Introduction
Colorectal cancer (CRC) is one of the leading causes
of cancer-associated mortality and the most common type of cancer,
with >1 million incident cases diagnosed annually worldwide
(1,2).
Of patients with synchronous distant metastases, defined as stage
IV by Union for International Cancer Control tumor node metastasis
(TNM) staging 7th edition (3), at the
time of diagnosis, ~25% exhibit poor prognoses, with a 5-year
survival rate of ~12% (4,5). Amongst patients diagnosed with a stage
IV CRC, liver metastases is the most common type, occurring in
20–30% of patients, whereas peritoneal and lung metastases occur in
10–15% and 10–25% patients, respectively, and other non-rectal or
non-colon metastases occur rarely (6). Amongst patients with synchronous distant
metastases, ~80% exhibit metastases that cannot be curatively
resected and the 10–30% who undergo resection of the primary tumor
experience complications such as perforation or hemorrhage
(7).
Previously, several prospective studies revealed
that initial tumor resection is an important step toward improving
the overall survival rates (OS) of patients with stage IV CRC
(8,9).
However, the optimal timing of primary tumor resection remains
controversial. Poultsides et al (10) demonstrated that upfront systemic
therapy may be safely administered to patients with stage IV CRC,
avoiding the need for palliative primary tumor resection in the
majority of cases. Additionally, a randomized phase III study
[European Organisation for Research and Treatment of Cancer (EORTC)
40983] demonstrated that amongst patients with initially resectable
liver metastases, including patients with stage IV disease and
those with tumor recurrence, perioperative (pre- and postoperative)
chemotherapy with folinic acid, fluorouracil and oxaliplatin
(FOLFOX4) significantly improved progression-free survival (PFS)
compared with surgery alone, although no differences in OS were
observed (11,12). The aforementioned study suggested that
perioperative chemotherapy with FOLFOX4 reduced the risk of
progression in a subset of patients with initially resectable liver
metastases. However, the molecular characteristics of these
patients were not examined, and the requirements of initial tumor
resection and perioperative chemotherapy remain debatable for
patients with stage IV CRC.
CRC progresses through a series of well-defined
steps that are associated with characteristic mutations, including
genetic and epigenetic alterations in various oncogenes and tumor
suppressor genes (13–15). Point mutations in the KRAS
oncogene are typically observed in codons 12 and 13 and less
frequently in codons 59, 61, 117 and 144. Additionally,
pathogenetic activating point mutations are primarily observed in
codon 600 of the BRAF oncogene (16). These mutations in the KRAS and
BRAF oncoproteins activate signaling cascades that mediate
cellular responses such as cell proliferation, apoptosis, adhesion,
invasion and angiogenesis (17,18).
Previously, mutations in the KRAS gene, including minor
mutations, have been associated with a resistance to anti-EGFR
antibodies (16,19,20).
Although the BRAF gene is located downstream of KRAS,
the activating V600E BRAF mutation is not considered a
predictive biomarker for resistance to anti-EGFR antibodies.
However, mutations in this gene have been suggested to be strong
prognostic indicators of poor prognoses in patients with stage II
and III CRC subsequent to curative resection, and in patients with
unresectable metastatic CRC (16,21–25).
The present study hypothesized that mutations in
BRAF and KRAS genes may also indicate the appropriate
treatment strategies for patients with stage IV CRC. Thus, the
presence of mutations in these genes was determined in a
consecutive series of patients with stage IV CRC, including those
with resectable and unresectable metastatic lesions at diagnosis,
and determined their clinical significance using correlations with
clinicopathological characteristics that are associated with
patient outcomes and survival.
Materials and methods
Study population
A total of 113 consecutively diagnosed patients with
stage IV CRC were treated with colectomy or proctectomy at the
Okayama University Hospital, Okayama, Japan, between May 2000 and
February 2013. All cases were histologically confirmed as
adenocarcinoma, and all familial CRC, such as Lynch syndrome and
familial adenomatous polyposis, were excluded.
The present study was approved by the Institutional
Review Board of the Okayama University Hospital. All patients gave
written informed consent for the use of tissues and clinical data
for research purposes. Histological diagnoses of tumors were made
according to the World Health Organization International
Histological Classification of tumors (26), and tumors were subclassified as
differentiated (well and moderately differentiated tubular
adenocarcinoma) or undifferentiated types (poorly differentiated
adenocarcinoma and mucinous adenocarcinoma) (27). Pathological stage was determined
according to the 7th edition Union for International Cancer Control
TNM classification of malignant tumors (5).
Analysis of KRAS and BRAF
mutations
Direct sequencing was performed to identify
mutations in KRAS exon 2, including codon 12 and 13, and
BRAF exon 15, including codon 600, using purified DNA from
formalin-fixed and paraffin-embedded tissues or from fresh frozen
tissues from each case. Primer sequences for KRAS and
BRAF and polymerase chain reaction (PCR) conditions were
described previously (28). PCR
products were purified using a QIAquick PCR purification kit
(Qiagen, Inc., Valencia, CA, USA) and directly sequenced using an
ABI PRISM® 310-Avant™ and a 310R Genetic Analyzer
(Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
Analysis of microsatellite status
Multiplex PCRs with the mononucleotide
microsatellite markers BAT26, NR27 and NR21 were performed to
determine the microsatellite instability (MSI) status of the CRC
tissues. Tumors exhibiting genomic instability in ≥1 mononucleotide
markers were classified as MSI, and types of cancer with no
mutations in these markers were categorized as microsatellite
stable (MSS). Previously, we demonstrated that data analyses with
the mononucleotide markers BAT26, NR27 and NR21 were comparable or
superior compared with those with the five markers recommended by
the National Cancer Institute workshop for detecting high MSI, or
mismatch deficiencies, in CRC (29).
