Introduction
Breast cancer (BC) is an important global health
problem (1). According to estimates
by the American Cancer Society in 2015, BC is a common cause of
cancer-associated mortality in females in the United States and is
predicted to account for 29% of all new female cancer diagnoses
(2). As with other types of cancer,
BC occurs due to an interaction between a lifestyle factor
(drinking alcohol, hormone replacement therapy during menopause and
ionizing radiation) (3,4) and a genetically susceptible host, such
as in patients with breast cancer susceptibility protein family
gene mutations (5–7). Current therapeutic options, including
adjuvant chemotherapy, radiotherapy, hormone therapies and surgery
are appropriate for patients with BC (8). These treatments have significant side
effects, including decreased immunity, nausea, vomiting, anemia,
leukopenia and myelosuppression (9).
However, further investigation is required for developing better
and more effective therapeutic agents.
A number of recently conducted studies have
recognized several potential agents (10), biomarkers (11) and candidate pathways (12) that may be used to treat patients with
BC. Among these approaches, cancer chemoprevention using synthetic
or natural complexes to treat, slow, suppress or reverse the
progression of tumorigenesis is one of the most promising
anticancer strategies. A growing number of natural products and raw
materials are being synthesized into potential therapeutic agents
for the management of various types of cancer (10–12).
Ursolic acid (UA) is a pentacyclic triterpenoid complex extracted
from naturally growing herbs (13).
Previous studies have demonstrated that UA has antiviral,
antibacterial, immunomodulatory and hepatoprotective properties
(14,15). Several studies have confirmed that UA
has anti-proliferative properties in a variety of cancer cell
lines, including those of colorectal cancer (16), endometrial cancer (17), squamous skin cancer (18) and BC (19). Furthermore, UA exhibits a wide range
of activities that have an effect on the development of cancer,
including repression of proliferation, angiogenesis, invasion,
metastasis, differentiation and induction of tumor cell apoptosis
(20). However, the poor
bioavailability and tumor-targeting specificity of UA limits its
clinical application in BC treatment (21).
A wide range of chemically modified compounds of UA
has been used to increase its bioavailability and antitumor
efficacy (12). The combination of
acyl piperazine moiety at C-28 has been demonstrated to improve the
antitumor activity of UA derivatives (22,23).
Additionally, a number of studies have described that several UA
modified derivatives at the C-3 and/or 17-COOH positions have
significantly improved antitumor effects (24–26).
Modifications at C-28 and C-3 of UA may therefore lead to improved
anti-BC activity. The current study investigated the anticancer
functions of UA and its novel derivatives, with a
nitrogen-containing heterocyclic scaffold and the privileged
fragment at the C-28 position on apoptosis induction, cell
proliferation and cell cycle in human BC lines. The aim of the
current study was to identify novel UA derivative compounds with
limited side effects, high selectivity, low toxicity and improved
anticancer activity.
Materials and methods
Preparation of UA derivative
FZU3010
3-acetyl UA (Sinopharm Chemical Reagent Co., Ltd.,
Shanghai, China) was produced by treating UA (TGI) with acetic
anhydride in dry pyridine under the existing
4-dimethylaminopyridine at room temperature for 2 h. 3-acetyl UA
was treated with oxalyl chloride at room temperature for 3 h to
produce an intermediary 28-acyl chloride. This compound was then
mixed at room temperature for 2 h with piperazine (Sinopharm
Chemical Reagent Co., Ltd.) to synthesize FZU3010.
Cell culture
BC SUM149PT and HCC1937 cell lines were obtained
from Key Laboratory of Animal Models and Human Disease Mechanisms
at the Kunming Institute of Zoology (Chinese Academy of Sciences,
Kunming, China). SUM149PT cells were maintained in RPMI-1640
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
HCC1937 cells were cultured in Ham's F12 medium (Lonza Group, Ltd.,
Basel, Switzerland) at 37°C. The medium was supplemented with 1%
antibiotics (100 mg/ml streptomycin sulfate and 100 U/ml
penicillin) and 10% fetal bovine serum (FBS; GE Healthcare Life
Sciences, Logan, UT, USA). Cells were cultivated at 37°C with a
humidified atmosphere of 5% CO2. Dimethyl sulfoxide
(DMSO) was used to prepare the stock solutions and additional
dilutions were produced using fresh culture medium. The
concentration of DMSO was 1% in the final culture medium.
Cell viability/proliferation
assay
Cell proliferation was determined using a
sulforhodamine B (SRB) assay kit (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). SUM149PT and HCC1937 cells were cultured in
96-well plates at a density of 2,000 cells/well for 48 h at 37°C.
Cells were then treated with seven different concentrations of
FZU3010 and UA (1, 2, 4, 6, 8, 10 and 20 µM) for 48 h; DMSO served
as a negative control. Cells were fixed in 100 ml 10%
trichloroacetic acid for 1 h at 37°C and then washed with deionized
water five times. Cells were stained for 5 min with 50 ml 0.4%
(W/V) SRB in 1% acetic acid in the dark at room temperature and the
plates were then washed five times with 1% acetic acid prior to
being dried. A total of 100 ml 10 mM Tris base was then added to
each well. Optical densities were determined at a wavelength of 530
nm using a spectrophotometric plate reader. Cell viability values
at different drug dosages were plotted and half-maximal inhibitory
concentration (IC50) values were obtained from the
graphs created.
