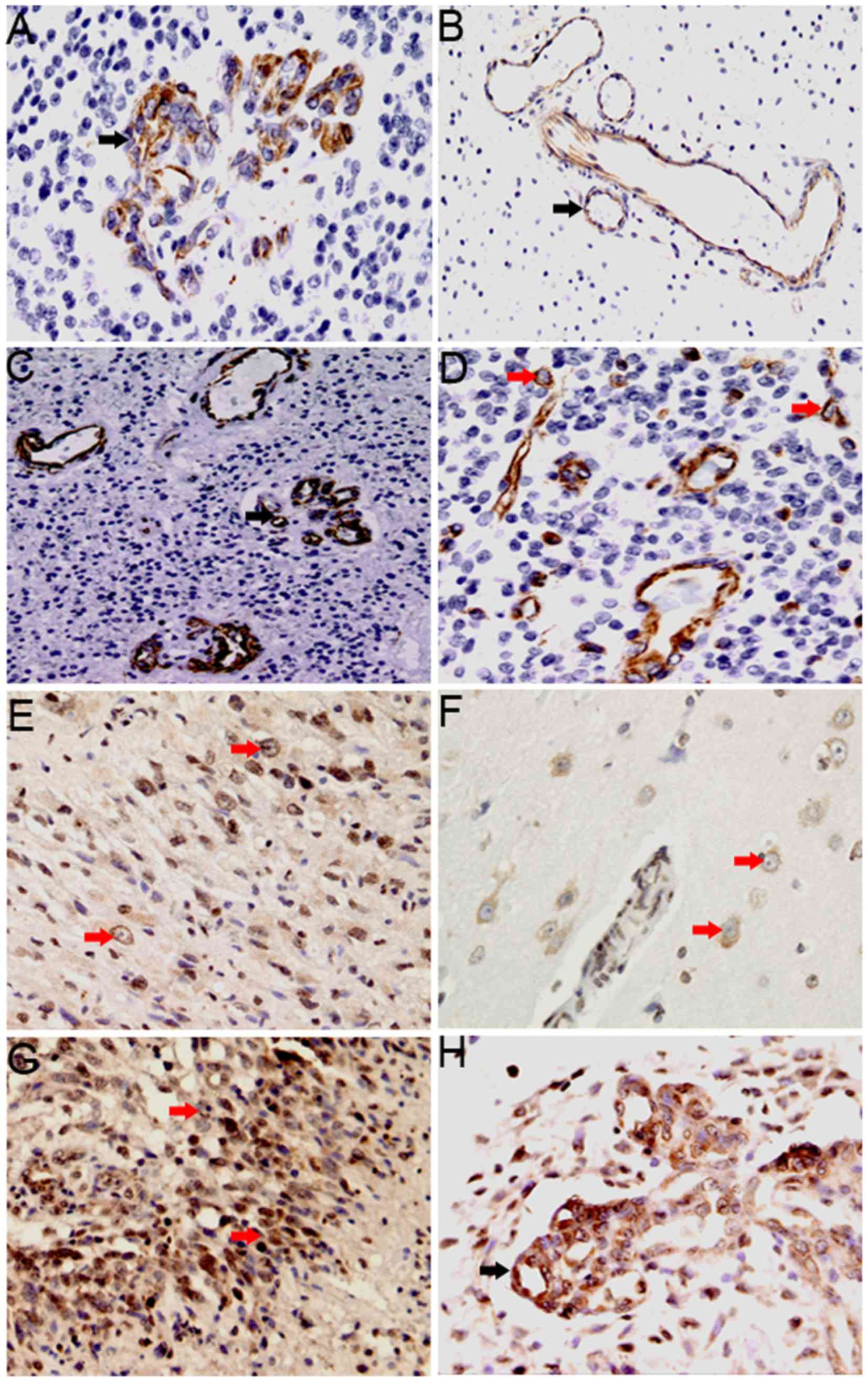 | Figure 2.Degree and cellular distribution of
CXCL12 and CXCR4 expression in glioma tissues. (A-D) CXCL12
expression in vascular endothelial cells (black arrow) and in tumor
cells (red arrow). (A) CXCL12 expression in glomeruloid vessels
(magnification, ×200). (B) CXCL12 expression in the non-glomerular
vessels of the peritumoral edema area (magnification, ×200). (C)
CXCL12 is expressed in vascular endothelial cells of the
surrounding necrotic area (magnification, ×200). (D) CXCL12
expression in tumor cells (magnification, ×200). (E-H) CXCR4
expression in tumor cells (red arrow) and endothelial cells (black
arrows). (E) CXCR4 expression in tumor cells (magnification, ×200).