Statistical analysis
Statistical analyses were performed using SPSS v.
20.0 software (IBM SPSS, Armonk, NY, USA). Categorical variables
were compared using Fisher's exact test, and continuous variables
were compared using the Kruskal-Wallis test. OS curves were
calculated using the Kaplan-Meier method, and differences in the
survival times amongst the subgroups were compared using the
log-rank test. Univariate and multivariate analyses were performed
using Cox proportional hazard regression models. Significant
factors from univariate analyses were included in multivariate
analysis to determine independent prognostic factors. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
Amongst the 113 patients with stage IV CRC, 57.5%
were male and 42.5% were female (Table
I), and the median age was 64 years, with a range of 35–88
years. The median serum CEA level was 34.0 ng/ml, with a range of
1.0–9092.0 ng/ml. Tumor locations were categorized as proximal
colon, from the cecum to the splenic flexure of the transverse
colon, or distal colon, from the splenic flexure of the descending
colon to the rectum. A total of 24.8% (28) tumors were in the proximal colon and
75.2% (85) tumors were in the distal colon. The majority of tumors,
85.8% (97/113) were histologically diagnosed as differentiated
adenocarcinoma, and 14.2% (16/113) of tumors were categorized as
undifferentiated adenocarcinoma. Distant metastatic lesions in a
single organ such as the liver or the lung occurred in 64.6%
(73/113) of patients and 35.4% (40/113) of patients exhibited
metastases in multiple organs (Table
I). Chemotherapy including fluoropyrimidine plus oxaliplatin or
irinotecan was administrated to 68.1% (77/113) of patients. Of
these, 77 were treated with chemotherapy: 23 received chemotherapy
prior and subsequent to resection of the primary tumor (upfront
chemotherapy) and 54 received chemotherapy subsequent to resection
of the primary tumor (postoperative chemotherapy). Amongst all
patients with stage IV CRC, 63.7% (72) of patients received
curative resection of primary and metastatic sites, defined by the
absence of residual disease; the remaining 36.3% (41) of patients received local excisions of
primary tumors alone, defined by the presence of residual
disease.
 | Table I.Characteristics of 113 patients with
stage IV colorectal cancer in relation to the mutational status of
BRAF and KRAS genes. |
Table I.
Characteristics of 113 patients with
stage IV colorectal cancer in relation to the mutational status of
BRAF and KRAS genes.
| Characteristic | Total (n=113) | BRAF-mutant
(n=7) | KRAS-mutant
(n=31) | Wild-type
(n=75) | P-value |
|---|
| Age (years) |
|
|
|
| 0.849a |
| Median
(Range) | 64 (35–88) | 64 (40–79) | 61 (35–88) | 65 (41–85) |
|
| Gender (%) |
|
|
|
| 0.595b |
|
Male | 65 (57.5) | 3 (42.9) | 17 (54.8) | 45 (60.0) |
|
|
Female | 48 (42.5 | 4 (57.1) | 14 (45.2) | 30 (40.0) |
|
| Serum
carcinoembryonic antigen level (ng/ml) |
|
|
|
| 0.574a |
| Median
(Range) | 34
(1.0–9092.0) | 32
(2.0–1385.0) | 31
(1.0–1353.0) | 42
(1.0–9092.0) |
|
| Tumor
Locationc (%) |
|
|
|
|
<0.001b |
|
Proximal colon | 28 (24.8) | 6 (85.7) | 13 (41.9) | 9 (12.0) |
|
| Distal
colon | 85 (75.2) | 1 (14.3) | 18 (58.1) | 66 (88.0) |
|
| Histology (%) |
|
|
|
| 0.016b |
|
Differentiated | 97 (85.8) | 3 (42.9) | 28 (90.3) | 66 (88.0) |
|
|
Undifferentiated | 16 (14.2) | 4 (57.1) | 3 (9.7) | 9 (12.0) |
|
| No. of distant
metastatic sites (%) |
|
|
|
| 0.114b |
|
Single | 73 (64.6) | 2 (28.6) | 22 (71.0) | 49 (65.3) |
|
|
Multiple | 40 (35.4) | 5 (71.4) | 9 (29.0) | 26 (34.7) |
|
|
Chemotherapyd (%) |
|
|
|
| 0.431b |
|
Upfronte | 23 (20.3) | 2 (28.6) | 9 (29.0) | 12 (16.0) |
|
|
Postoperative | 54 (47.8) | 2 (28.6) | 14 (45.2) | 38 (50.7) |
|
|
None | 36 (31.9) | 3 (42.8) | 8 (25.8) | 25 (33.3) |
|
| Molecularly
targeted therapy (%) |
|
|
|
| 0.663b |
|
Yes | 52 (46.0) | 2 (28.6) | 14 (45.2) | 36 (48.0) |
|
| No | 61 (54.0) | 5 (71.4) | 17 (54.8) | 39 (52.0) |
|
| Residual disease
(%) |
|
|
|
| 0.045b |
|
Present | 72 (63.7) | 7 (100.0) | 16 (51.6) | 49 (65.3) |
|
|
Absent | 41 (36.3) | 0 (0.0) | 15 (48.4) | 26 (34.7) |
|
Frequencies of MSI and mutations in
BRAF and KRAS genes in stage IV CRC
In the present cohort of patients with stage IV CRC,
no tumor displayed MSI, and all 113 tumors were categorized as MSS.