Cell cycle analysis
A cell-cycle cytotoxicity assay was used to
determine the suppression of cancer cell development. SUM149PT and
HCC1937 cells were seeded onto 96-well plates at a density of
1×104 cells/well and were treated for 24 h with FZU3010
(2.5 and 5 µM), UA (5 µM) or 0.1% DMSO, which was used as a
vehicle-treated control. SUM149PT and HCC1937 (1×104)
cells were collected using trypsinization and ice-cold PBS was then
used to wash the cells. Cells were fixed with ice-cold 70% methanol
overnight at 4°C. All cells were centrifuged (4°C, 13,000 × g) and
placed in ice-cold PBS suspension prior to being incubated with
RNase (Sigma-Aldrich; Merck KGaA) for 30 min at 37°C. Cells were
then stained with 1 mg/ml propidium iodide (PI) (Sigma-Aldrich;
Merck KGaA) in the dark at 4°C for 30 min. A FACScan flow cytometer
337452 Rev system (BD Biosciences, Franklin Lakes, NJ, USA) was
then used to analyze cell cycle distribution. All data were
analyzed using CELL Quest and ModFit LT software for Mac V1.01
(Verity Software House, Inc., Topsham, ME, USA).
Apoptosis analysis
The Annexin V-fluoroscein isothiocyanate (FITC)/PI
test was used to determine the rate of apoptosis in the BC cell
lines. Doxorubicin (DTX; Sigma-Aldrich; Merck KGaA) served as a
positive control. Cells were cultured in 6-well plates at a density
of 2×106/well in 10% FBS-Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc.) and treated at room
temperature with different concentrations of UA (0, 1.25, 2.5 or 5
µM) and 5 µM DTX for 48 h. Cold PBS was then used to wash the cells
twice for 30 min and cells were resuspended into 1X binding buffer
(1.4 M NaCl, 25 mM CaCl2, 0.1 M Hepes/NaOH; pH 7.4) at a
concentration of 1×106 cells/ml. A total of 100 µl
solution (1×105 cells) was transferred into a 5-ml
culture tube and 5 µl Annexin V-FITC (BD Biosciences) and 5 µl PI
was added to each tube. Cells were gently vortexed and incubated at
room temperature for 30 min in the dark. A total of 200 ml PBS was
then added to each tube. A FACSCalibur flow cytometer with
FACSLoader (BD Biosciences) was used to analyze the cells at
emission and excitation wavelengths of 488 and 570 nm,
respectively. Apoptosis was determined using the Annexin V-FITC/PI
apoptosis kit (Biovision, Inc., Milpitas, CA, USA) according to the
manufacturers protocol as per the manufacturer's instructions.
Statistical analysis
Data are expressed as the mean ± standard deviation
from at least three experiments. One-way analysis of variance was
used to analyze and compare multiple drug concentrations within the
same group, and unpaired two-tailed tests were used to compare
differences between groups. The least significant difference post
hoc method was used to perform mean separations. IC50
concentrations/curves were calculated and drawn using Excel 2007
(Microsoft Corporation, Redmond, WA, USA). All statistical analyses
were performed using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was determined to indicate statistically significant
difference.
Results
Chemistry of FZU3010
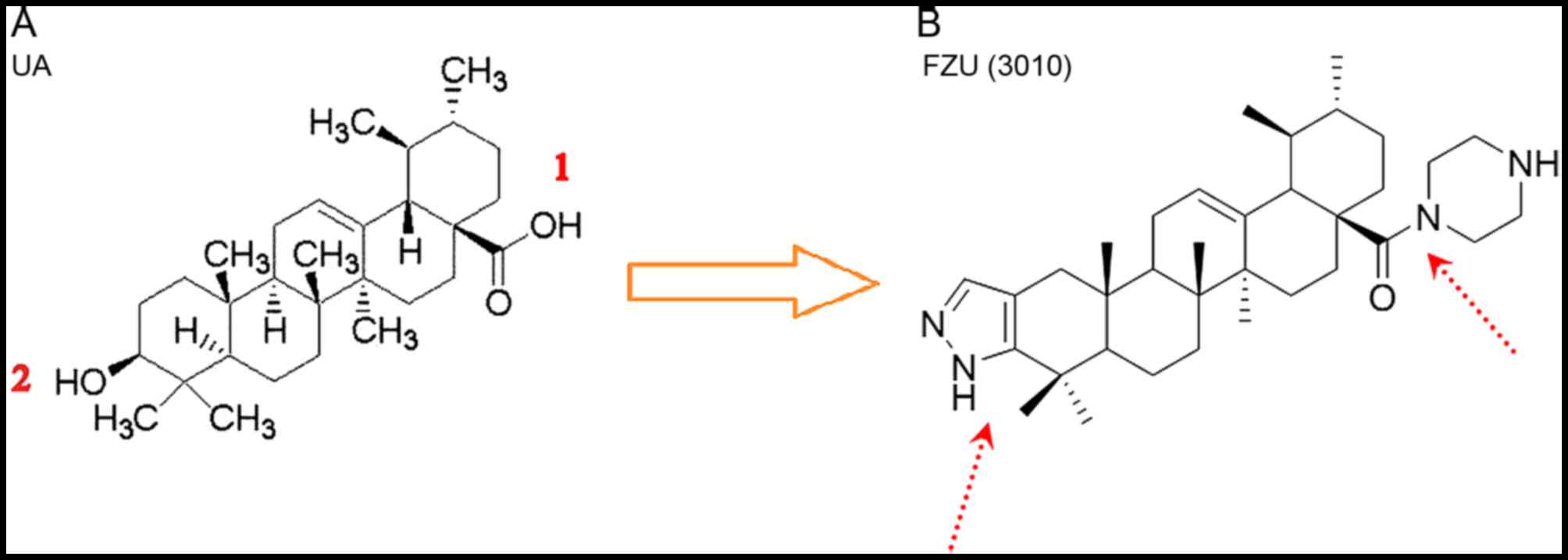
UA was used as the parent compound in the present
study and modifications were made to its structure at the C-3
position and C-28 position. Synthesis of the UA derivative FZU3010
is presented in Fig. 1.