(F) CXCR4 expression in perivascular tumor cells of the peritumoral
edema area (magnification, 400). (G) CXCR4 expression in tumor
cells in the surrounding necrotic area (magnification, ×200). (H)
CXCR4 expression in glomeruloid vessels (magnification, ×200).
CXCR4, C-X-C receptor 4; CXCL12, CXC-motif-chemokine 12. |
CXCR4 expression was detected in 52/58 glioma
tissues, mainly in tumor cells (Fig. 2E
and F), with particularly high expression in areas of tumor
adjacent to necrosis (Fig. 2G). In
addition, CXCR4 with low levels of expression also observed in
vascular endothelial cells (Fig. 2H).
Low CXCR4 expression in tumor cells was detected in 19 samples and
high expression was detected in 39 samples. In vascular endothelial
cells, low expression was detected in 36 samples and high
expression was detected in 22 samples.
Notably, in tumor cells with high CXCR4 expression,
the expression of CXCL12 in vascular endothelial cells was also
high in the same specimens. It was also revealed that CXCR4 was
highly expressed in tumor cells, and CXCL12 was lowly expressed in
tumor cells in the same tissue. Similarly, in vascular endothelial
cells, high CXCL12 expression was associated with low CXCR4
expression in tumor cells.
Association between CXCL12 and CXCR4
expression and clinicopathological features
The associations between CXCL12 expression and
clinicopathological features are listed in Table I. CXCL12 expression in vascular
endothelial cells was significantly associated with age
(P<0.0001) and pathological grade (P<0.0001), while the level
of CXCL12-positive tumor cells was significantly associated only
with pathological grade (P=0.003). However, no association
was observed between patient sex and CXCL12 expression in
endothelial or tumor cells.
 | Table I.Association between CXCL12 and
characteristics of patients. |
Table I.
Association between CXCL12 and
characteristics of patients.
|
| Tumoral CXCL12,
n |
| Endothelial CXCL12,
n |
|
|---|
|
|
|
|
|
|
|---|
| Patients
features | Low | High | P-value | Low | High | P-value |
|---|
| Age |
|
| 0.473 |
|
| <0.0001 |
| <44.57
years | 25 | 3 |
| 20 | 8 |
|
| ≥44.57
years | 24 | 6 |
| 6 | 24 |
|
| Gender |
|
| 0.426 |
|
| 0.778 |
| Male | 36 | 5 |
| 19 | 22 |
|
|
Female | 13 | 4 |
| 7 | 10 |
|
| Pathological
grade |
|
| 0.003 |
|
| <0.0001 |
| II | 19 | 1 |
| 16 | 4 |
|
| III | 16 | 0 |
| 8 | 8 |
|
| IV | 14 | 8 |
| 2 | 20 |
|
| Edema degree |
|
| 0.067 |
|
| 0.033 |
|
Slight | 23 | 1 |
| 15 | 9 |
|
|
Severe | 26 | 8 |
| 11 | 23 |
|
| Edema
morphology |
|
| 0.167 |
|
| 0.033 |
| Ring
form | 24 | 2 |
| 16 | 10 |
|
|
Irregular form | 25 | 7 |
| 10 | 22 |
|
CXCR4 expression in tumor cells was significantly
associated with age (P=0.002) and pathological grade
(P<0.0001). The level of CXCR4-positive vascular endothelial
cells was also significantly associated with age (P=0.016)
and pathological grade (P<0.0001). However, CXCR4 expression was
not associated with patient sex in tumor or endothelial cells
(Table II).
 | Table II.Association between CXCR4 and
characteristics of patients. |
Table II.