KRAS and BRAF mutation analyses were successful in
113 specimens, and mutations in the two genes occurred in a
mutually exclusive manner, with no tumors exhibiting simultaneous
mutations in the two genes. Mutated BRAF was revealed in
6.2% (7) tumors and encoded the V600E
mutation. Mutations in codons 12 or 13 of the KRAS gene were
revealed in 27.4% (31) of tumors.
Amongst the 31 tumors with KRAS exon 2 mutations, all
exhibited single mutations and the most prevalent types of
mutations were GGT to GAT (G12D) in 13.3% (15/113) of tumors,
followed by GGT to GTT (G12V) in 6.2% (7/113) of tumors, GGC to GAC
(G13D) in 4.4% (5/113) of tumors, GGT to AGT (G12S) in 2.7% (3/113)
of tumors, and GGT to TGT (G12C) in 0.9% (1/113) of tumors. Based
on the presence or absence of mutations in these two genes, all 113
patients with stage IV CRC were classified as BRAF-mutant,
KRAS-mutant, or wild-type (Table
I).
Associations between genetic profiles
and clinicopathological characteristics
BRAF-mutant tumors were observed
significantly more frequently in the proximal colon, 6 in the
proximal colon vs. 1 in the distal colon, and BRAF mutations
were associated with undifferentiated histological phenotypes
(P=0.016). Distant metastases in multiple organs were more common
in patients with BRAF-mutant CRC (71.4%; 5/7) compared with
those with KRAS-mutant (29.0%; 9/31) and wild-type cancers
(34.7%; 26/75; P=0.095).
A total of 74.2% (23/31) patients with
KRAS-mutant tumors and 57.1% (4/7) patients with
BRAF-mutant tumors had received one or more course of
fluoropyrimidine-based chemotherapy. Upfront chemotherapy was
administrated in 28.6% (2/7) patients with BRAF-mutant
tumors, 29.0% (9/31) of patients with KRAS-mutant tumors,
and 16.0% (12/75) of patients with wild-type tumors. Postoperative
chemotherapy was administrated in 28.6% (2/7) of patients with
BRAF V600E mutations, 45.2% (14/31) of patients with
KRAS mutations, and 50.7% (38/75) of patients with wild-type
tumors. In contrast, 42.8% (3 of 7) of patients with BRAF
mutations, 25.8% (8/31) of patients with KRAS mutations, and