Effect of UA and FZU3010 on cell
viability
An SRB assay was used to determine the effect of
FZU3010 and UA on the viability of SUM149PT and HCC1937 cells.
Cells were incubated for 48 h with 0, 1, 2, 4, 6, 8, 10 and 20 µM
FZU3010 or UA. The IC50 values for UA to suppress cell
viability were 8–10 µM in the two cancer cell lines (Fig. 2). FZU3010 repressed the viability of
the two cancer cell lines compared with the UA-treated cells and
exhibited an IC50 of 4–6 µM. FZU3010 has also been
demonstrated to be non-toxic to the normal human HELF cell line in
previous studies (27,28). Furthermore, the results of the present
study were consistent with other findings that the IC50
of FZU3010 was 4–6 µM in the human body (29,30).
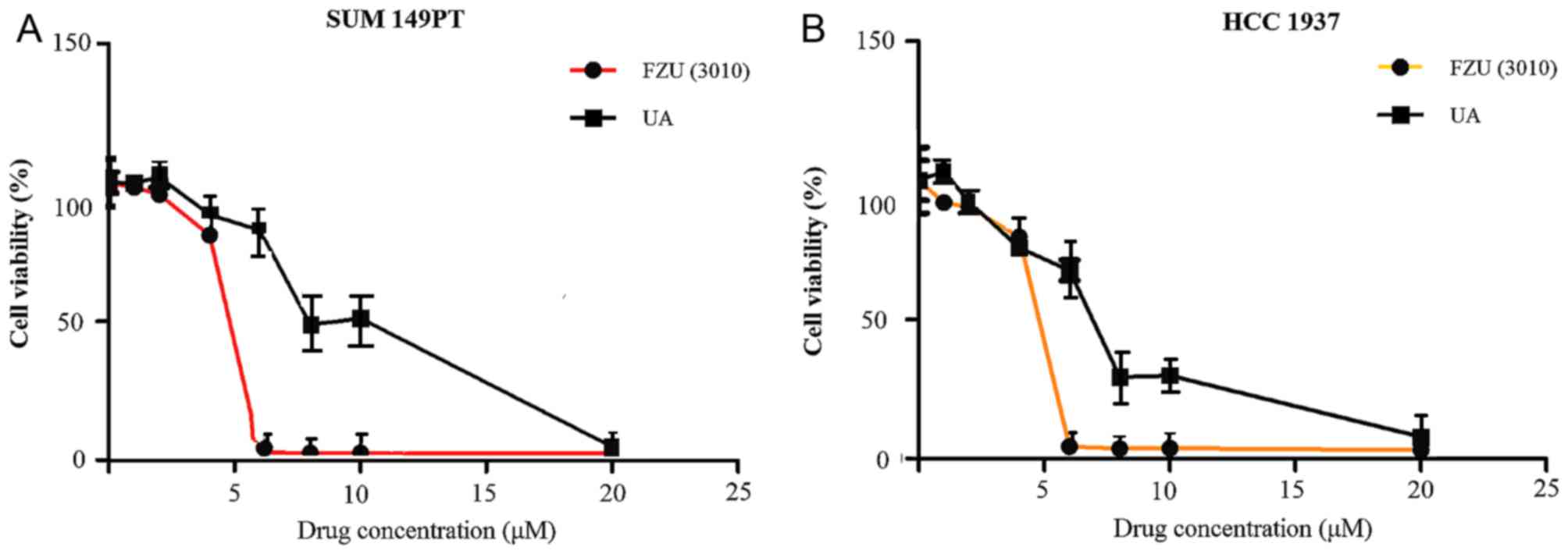 | Figure 2.Dose-response curves of the
anti-proliferative effect of FZU3010 and UA. A sulforhodamine B
assay was used to determine the capability of FZU3010 and UA to
inhibit the viability of SUM149PT and HCC1937 cells. FZU3010 (0, 1,
2, 4, 6, 8, 10 and 20 µM) and UA (0, 1, 2, 4, 6, 8, 10 and 20 µM)
was used to treat (A) SUM149PT and (B) HCC1937 cells for 48 h. Data
are expressed as the mean ± standard deviation from at least three
experiments. Unpaired two-tailed test was used to compare
differences between groups. UA, ursolic acid. |
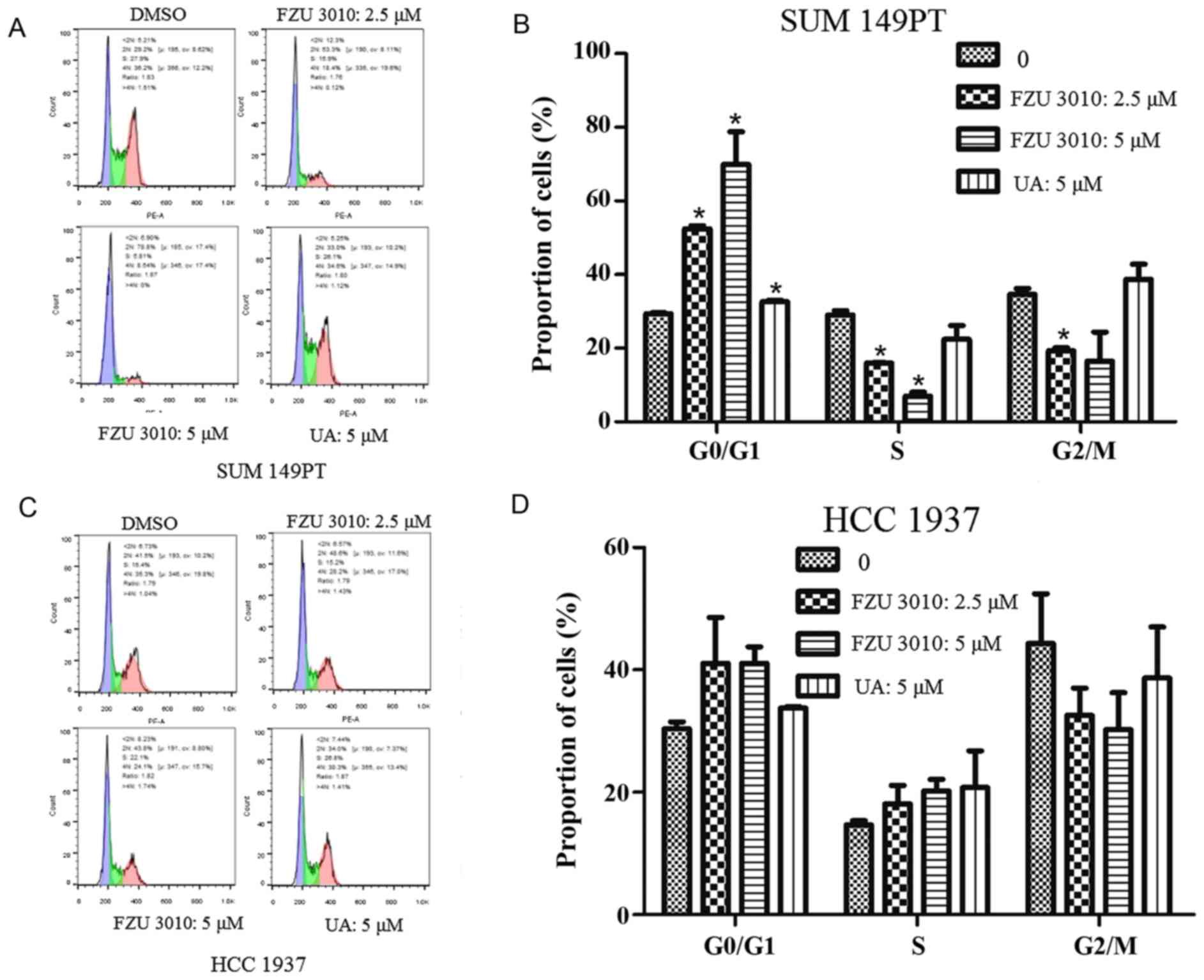
Effect of FZU3010 on cell cycle
distribution