Association between CXCR4 and
characteristics of patients.
|
| Tumoral CXCL12,
n |
| Endothelial CXCL12,
n |
|
|---|
|
|
|
|
|
|
|---|
| Patients
features | Low | High | P-value | Low | High | P-value |
|---|
| Age |
|
| 0.002 |
|
| 0.016 |
|
<44.57 years | 15 | 13 |
| 22 | 6 |
|
| ≥44.57
years | 4 | 26 |
| 14 | 16 |
|
| Gender |
|
| 1.000 |
|
| 0.773 |
|
Male | 14 | 27 |
| 26 | 15 |
|
|
Female | 5 | 12 |
| 10 | 7 |
|
| Pathological
grade |
|
| <0.0001 |
|
| <0.0001 |
| II | 13 | 7 |
| 19 | 1 |
|
|
III | 4 | 12 |
| 9 | 7 |
|
| IV | 2 | 20 |
| 8 | 14 |
|
| Edema degree |
|
| 0.001 |
|
| 0.030 |
|
Slight | 14 | 10 |
| 19 | 5 |
|
|
Severe | 5 | 29 |
| 17 | 17 |
|
| Edema
morphology |
|
| 0.001 |
|
| 0.106 |
| Ring
form | 14 | 12 |
| 19 | 7 |
|
|
Irregular form | 4 | 28 |
| 16 | 16 |
|
Association between CXCL12 and CXCR4
expression and PTBE
PTBE is one of the most important features of
gliomas on preoperative MRI; therefore, the association between
CXCL12 and CXCR4 expression and PTBE in gliomas was investigated.
CXCL12 expression in vascular endothelial cells was significantly
associated with edema degree (P=0.033) and edema morphology
(P=0.033), while the level of CXCL12-positive tumor cells
was not significantly associated with edema degree (P=0.067)
or morphology (P=0.167; Table
I).
For CXCR4, the degree (P=0.001) and
morphology (P=0.001) of PTBE were positively-associated with
the expression of CXCR4 in tumor cells. The level of CXCR4-positive
vascular endothelial cells was significantly associated with edema
degree (P=0.030), while no association was observed between