33.3% (25/75) of patients with wild-type tumors did not receive
chemotherapy prior or subsequent to resection of the primary tumor.
The molecular targeted agent bevacizumab was administered to 54.8%
(17/31) of patients with KRAS-mutant tumors, and 28.6% (2/7)
of patients with BRAF-mutant tumors received bevacizumab and
cetuximab. Amongst all patients with stage IV CRC, 63.7% (72
patients) received curative resections that included metastatic
sites, as defined by the absence of residual disease, whereas the
remaining 36.3% (41 patients) received local excisions of primary
tumors alone, as defined by the presence of residual disease. No
patients with BRAF-mutant tumors received curative
resection, whereas 48.4% (15/31) of patients with
KRAS-mutant tumors and 34.7% (26/75) of patients with
wild-type tumors received curative resection (P=0.047).
Survival analyses in stage IV CRC
patients with BRAF mutations
The OS in patients with stage IV CRC with mutations
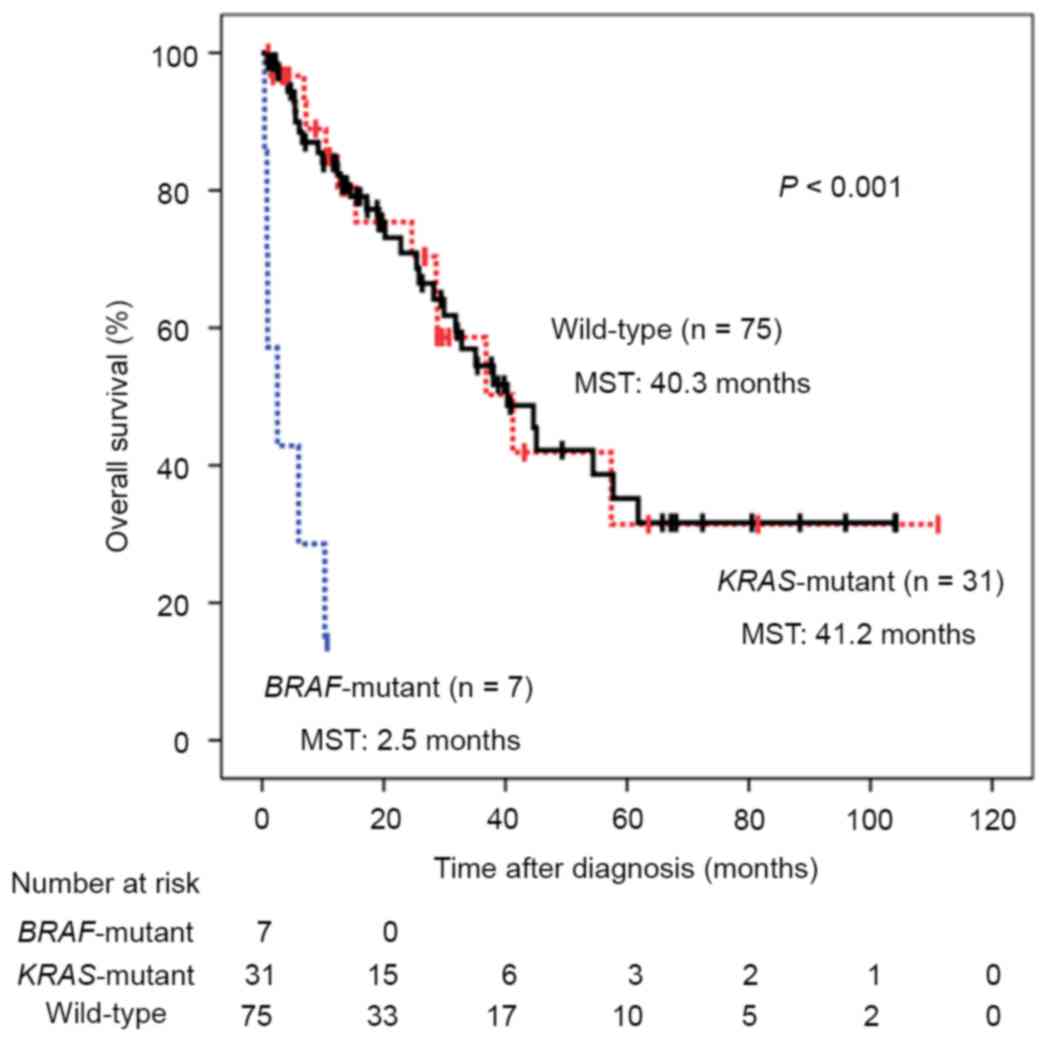
in KRAS and BRAF genes is illustrated in Fig. 1. The median follow-up duration was
17.3 months and patients with BRAF mutations exhibited
significantly poorer prognoses compared with those with KRAS
mutations or wild-type tumors, with median survival times (MSTs) of
2.5, 41.2 and 40.3 months, respectively (P<0.001). Univariate
analysis revealed several factors associated with poor prognosis,
including tumors with undifferentiated histology, multiple
metastatic sites, residual disease, no chemotherapy, therapy with
molecular targeted drugs and the presence of BRAF mutations
(Table II). Similarly, multivariate
analysis revealed that undifferentiated tumor histology, residual
disease, no chemotherapy and mutations in the BRAF gene were
statistically significant predictors of survival and independent
prognostic factors for poor outcomes of stage IV CRC (Hazard ratio;
8.42, P<0.0001; Table III).
 | Table II.Univariate analysis for survival
outcomes in 113 patients with metastatic colorectal cancer. |
Table II.
Univariate analysis for survival