The cell cycle consists of four different phases:
G0, which is a rest phase in which the cell stops
dividing and has left the cell cycle; G1, which is a
phase in which the cell prepares energy and material for DNA
replication; S, which is the synthesis phase; G2, which is known as
the interphase where preparation for the M phase occurs; and M,
which is a ‘mitosis’ phase, in which nuclear and cytoplasmic
division occurs.
The FZU3010-induced suppression of cancer cell
viability due to the arrest of cell cycle progression was confirmed
using a cell-cycle cytotoxicity assay by treating SUM149PT and
HCC1937 cells for 24 h with FZU3010 at different doses (0, 1.25,
2.5 or 5 µM), UA (5 µM) and 0.1% supplemented DMSO medium, which
served as a control (Fig. 3). The
results demonstrated that FZU3010 administration significantly
increased the percentage of cells in G0/G1
compared with the DMSO-treated control SUM149PT cells. The arrest
of G0/G1 was highest in cells treated with 5
µM FZU3010.
Effect of FZU3010 on cell
apoptosis
SUM149PT and HCC1937 cell apoptosis was investigated
to validate the anticancer activity of FZU3010 using Annexin
V-FITC/PI double staining, with the chemotherapy drug DTX serving
as a positive control (Fig. 4). The
number of apoptotic SUM149PT and HCC1937 cells was measured
following treatment for 48 h with FZU3010 at concentrations of 0,
1.25, 2.5 and 5 µM and UA at a concentration of 5 µM, respectively.