edema morphology and CXCR4 expression in vascular endothelial cells
(P=0.106; Table II).
Association between CXCL12 and CXCR4
expression and patient clinical outcome
To investigate the association between CXCL12 and
CXCR4 expression and patient prognosis, corresponding PFS and OS
were calculated from the study patients (Table III). The median PFS was 18.12 months
(range, 1–50 months) and the median OS was 25.33 months (range,
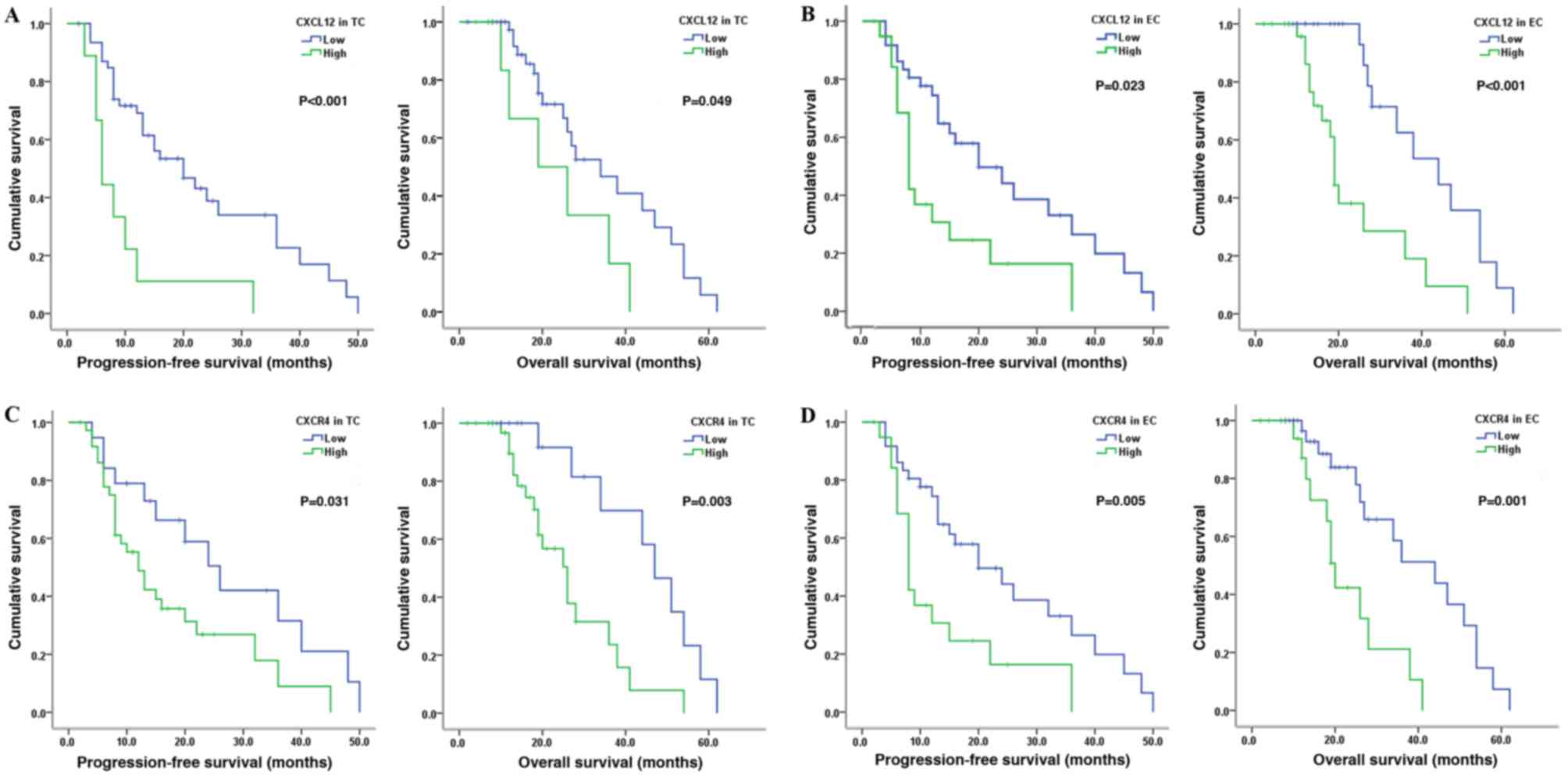
2–58 months). It was identified that high CXCL12 expression in
tumor cells in patients with glioma was associated with reduced PFS
(P<0.001) and OS (P=0.049; Fig.
3A). Similar results were observed in patients with high CXCL12
expression in vascular endothelial cells (PFS, P=0.023; OS,
P<0.001; Fig. 3B). For CXCR4, the
PFS (P=0.031) and the OS (P=0.003) were short in
patients with high CXCR4 expression in tumor cells (Fig. 3C). Furthermore, a similar association
between high CXCR4 expression in vascular endothelial cells and
short PFS (P=0.005) and OS (P=0.001) times was
observed (Fig. 3D). Multivariate
analysis showed that, regardless of the level or site of
expression, CXCL12 and CXCR4 were not independent prognostic
factors in patients with glioma; only pathological grade was found
to be an independent prognostic factor.
 | Table III.Cox proportional hazards regressions
for PFS and OS in gliomas. |
Table III.
Cox proportional hazards regressions
for PFS and OS in gliomas.
|
| PFS | OS |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (≥44.57 vs.