outcomes in 113 patients with metastatic colorectal cancer.
| Variable | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Age (years):
≥64/≤63 |
1.16 | 0.67–2.03 | 0.594 |
| Gender:
Male/Female |
0.94 | 0.53–1.64 | 0.820 |
| Serum
carcinoembryonic antigen level (ng/ml): ≥34/<34 |
0.98 | 0.56–1.71 | 0.945 |
| Tumor location:
Distal/Proximal |
0.97 | 0.55–1.71 | 0.910 |
| Histology:
Undifferentiated/Differentiated |
5.00 | 2.48–10.10 | <0.001 |
| No. of metastatic
sites: Multiple/Single |
3.27 | 1.86–5.75 | <0.001 |
| Residual Disease:
Present/Absent |
4.46 | 2.29–8.70 | <0.001 |
| Chemotherapy:
No/Yes |
2.70 | 1.53–4.76 | <0.001 |
| Molecularly
targeted therapy: No/Yes |
2.02 | 1.11–3.68 | 0.021 |
| KRAS
mutation: Yes/No |
0.86 | 0.45–1.65 | 0.651 |
| BRAF
mutation: Yes/No | 11.88 | 4.55–31.00 | <0.001 |
 | Table III.Multivariate analysis for survival
outcomes in 113 patients with metastatic colorectal cancer. |
Table III.
Multivariate analysis for survival
outcomes in 113 patients with metastatic colorectal cancer.
| Variable | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Histology:
Undifferentiated/Differentiated | 2.61 | 1.10–6.21 | 0.030 |
| No. of metastatic
sites: Multiple/Single | 1.47 | 0.74–2.94 | 0.274 |
| Residual disease:
Present/Absent | 5.65 | 2.41–13.16 | <0.001 |
| Chemotherapy:
No/Yes | 3.44 | 1.60–7.39 | 0.002 |
| Molecularly
targeted therapy: No/Yes | 1.66 | 0.81–3.38 | 0.167 |
| BRAF
mutation: Yes/No | 8.42 | 2.72–26.02 | <0.001 |
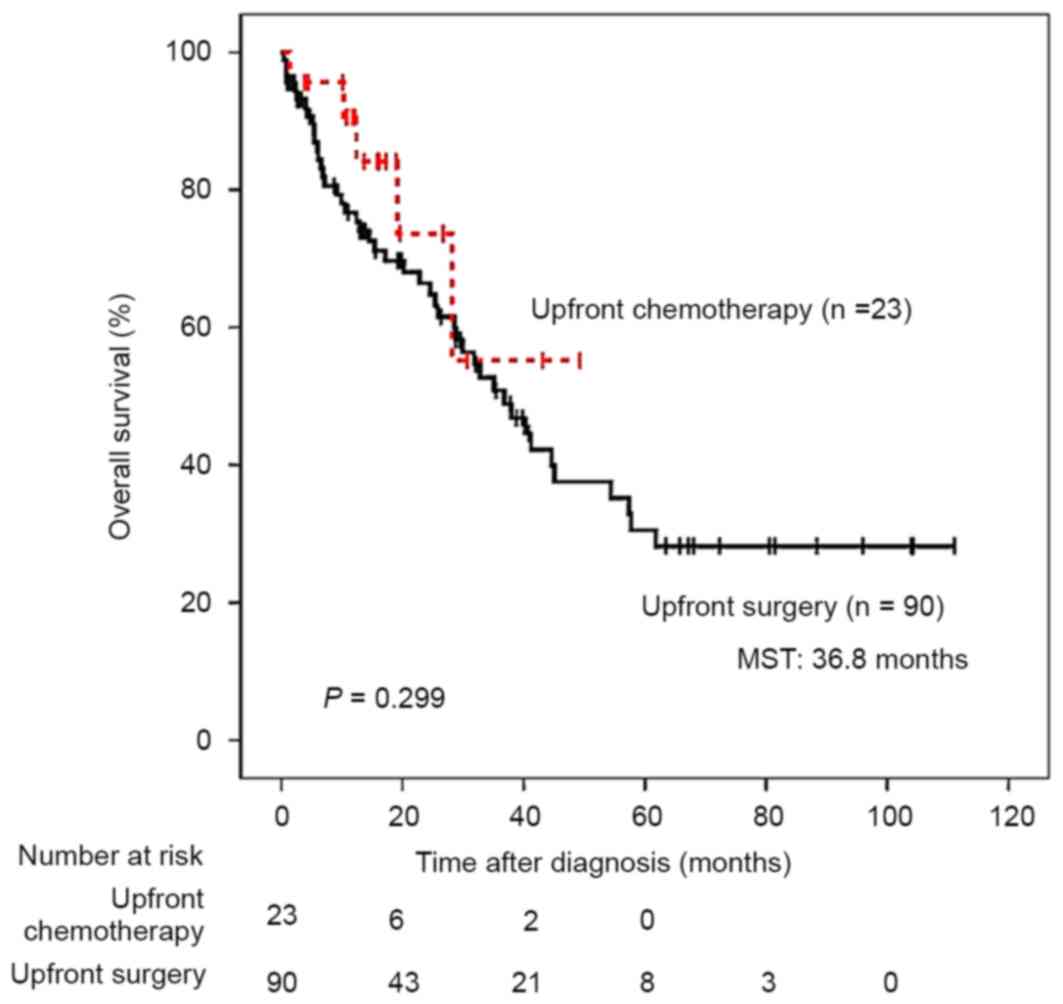
Subsequent to correcting for the administration of
upfront chemotherapy, clinical outcomes did not differ between
patients with stage IV CRC with and without upfront chemotherapy
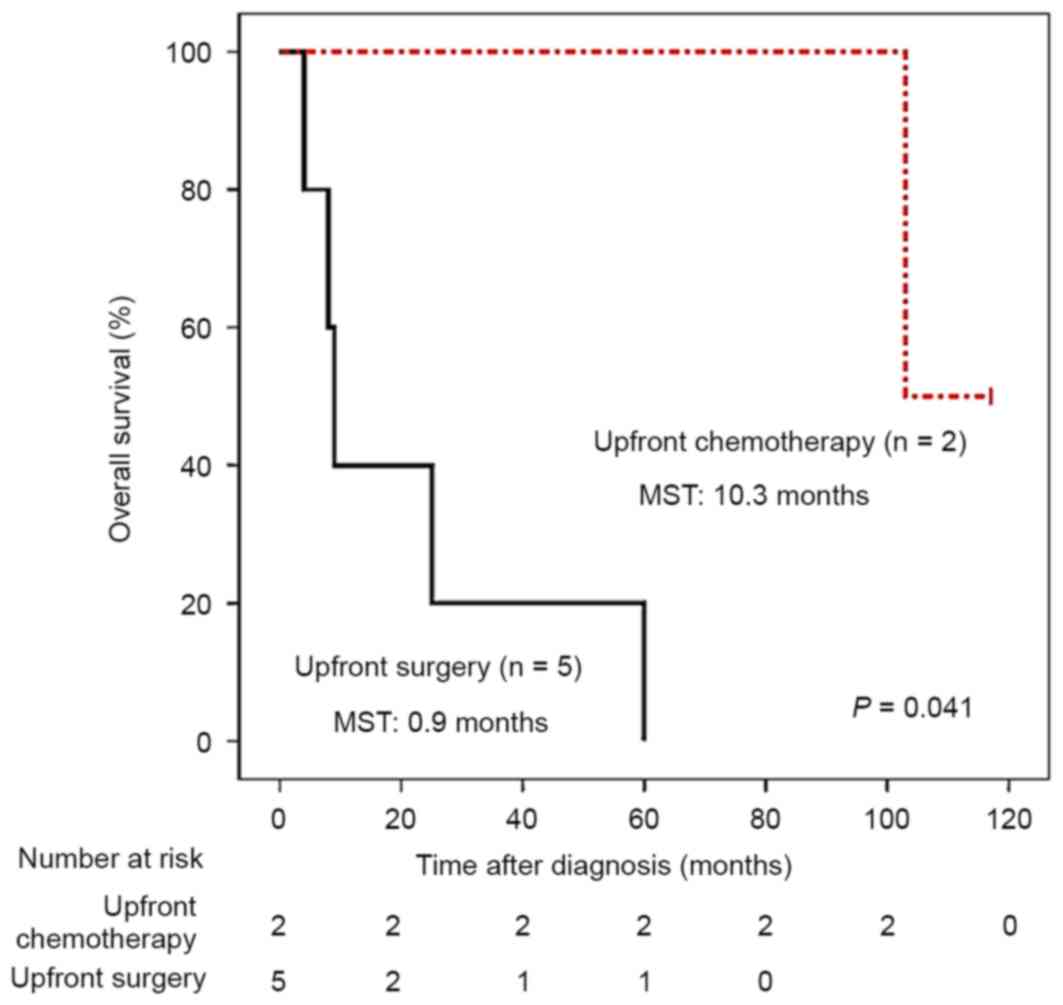
(Fig. 2). However, amongst 7 patients
with BRAF V600E mutations in primary tumors, 2 received
upfront chemotherapy and demonstrated improved survival compared
with the 5 patients who did not receive upfront chemotherapy
(Fig. 3), highlighting the prognostic
value of BRAF mutations in patients with stage IV CRC.