The percentage of Annexin V-positive SUM149PT and HCC1937 apoptotic
cells was significantly increased following the administration with
5 µM FZU3010 compared with cells treated with 0 µM (normal
control); however, the difference between cells treated with the
lower concentrations of FZU3010 and the normal control cells was
not significant. The capability of FZU3010 to induce apoptosis in
SUM 149PT (P=0.008) and HCC 1937 (P=0.01) cells was significantly
higher in cells treated with 5 µM FZU3010 compared with cells
treated with 5 µM UA. Furthermore, the apoptotic cells in SUM149PT
(P=0.049) and HCC 1937 (P=0.038) cells treated with 5 µM FZU3010
were significantly increased compared with those treated with 5 µM
DTX.
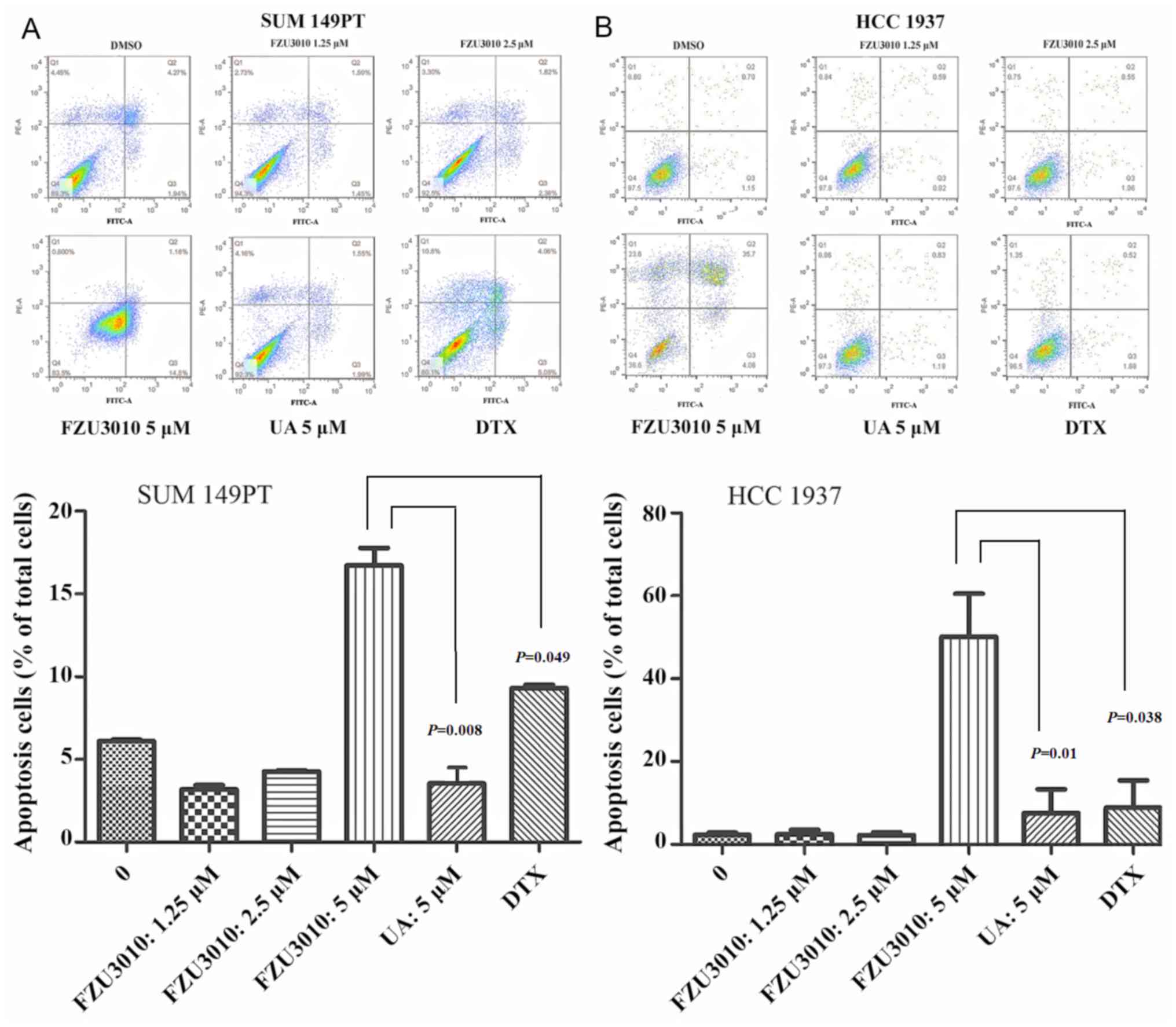 | Figure 4.Effect of FZU3010 on cell apoptosis.
The percentage of apoptotic cells was determined for cells treated
for 48 h with FZU3010 (0, 1.25, 2.5, 5 µM), UA (5 µM) and DTX (5
µM). (A) Flow cytometry analysis of apoptosis in SUM149PT cells.