<44.57 years) | 1.14
(0.46–2.84) | 0.772 | 2.46
(0.80–7.59) | 0.851 |
| Gender (male vs.
female) | 1.66
(0.69–4.02) | 0.258 | 1.11
(0.37–3.31) | 0.119 |
| Pathological grade
(II vs. III vs. IV) | 3.77
(1.88–7.58) | <0.0001 | 2.98
(1.12–7.49) | 0.020 |
| Edema (slight vs.
severe) | 12.66
(1.96–81.20) | 0.007 | 0.50
(0.05–5.32 | 0.564 |
| Edema (ring form
vs. irregular form) | 0.16
(0.03–0.84) | 0.030 | 3.43
(0.37–31.95) | 0.278 |
| CXCL12 expression
in TC (high vs. low) | 1.90
(0.75–4.95) | 0.172 | 0.41
(0.11–1.51) | 0.181 |
| CXCL12 expression
in EC (high vs. low) | 0.85
(0.34–2.12) | 0.719 | 3.15
(0.96–10.31) | 0.058 |
| CXCR4 expression in
TC (high vs. low) | 0.69
(0.26–1.84) | 0.460 | 1.72
(0.47–6.27) | 0.414 |
| CXCR4 expression in
EC (high vs. low) | 0.74
(0.30–1.82) | 0.513 | 0.47
(0.11–2.07) | 0.315 |
Discussion
PTBE is a type of vasogenic edema, which results
from interactions among cytokines secreted by nests of tumor and
endothelial cells (6). CXCR4 is an a
chemokine G-protein coupled receptor with seven-transmembrane alpha
helices and 352 residues (17). The
associated ligand CXCL12, also termed stromal-derived factor-1a,
has long been known to regulate lymphocyte chemotaxis and the
development of lymphoid organ structure (18). The binding of CXCR4 and CXCL12
activates downstream signaling molecules, including extracellular
signal-regulated kinase-1/2, mitogen-activated protein kinase,
protein kinase B/phosphoinositide 3-kinase, nuclear factor-κB and
c-Jun N-terminal kinase, which promote the differentiation and
invasion of tumor cells (19–22). In addition, activation of the
CXCL12-CXCR4signaling pathway induces angiogenesis in glioma
(23,24), and blockade of this pathway has been
demonstrated to reduce the density of tumor angiogenesis (25,26).
Therefore, the CXCR4-CXCL12 signaling pathway may affect the degree
and morphological characteristics of PTBE.
In the present study, immunohistochemical staining
demonstrated that CXCL12 was expressed mainly in vascular
endothelial cells, while CXCR4 was mainly expressed in tumor cells,
with particularly strong staining around the necrotic area. The
degree and form of PTBE reflected the expression levels of CXCL12
in vascular endothelial cells and CXCR4 in tumor cells. In
addition, CXCL12-positive endothelial cells were observed at the
site of peritumoral edema and CXCR4-positive tumor cells were
detected within the area of peritumoral edema or perivascular
regions. These results indicated that the expression levels of
CXCL12 in vascular endothelial cells and CXCR4 in tumor cells are
associated with PTBE.
In addition, the association between CXCL12/CXCR4
expression and clinicopathological features was analyzed. Previous
studies in patients with glioma have revealed an association
between CXCR4 and clinicopathological features or patient survival
time (10,27). In the present study, it was found that
age was associated with CXCR4 expression in tumor cells and
vascular endothelial cells. However, only patient age was
associated with CXCL12 expression in vascular endothelial cells.
These data are consistent with the observation by Schoenegger et
al (2) that the incidence of PTBE
is associated with patient age. In the present study, CXCL12/CXCR4
expression in tumor cells and vascular endothelial cells was
associated with pathological grade. In addition, Kaplan-Meier
survival curve analysis revealed significantly shorter PFS and OS
for patients with high CXCL12 and CXCR4 expression in tumor cells
and/or endothelial cells. However, multivariate analysis found that
CXCL12 and CXCR4 expression in tumor and endothelial cells were not
independent prognostic factors for patients with glioblastoma.