Discussion
The present study identified the presence of the
BRAF V600E mutation in primary tumor tissues and produced
data that supported the hypothesis of the potential for
individualized treatment strategies for patients with stage IV
CRC.
Several studies investigating stage IV CRC have
demonstrated that initial tumor resection improves survival
(6,8,9,30). However, in certain cases, surgical
removal of the primary tumor is accompanied by exceptionally rapid
outgrowth of distant metastases (31,32),
suggesting that primary tumors inhibit the growth of metastatic
lesions in a limited number of cases (33–35). The
data of the present study indicate that this subset of patients
with stage IV CRC may include those with the BRAF V600E
mutation, with rapid outgrowth of distant metastases subsequent to
the surgical removal of primary tumors.
The importance of upfront systemic chemotherapy was
reported in a previous randomized phase III study (EORTC 40983)
(11,12), although perioperative combination
chemotherapy with FOLFOX4 increased PFS compared with surgery alone
in a subset of patients, no differences in overall survival were
observed (11,12). Therefore, perioperative chemotherapy
may reduce the risk of PFS events in certain patients with stage IV
CRC with initially resectable liver metastases. In agreement with
this, the data of the present study demonstrates that patients with
V600E BRAF-mutant advanced CRC are the most likely type of
patient to benefit from perioperative chemotherapy.
In the present cohort of patients with stage IV
disease, no primary tumors exhibited MSI, which typically indicates
defective DNA mismatch repair systems (36). Clinically, CRC with MSI include
patients with Lynch syndrome and sporadic MSI cancer (36,37). Lynch
syndrome is hereditary and reflects germline mutations in the DNA
mismatch repair genes MLH1, PMS2, MSH2 or
MSH6 (36,37). In contrast, sporadic MSI usually
reflects hypermethylation of the MLH1 promoter, which causes
transcriptional silencing of this proofreading gene (15,28). In
the present study, patients with Lynch syndrome were excluded and
the entire cohort of stage IV patients exhibited sporadic CRC.
Typical features of sporadic MSI CRC include older age, female sex,
proximal tumor location, undifferentiated histology, lower clinical
stage, slow growth and better overall prognosis (38–40).
Therefore, it is unlikely that the tumors in the present study
displayed MSI, which is usually uncharacteristic of
advanced/metastatic CRC.
Mutations in the BRAF oncogene were first
identified in 2002 and were demonstrated to be initially associated
with MSI CRC, particularly sporadic MSI tumors (15,41,42).
Subsequently, BRAF-mutant CRC were recognized as either
sporadic MSI tumors or MSS tumors, and the BRAF V600E
mutation was exhibited in >50% of sporadic MSI tumors and in
certain MSS tumors (28). In a
retrospective study of several clinical trials, the presence of the
BRAF V600E mutation was a strong negative prognostic factor
for OS in patients with stage II/III CRC, particularly in patients
with colorectal tumors with low or stable microsatellite
instability, MSI-L MSS or no MSI (25). Several studies demonstrated that
patients with CRC with MSI tumors carrying BRAF mutations
exhibited significantly better prognoses compared with those with
BRAF-mutant MSS tumors (43,44).
Similarly, amongst patients with metastatic CRC treated with
combination chemotherapy using molecular targeted agents,
BRAF mutations were associated with significantly poorer
prognoses (16,21–23).
In the prospective FFCD 9601 trial, which examined
the benefit of primary tumor resection on survival of patients with
CRC with synchronous metastases, stage IV, treated by chemotherapy,
survival outcomes were better in patients with distal primary
lesions (9). Although the mechanisms
behind these observations remain unclear, the authors emphasized
that the primary tumor locations and resections were critical
clinical factors in the therapeutic management of these patients
with CRC. In the present study, BRAF-mutant CRC were
significantly associated with proximally located primary tumors and
poor prognosis, with a median overall survival time of 2.5 months.
Therefore, the absence of the BRAF V600E mutation may
explain the improved survival outcomes in patients with stage IV
CRC with distal cancers. Additionally, the present data demonstrate
that patients with V600E BRAF-mutant stage IV tumors tend to
exhibit distant metastases in multiple organs, inhibiting the
success of curative resection. Accordingly, none of the present
patients with BRAF mutations received curative resection, as
indicated by the significantly worse prognoses for
BRAF-mutant CRC.
Although there were only seven CRC exhibiting the
BRAF V600E mutation, 6.2%, of the 113 stage IV patients with
CRC, they demonstrated a trend of improved responses to upfront
systematic chemotherapy, which improved prognoses. In contrast,
patients with BRAF-mutant tumors who received upfront
surgical resection of primary tumors instead of chemotherapy
exhibited limited MSTs of 0.9 months. These data indicate that
intensive upfront chemotherapy improves prognoses of patients with
stage IV CRC with BRAF mutations.
In conclusion, although the present study was
limited to 113 patients, the presence of BRAF mutations in
primary tumors from patients with stage IV CRC was a significant
negative prognostic factor. The present data suggest that intensive
upfront chemotherapy enhances survival rates in patients with
advanced CRC exhibiting BRAF mutations.
Acknowledgements
The authors would like to thank Mr. Toru Nakai, Ms.