(B) Flow cytometry analysis of apoptosis in HCC1937 cells. Data are
expressed as the mean ± standard deviation of at least three
experiments. FZU3010 induced apoptosis in BC SUM 149PT (P=0.008)
and HCC1937 (P=0.01) cells was significantly higher in cells
treated with 5 µM FZU3010 compared with cells treated with 5 µM UA,
respectively. Lower left quadrant, viable cells (Annexin V-/PI-);
lower right quadrant, early apoptotic cells (Annexin V+/PI-); upper
right quadrant, late apoptotic cells (Annexin V+/PI+); upper left
quadrant, necrotic cells (Annexin V-/PI+). One-way analysis of
variance was used to analyze multiple comparisons between different
drug concentrations for the same group. Comparisons indicated by
lines. UA, ursolic acid; DTX, doxorubicin. |
Discussion
UA is a pentacyclic triterpenoid composite isolated
from natural plants or traditional medicinal herbs, exhibiting a
wide range of pharmacological activities (12–20). The
anti-inflammatory and anti-oxidative functions of UA, including
cardiovascular protection, neuroprotection and hepatoprotection,
have been demonstrated previously (31). The anticancer activity of UA has also
been reported in different types of cancer cell lines (10–12). UA is
therefore an effective anticancer agent to which extensive
structural changes have been made in order to further increase its
anticancer activity (32). Several
studies have reported that modified derivatives of UA with
functional groups at the C-3 and/or C-28 positions exhibit
significant bioactivity (33–35).
The results of the present study demonstrated that
FZU3010 significantly repressed the viability of SUM149PT and
HCC1937 cells with an IC50 of 4–6 µM, exhibiting a lower
cytotoxicity than DMSO-treated cell lines. This result indicates
that the modification at C-28 and 3-OH in the UA core significantly
increases the antitumor activities of UA. These results are
consistent with a previous study by Chen et al (36), which demonstrated that the structural
changes in position 3 and/or 28 of UA were crucial for its
cytotoxic activity. In another study, UA-benzylidine derivatives
exhibited strong cytotoxic activity and amino acid linkage
(37), and UA also caused significant
cytotoxic effects in different cancer cell lines. Furthermore, Liu
et al (38) proposed that the
inclusion of an acyl piperazine moiety at C-28 of UA, keeping the
polar group at C-3, significantly increased the antitumor activity
of the molecule. To the best of our knowledge, the present study
was the first to incorporate piperazine and thiourea into the C-28
and C-3 of UA, with the results indicating that FZU3010
significantly inhibits BC cell viability.
Clinically, it has been suggested that the cell
cycle is a primary target for cancer treatment (39). The results of the present study
demonstrated that FZU3010 arrested cells in the
G0/G1 phase and prevented them from
transitioning to the S phase. G0 is the resting phase in
which cells stop dividing and leave the cell cycle, while cells
prepare energy and material for DNA replication in G1
phase (40). Therefore, arrest of
cells in the G0/G1 phase resulted in the
obstruction of mitosis and cellular DNA synthesis. The role of the
UA derivative FZU3010 in inducing apoptosis as part of its
anticancer activity was investigated by measuring the percentage of
apoptotic SUM149PT and HCC1937 cells following treatment with
FZU3010. The results of this treatment indicated that the apoptotic
cell rates were highest in cells treated with 5 µM FZU3010. The
capability of FZU3010 to induce apoptosis in BC SUM 149PT (P=0.008)
and HCC 1937 (P=0.01) cells was significantly higher in cells
treated with 5 µM FZU3010 compared with cells treated with 5 µM UA.
Furthermore, the apoptotic cells in SUM149PT (P=0.049) and HCC 1937
(P=0.038) cells treated with 5 µM FZU3010 were significantly
increased compared with cells treated with 5 µM DTX.
A number of potential molecular mechanisms
underlying the anticancer properties of UA have been elucidated.
The results of a previous in vitro study indicated that UA
decreases the proliferation of several types of cancer cells by
inhibiting the signal transducer and activator of transcription 3
activation pathway and increasing the rate of apoptosis (41). Furthermore, UA upregulates the
pro-apoptosis factor Bcl-associated X and downregulates the
anti-apoptosis factor B-cell lymphoma-2, leading to the induction
of apoptosis (33). UA-induced
apoptosis serves a role in the secretion of cytochrome c in
the mitochondrial death pathway (42).
In conclusion, the UA piperazine derivative FZU3010
was designed and synthesized for the current study. The results of
subsequent experiments indicate that FZU3010 has the potential to
impede BC cell progression by inducing apoptosis and cell cycle
arrest at S and G0/G1 phase. However, future
studies are required to identify the mechanisms involved in the
viability of BC cell lines. Therefore, FZU3010 is a promising
therapeutic agent for the treatment of BC.
Acknowledgements
The current study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81272930
and 81322038). The authors also wish to thank Dr Zulqarnain Baloch
(Kunming University of Science and Technology, China) for
assistance with revising the original manuscript.
References
|
1
|
McGuire S: World Cancer Report 2014.
Geneva, Switzerland: World health organization, international
agency for research on cancer, WHO press, 2015. Adv Nutr.
7:418–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu X, Zou T, Cao N, Ni J, Xu W, Zhou T and
Wang X: Plasma homocysteine levels and genetic polymorphisms in
folate metablism are associated with breast cancer risk in chinese
women. Hered Cancer Clin Pract. 12:22014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hilakivi-Clarke L, de Assis S, Warri A and
Luoto R: Pregnancy hormonal environment and mother's breast cancer
risk. Horm Mol Biol Clin Investig. 9:11–23. 2012.PubMed/NCBI
|
|
5
|
Aloraifi F, Boland MR, Green AJ and
Geraghty JG: Gene analysis techniques and susceptibility gene
discovery in non-BRCA1/BRCA2 familial breast cancer. Surg Oncol.