In summary, the present data indicated that the
expression levels of CXCL12 in vascular endothelial cells and CXCR4
expression in tumor cells are associated with the degree and
morphological characteristics of PTBE, while CXCL12/CXCR4
expression is not an independent prognostic factor for glioma
patients. However, it should be noted that the present study is
subject to the limitations of a retrospective study design,
potentially inaccurate data collection that relied on patient
memory and a small sample size. Large-scale randomized multicenter
studies are required to analyze the function of the CXCL12-CXCR4
signaling pathway in patients with glioma.
Acknowledgements
The authors would like to thank the Department of
Pathology, the First Affiliated Hospital of Fujian Medical
University (Fuzhou, China) for help in immunohistochemistry design
and the Public Health School, Fujian Medical University, for
assistance in data processing and statistical analysis. The present
study was supported by the National Natural Science Foundation of
China (grant no. 21435002).
References
|
1
|
Seidel C, Dörner N, Osswald M, Wick A,
Platten M, Bendszus M and Wick W: Does age matter?-A MRI study on
peritumoral edema in newly diagnosed primary glioblastoma. BMC
Cancer. 11:1272011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schoenegger K, Oberndorfer S, Wuschitz B,
Struhal W, Hainfellner J, Prayer D, Heinzl H, Lahrmann H, Marosi C
and Grisold W: Peritumoral edema on MRI at initial diagnosis: An
independent prognostic factor for glioblastoma? Eur J Neurol.
16:874–878. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng L, Huang Z, Zhou W, Wu Q, Donnola S,
Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, et al: Glioblastoma
stem cells generate vascular pericytes to support vessel function
and tumor growth. Cell. 153:139–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Soda Y, Myskiw C, Rommel A and Verma IM:
Mechanisms of neovascularization and resistance to anti-angiogenic
therapies in glioblastoma multiforme. J Mol Med (Berl). 91:439–448.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu S, Ahn D, Johnson G, Law M, Zagzag D
and Grossman RI: Diffusion-tensor MR imaging of intracranial
neoplasia and associated peritumoral edema: Introduction of the
tumor infiltration index. Radiology. 232:221–228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin ZX: Glioma-related edema: New insight
into molecular mechanisms and their clinical implications. Chin J
Cancer. 32:49–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hartmann M, Jansen O, Egelhof T, Forsting
M, Albert FK and Sartor K: Effect of brain edema on the recurrence
pattern of malignant gliomas. Radiologe. 38:948–953. 1998.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Engelhorn T, Savaskan NE, Schwarz MA,
Kreutzer J, Meyer EP, Hahnen E, Ganslandt O, Dörfler A, Nimsky C,
Buchfelder M and Eyüpoglu IY: Cellular characterization of the
peritumoral edema zone in malignant brain tumors. Cancer Sci.
100:1856–1862. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou Y, Larsen PH, Hao C and Yong VW:
CXCR4 is a major chemokine receptor on glioma cells and mediates
their survival. J Biol Chem. 277:49481–49487. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bian XW, Yang SX, Chen JH, Ping YF, Zhou
XD, Wang QL, Jiang XF, Gong W, Xiao HL, Du LL, et al: Preferential
expression of chemokine receptor CXCR4 by highly malignant human
gliomas and its association with poor patient survival.
Neurosurgery. 61:570–579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ehtesham M, Winston JA, Kabos P and
Thompson RC: CXCR4 expression mediates glioma cell invasiveness.
Oncogene. 25:2801–2806. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stevenson CB, Ehtesham M, McMillan KM,
Valadez JG, Edgeworth ML, Price RR, Abel TW, Mapara KY and Thompson
RC: CXCR4 expression is elevated in glioblastoma multiforme and
correlates with an increase in intensity and extent of peritumoral
T2-weighted magnetic resonance imaging signal abnormalities.