Tae Yamanishi and Mr. Akihiro Nyuya for technical assistance. The
authors would also like to thank Dr. Margaret Hinshelwood, of the
Office of Scientific Publications at the Baylor Charles A. Sammons
Cancer Center at Dallas for her helpful editorial comments. The
present study was supported by KAKENHI (grant nos. 20590572,
25860409, 26462016 and 15H03034).
References
|
1
|
Soerjomataram I, Lortet-Tieulent J, Parkin
DM, Ferlay J, Mathers C, Forman D and Bray F: Global burden of
cancer in 2008: A systematic analysis of disability-adjusted
life-years in 12 world regions. Lancet. 380:1840–1850. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sobin L GM and Wittekind C: TNM
Classification of Malignant Tumours. 7th edition. Wiley-Blackwell;
Chichester: 2009
|
|
4
|
Van Cutsem E, Nordlinger B and Cervantes
A: ESMO Guidelines Working Group: Advanced colorectal cancer: ESMO
clinical practice guidelines for treatment. Ann Oncol. 21 Suppl
5:v93–v97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wittekind C: 2010 TNM system: On the 7th
edition of TNM classification of malignant tumors. Pathologe.
31:331–332. 2010.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cirocchi R, Trastulli S, Abraha I,
Vettoretto N, Boselli C, Montedori A, Parisi A, Noya G and Platell
C: Non-resection versus resection for an asymptomatic primary
tumour in patients with unresectable stage IV colorectal cancer.
Cochrane Database Syst Rev: CD008997. 2012. View Article : Google Scholar
|
|
7
|
Ruo L, Gougoutas C, Paty PB, Guillem JG,
Cohen AM and Wong WD: Elective bowel resection for incurable stage
IV colorectal cancer: Prognostic variables for asymptomatic
patients. J Am Coll Surg. 196:722–728. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Venderbosch S, de Wilt JH, Teerenstra S,
Loosveld OJ, van Bochove A, Sinnige HA, Creemers GJ, Tesselaar ME,
Mol L, Punt CJ and Koopman M: Prognostic value of resection of
primary tumor in patients with stage IV colorectal cancer:
Retrospective analysis of two randomized studies and a review of
the literature. Ann Surg Oncol. 18:3252–3260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferrand F, Malka D, Bourredjem A, Allonier
C, Bouché O, Louafi S, Boige V, Mousseau M, Raoul JL, Bedenne L, et
al: Impact of primary tumour resection on survival of patients with
colorectal cancer and synchronous metastases treated by
chemotherapy: Results from the multicenter, randomised trial
Federation Francophone de Cancérologie Digestive 9601. Eur J
Cancer. 49:90–97. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Poultsides GA, Servais EL, Saltz LB, Patil
S, Kemeny NE, Guillem JG, Weiser M, Temple LK, Wong WD and Paty PB:
Outcome of primary tumor in patients with synchronous stage IV
colorectal cancer receiving combination chemotherapy without
surgery as initial treatment. J Clin Oncol. 27:3379–3384. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nordlinger B, Sorbye H, Glimelius B,
Poston GJ, Schlag PM, Rougier P, Schlag PM, Rougier P, Bechstein
WO, Primrose JN, et al: Perioperative chemotherapy with FOLFOX4 and
surgery versus surgery alone for resectable liver metastases from
colorectal cancer (EORTC Intergroup trial 40983): A randomised
controlled trial. Lancet. 371:1007–1016. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nordlinger B, Sorbye H, Glimelius B,
Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole
ET, Finch-Jones M, et al: Perioperative FOLFOX4 chemotherapy and
surgery versus surgery alone for resectable liver metastases from
colorectal cancer (EORTC 40983): Long-term results of a randomised,
controlled, phase 3 trial. Lancet Oncol. 14:1208–1215. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cancer Genome Atlas Network: Comprehensive
molecular characterization of human colon and rectal cancer.
Nature. 487:330–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lao VV and Grady WM: Epigenetics and
colorectal cancer. Nat Rev Gastroenterol Hepatol. 8:686–700. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagasaka T, Sasamoto H, Notohara K,
Cullings HM, Takeda M, Kimura K, Kambara T, MacPhee DG, Young J,
Leggett BA, et al: Colorectal cancer with mutation in BRAF, KRAS,
and wild-type with respect to both oncogenes showing different
patterns of DNA methylation. J Clin Oncol. 22:4584–4594. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Panitumumab-FOLFOX4 treatment and RAS mutations
in colorectal cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Downward J: Targeting RAS signalling
pathways in cancer therapy. Nat Rev Cancer. 3:11–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wong JJ, Hawkins NJ and Ward RL:
Colorectal cancer: A model for epigenetic tumorigenesis. Gut.