24:100–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tung N, Battelli C, Allen B, Kaldate R,
Bhatnagar S, Bowles K, Timms K, Garber JE, Herold C, Ellisen L, et
al: Frequency of mutations in individuals with breast cancer
referred for BRCA1 and BRCA2 testing using next-generation
sequencing with a 25-gene panel. Cancer. 121:25–33. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Castro MA, de Santiago I, Campbell TM,
Vaughn C, Hickey TE, Ross E, Tilley WD, Markowetz F, Ponder BA and
Meyer KB: Regulators of genetic risk of breast cancer identified by
integrative network analysis. Nat Genet. 48:12–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Holmes MD, Chen WY, Li L, Hertzmark E,
Spiegelman D and Hankinson SE: Aspirin intake and survival after
breast cancer. J Clin Oncol. 28:1467–1472. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bilgin B, Sendur MAN, Şener Dede D, Akinci
MB and Yalçın B: A current and comprehensive review of
cyclin-dependent kinase inhibitors for the treatment of metastatic
breast cancer. Curr Med Res Opin. 33:1559–1569. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Dong B, Tan Y, Yu S and Bao YX: A
study on the immunomodulation of polysaccharopeptide through the
TLR4-TIRAP/MAL-MyD88 signaling pathway in PBMCs from breast cancer
patients. Immunopharmacol Immunotoxicol. 35:497–504. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Toi M, Masuda N, Ishiguro H, Saji S, Ohno
S and Chow LW: Development of breast cancer therapy:
Biomarker-driven and response-guided approaches in a neoadjuvant
setting. Int J Biol Markers. 30:e252–e253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramalingam S, Gediya L, Kwegyir-Afful AK,
Ramamurthy VP, Purushottamachar P, Mbatia H and Njar VC: First MNKs
degrading agents block phosphorylation of eIF4E, induce apoptosis,
inhibit cell growth, migration and invasion in triple negative and
Her2-overexpressing breast cancer cell lines. Oncotarget.
5:530–543. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cargnin ST and Gnoatto SB: Ursolic acid
from apple pomace and traditional plants: A valuable triterpenoid
with functional properties. Food Chem. 220:477–489. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mancha-Ramirez AM and Slaga TJ: Ursolic
acid and chronic disease: An overview of UA's effects on prevention
and treatment of obesity and cancer. Adv Exp Med Biol. 928:75–96.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hussain H, Green IR, Ali I, Khan IA, Ali
Z, Al-Sadi AM and Ahmed I: Ursolic acid derivatives for
pharmaceutical use: A patent review (2012–2016). Expert Opin Ther
Pat. 27:1061–1072. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garay CA and Engstrom PF: Chemoprevention
of colorectal cancer: Dietary and pharmacologic approaches.
Oncology (Williston Park). 13:89–100, 105. 1999.PubMed/NCBI
|
|
17
|
Ko EY and Moon A: Natural products for
chemoprevention of breast cancer. J Cancer Prev. 20:223–231. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xavier CP, Lima CF, Pedro DF, Wilson JM,
Kristiansen K and Pereira-Wilson C: Ursolic acid induces cell death
and modulates autophagy through JNK pathway in apoptosis-resistant
colorectal cancer cells. J Nutr Biochem. 24:706–712. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Achiwa Y, Hasegawa K, Komiya T and Udagawa
Y: Ursolic acid induces Bax-dependent apoptosis through the
caspase-3 pathway in endometrial cancer SNG-II cells. Oncol Rep.
13:51–57. 2005.PubMed/NCBI
|
|
20
|
Yang X, Li Y, Jiang W, Ou M, Chen Y, Xu Y,
Wu Q, Zheng Q, Wu F, Wang L, et al: Synthesis and biological
evaluation of novel ursolic acid derivatives as potential
anticancer prodrugs. Chem Biol Drug Des. 86:1397–1404. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yarla NS, Bishayee A, Sethi G, Reddanna P,
Kalle AM, Dhananjaya BL, Dowluru KS, Chintala R and Duddukuri GR:
Targeting arachidonic acid pathway by natural products for cancer
prevention and therapy. Semin Cancer Biol. 41:48–81. 2016.