Neurosurgery. 63:560–570. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barbero S, Bonavia R, Bajetto A, Porcile
C, Pirani P, Ravetti JL, Zona GL, Spaziante R, Florio T and
Schettini G: Stromal cell-derived factor 1alpha stimulates human
glioblastoma cell growth through the activation of both
extracellular signal-regulated kinases 1/2 and Akt. Cancer Res.
63:1969–1974. 2003.PubMed/NCBI
|
|
14
|
Lin ZX: Patterns in the occurrence and
development of tumors. Chin Med J (Engl). 124:1097–1104.
2011.PubMed/NCBI
|
|
15
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zagzag D, Esencay M, Mendez O, Yee H,
Smirnova I, Huang Y, Chiriboga L, Lukyanov E, Liu M and Newcomb EW:
Hypoxia- and vascular endothelial growth factor-induced stromal
cell-derived factor-1alpha/CXCR4 expression in glioblastomas: One
plausible explanation of Scherer's structures. Am J Pathol.
173:545–560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu B, Chien EY, Mol CD, Fenalti G, Liu W,
Katritch V, Abagyan R, Brooun A, Wells P, Bi FC, et al: Structures
of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide
antagonists. Science. 330:1066–1071. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshie O, Imai T and Nomiyama H: Novel
lymphocyte-specific CC chemokines and their receptors. J Leukoc
Biol. 62:634–644. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Furusato B, Mohamed A, Uhlén M and Rhim
JS: CXCR4 and cancer. Pathol Int. 60:497–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun X, Cheng G, Hao M, Zheng J, Zhou X,
Zhang J, Taichman RS, Pienta KJ and Wang J: CXCL12/CXCR4/CXCR7
chemokine axis and cancer progression. Cancer Metastasis Rev.
29:709–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hong X, Jiang F, Kalkanis SN, Zhang ZG,
Zhang XP, DeCarvalho AC, Katakowski M, Bobbitt K, Mikkelsen T and
Chopp M: SDF-1 and CXCR4 are up-regulated by VEGF and contribute to
glioma cell invasion. Cancer Lett. 236:39–45. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu DY, Tang CH, Yeh WL, Wong KL, Lin CP,
Chen YH, Lai CH, Chen YF, Leung YM and Fu WM: SDF-1alpha
up-regulates interleukin-6 through CXCR4, PI3K/Akt, ERK, and
NF-kappaB-dependent pathway in microglia. Eur J Pharmacol.
613:146–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kryczek I, Lange A, Mottram P, Alvarez X,
Cheng P, Hogan M, Moons L, Wei S, Zou L, Machelon V, et al: CXCL12
and vascular endothelial growth factor synergistically induce
neoangiogenesis in human ovarian cancers. Cancer Res. 65:465–472.
2005.PubMed/NCBI
|
|
24
|
Mirshahi F, Pourtau J, Li H, Muraine M,
Trochon V, Legrand E, Vannier J, Soria J, Vasse M and Soria C:
SDF-1 activity on microvascular endothelial cells: Consequences on
angiogenesis in in vitro and in vivo models. Thromb Res.
99:587–594. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guleng B, Tateishi K, Ohta M, Kanai F,
Jazag A, Ijichi H, Tanaka Y, Washida M, Morikane K, Fukushima Y, et
al: Blockade of the stromal cell-derived factor-1/CXCR4 axis
attenuates in vivo tumor growth by inhibiting angiogenesis in a
vascular endothelial growth factor-independent manner. Cancer Res.
65:5864–5871. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tachibana K, Hirota S, Iizasa H, Yoshida
H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N,
Nishikawa S, et al: The chemokine receptor CXCR4 is essential for
vascularization of the gastrointestinal tract. Nature. 393:591–594.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lv S, Sun B, Zhong X, Dai C, Wang W, Ma X,
Song H, Shi R and Wang R: The clinical implications of chemokine
receptor CXCR4 in grade and prognosis of glioma patients: A
meta-analysis. Mol Neurobiol. 52:555–561. 2015. View Article : Google Scholar : PubMed/NCBI
|