56:140–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karapetis CS, Khambata-Ford S, Jonker DJ,
O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD,
Robitaille S, et al: K-ras mutations and benefit from cetuximab in
advanced colorectal cancer. N Engl J Med. 359:1757–1765. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Van Cutsem E, Kohne CH, Hitre E, Zaluski
J, Chien Chang CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Laurent-Puig P, Cayre A, Manceau G, Buc E,
Bachet JB, Lecomte T, Rougier P, Lievre A, Landi B, Boige V, et al:
Analysis of PTEN, BRAF, and EGFR status in determining benefit from
cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin
Oncol. 27:5924–5930. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tol J, Nagtegaal ID and Punt CJ: BRAF
mutation in metastatic colorectal cancer. N Engl J Med. 361:98–99.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Van Cutsem E, Köhne CH, Láng I, Folprecht
G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D,
Tejpar S, et al: Cetuximab plus irinotecan, fluorouracil, and
leucovorin as first-line treatment for metastatic colorectal
cancer: Updated analysis of overall survival according to tumor
KRAS and BRAF mutation status. J Clin Oncol. 29:2011–2019. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Douillard JY, Siena S, Cassidy J,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Final results from PRIME: Randomized phase 3
study of panitumumab with FOLFOX4 for first-line treatment of
metastatic colorectal cancer. Ann Oncol. 25:1346–1355. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roth AD, Tejpar S, Delorenzi M, Yan P,
Fiocca R, Klingbiel D, Dietrich D, Biesmans B, Bodoky G, Barone C,
et al: Prognostic role of KRAS and BRAF in stage II and III
resected colon cancer: Results of the translational study on the
PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 28:466–474.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hamilton SR and Aaltonen LA: Pathology and
genetics of tumours of the digestive systemWorld Health
Organization Classification of Tumours. IARCPress; Lyon: 2000
|
|
27
|
Seifert G, Brocheriou C, Cardesa A and
Eveson JW: WHO International Histological Classification of
Tumours. Tentative histological classification of salivary gland
tumours. Pathol Res Pract. 186:555–581. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nagasaka T, Koi M, Kloor M, Gebert J,
Vilkin A, Nishida N, Shin SK, Sasamoto H, Tanaka N, Matsubara N, et
al: Mutations in both KRAS and BRAF may contribute to the
methylator phenotype in colon cancer. Gastroenterology.
134(1950–1960): 1960. e12008.
|
|
29
|
Goel A, Nagasaka T, Hamelin R and Boland
CR: An optimized pentaplex PCR for detecting DNA mismatch
repair-deficient colorectal cancers. PLoS One. 5:e93932010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stillwell AP, Buettner PG and Ho YH:
Meta-analysis of survival of patients with stage IV colorectal
cancer managed with surgical resection versus chemotherapy alone.
World J Surg. 34:797–807. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Simpson-Herren L, Sanford AH and Holmquist
JP: Effects of surgery on the cell kinetics of residual tumor.
Cancer Treat Rep. 60:1749–1760. 1976.PubMed/NCBI
|
|
32
|
Fisher B, Gunduz N and Saffer EA:
Influence of the interval between primary tumor removal and
chemotherapy on kinetics and growth of metastases. Cancer Res.
43:1488–1992. 1983.PubMed/NCBI
|
|
33
|
Peeters CF, Westphal JR, de Waal RM,
Ruiter DJ, Wobbes T and Ruers TJ: Vascular density in colorectal
liver metastases increases after removal of the primary tumor in
human cancer patients. Int J Cancer. 112:554–559. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peeters CF, de Waal RM, Wobbes T, Westphal
JR and Ruers TJ: Outgrowth of human liver metastases after
resection of the primary colorectal tumor: A shift in the balance
between apoptosis and proliferation. Int J Cancer. 119:1249–1253.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Scheer MG, Stollman TH, Vogel WV, Boerman
OC, Oyen WJ and Ruers TJ: Increased metabolic activity of indolent
liver metastases after resection of a primary colorectal tumor. J
Nucl Med. 49:887–891. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Umar A, Boland CR, Terdiman JP, Syngal S,
de la Chapelle A, Rüschoff J, Fishel R, Lindor NM, Burgart LJ,
Hamelin R, et al: Revised bethesda guidelines for hereditary
nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite
instability. J Natl Cancer Inst. 96:261–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Imai K and Yamamoto H: Carcinogenesis and
microsatellite instability: The interrelationship between genetics
and epigenetics. Carcinogenesis. 29:673–680. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boland CR, Thibodeau SN, Hamilton SR,
Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA,
Fodde R, Ranzani GN and Srivastava S: A National Cancer Institute
Workshop on Microsatellite Instability for cancer detection and
familial predisposition: Development of international criteria for
the determination of microsatellite instability in colorectal
cancer. Cancer Res. 58:5248–5257. 1998.PubMed/NCBI
|
|
39
|
Hutchins G, Southward K, Handley K, Magill
L, Beaumont C, Stahlschmidt J, Richman S, Chambers P, Seymour M,
Kerr D, et al: Value of mismatch repair, KRAS, and BRAF mutations
in predicting recurrence and benefits from chemotherapy in
colorectal cancer. J Clin Oncol. 29:1261–1270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Popat S, Hubner R and Houlston RS:
Systematic review of microsatellite instability and colorectal
cancer prognosis. J Clin Oncol. 23:609–618. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rajagopalan H, Bardelli A, Lengauer C,
Kinzler KW, Vogelstein B and Velculescu VE: Tumorigenesis: RAF/RAS
oncogenes and mismatch-repair status. Nature. 418:9342002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang L, Cunningham JM, Winters JL,
Guenther JC, French AJ, Boardman LA, Burgart LJ, McDonnell SK,
Schaid DJ and Thibodeau SN: BRAF mutations in colon cancer are not
likely attributable to defective DNA mismatch repair. Cancer Res.
63:5209–5212. 2003.PubMed/NCBI
|
|
43
|
Lochhead P, Kuchiba A, Imamura Y, Liao X,
Yamauchi M, Nishihara R, Qian ZR, Morikawa T, Shen J, Meyerhardt
JA, et al: Microsatellite instability and BRAF mutation testing in
colorectal cancer prognostication. J Natl Cancer Inst.
105:1151–1156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Taieb J, Zaanan A, Le Malicot K, Julié C,
Blons H, Mineur L, Bennouna J, Tabernero J, Mini E, Folprecht G, et
al: Prognostic effect of BRAF and KRAS mutations in patients with
stage III colon cancer treated with leucovorin, fluorouracil and
oxaliplatin with or without cetuximab: A post hoc analysis of the
PETACC-8 trial. JAMA Oncol. 1–11. 2016.PubMed/NCBI
|