View Article : Google Scholar
|
|
22
|
Wen JH, Wei XH, Sheng XY, Zhou DQ, Peng
HW, Lu YN and Zhou J: Effect of ursolic acid on breast cancer
resistance protein-mediated transport of rosuvastatin in vivo and
vitro. Chin Med Sci J. 30:218–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen H, Gao Y, Wang A, Zhou X, Zheng Y and
Zhou J: Evolution in medicinal chemistry of ursolic acid
derivatives as anticancer agents. Eur J Med Chem. 92:648–655. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gu W, Jin XY, Li DD, Wang SF, Tao XB and
Chen H: Design, synthesis and in vitro anticancer activity of novel
quinoline and oxadiazole derivatives of ursolic acid. Bioorg Med
Chem Lett. 27:4128–4132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Monteath SAF Alves, Maciel MAM, Vega RG,
de Mello H, de Araújo Martins C, Esteves-Souza A, Gattass CR and
Echevarria A: Ultrasound-assisted extraction of ursolic acid from
the flowers of ixora coccinia Linn (Rubiaceae) and
antiproliferative activity of ursolic acid and synthesized
derivatives. Pharmacogn Mag. 13:265–269. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang C, Xu SH, Ma BL, Wang WW, Yu BY and
Zhang J: New derivatives of ursolic acid through the
biotransformation by Bacillus megaterium CGMCC 1.1741 as inhibitors
on nitric oxide production. Bioorg Med Chem Lett. 27:2575–2578.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Riaz S, Khan IU, Yar M, Ashraf M, Rehman
TU, Shaukat A, Jamal SB, Duarte VC and Alves MJ: Novel
pyridine-2,4,6-tricarbohydrazide derivatives: Design, synthesis,
characterization and in vitro biological evaluation as α- and
β-glucosidase inhibitors. Bioorg Chem. 57:148–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang XC, Jin L, Wang M, Liang D, Chen ZF,
Zhang Y, Pan YM and Wang HS: Design, synthesis and in vitro
evaluation of novel dehydroabietic acid derivatives containing a
dipeptide moiety as potential anticancer agents. Eur J Med Chem.
89:370–385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shao JW, Dai YC, Xue JP, Wang JC, Lin FP
and Guo YH: In vitro and in vivo anticancer activity evaluation of
ursolic acid derivatives. Eur J Med Chem. 46:2652–2661. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng QY, Li PP, Jin FS, Yao C, Zhang GH,
Zang T and Ai X: Ursolic acid induces ER stress response to
activate ASK1-JNK signaling and induce apoptosis in human bladder
cancer T24 cells. Cell Signal. 25:206–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leal AS, Wang R, Salvador JA and Jing Y:
Synthesis of novel ursolic acid heterocyclic derivatives with
improved abilities of antiproliferation and induction of p53,
p21waf1 and NOXA in pancreatic cancer cells. Bioorg Med Chem.
20:5774–5786. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang G, Pemp D, Stadtmüller P, Nimczick
M, Heilmann J and Decker M: Design, synthesis and in vitro
evaluation of novel uni- and bivalent ligands for the cannabinoid
receptor type 1 with variation of spacer length and structure.
Bioorg Med Chem Lett. 24:4209–4214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chavan HV, Adsul LK, Kotmale AS, Dhakane
VD, Thakare VN and Bandgar BP: Design, synthesis, characterization
and in vitro and in vivo anti-inflammatory evaluation of novel
pyrazole-based chalcones. J Enzyme Inhib Med Chem. 30:22–31. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong H, Yang X, Xie J, Xiang L, Li Y, Ou
M, Chi T, Liu Z, Yu S, Gao Y, et al: UP12, a novel ursolic acid
derivative with potential for targeting multiple signaling pathways
in hepatocellular carcinoma. Biochem Pharmacol. 93:151–162. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tu HY, Huang AM, Wei BL, Gan KH, Hour TC,
Yang SC, Pu YS and Lin CN: Ursolic acid derivatives induce cell
cycle arrest and apoptosis in NTUB1 cells associated with reactive
oxygen species. Bioorg Med Chem. 17:7265–7274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen J, Fu H, Wang Z, Yin F, Li J, Hua Y
and Cai Z: A new synthetic ursolic acid derivative IUA with
anti-tumor efficacy against osteosarcoma cells via inhibition of
JNK signaling pathway. Cell Physiol Biochem. 34:724–733. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dar BA, Lone AM, Shah WA and Qurishi MA:
Synthesis and screening of ursolic acid-benzylidine derivatives as
potential anti-cancer agents. Eur J Med Chem. 111:26–32. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu MC, Yang SJ, Jin LH, Hu DY, Xue W,
Song BA and Yang S: Synthesis and cytotoxicity of novel ursolic
acid derivatives containing an acyl piperazine moiety. Eur J Med
Chem. 58:128–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oura K, Tadokoro T, Fujihara S, Morishita
A, Chiyo T, Samukawa E, Yamana Y, Fujita K, Sakamoto T, Nomura T,
et al: Telmisartan inhibits hepatocellular carcinoma cell
proliferation in vitro by inducing cell cycle arrest. Oncol Rep.
38:2825–2835. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kang S, Kang MS, Ryu E and Myung K:
Eukaryotic DNA replication: Orchestrated action of multi-subunit
protein complexes. Mutat Res. S0027–S5107. 2017.PubMed/NCBI
|
|
41
|
Pathak AK, Bhutani M, Nair AS, Ahn KS,
Chakraborty A, Kadara H, Guha S, Sethi G and Aggarwal BB: Ursolic
acid inhibits STAT3 activation pathway leading to suppression of
proliferation and chemosensitization of human multiple myeloma
cells. Mol Cancer Res. 5:943–955. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yeh CT, Wu CH and Yen GC: Ursolic acid, a
naturally occurring triterpenoid, suppresses migration and invasion
of human breast cancer cells by modulating c-Jun N-terminal kinase,
Akt and mammalian target of rapamycin signaling. Mol Nutr Food Res.
54:1285–1295. 2010. View Article : Google Scholar : PubMed/NCBI
|