Introduction
T cell adoptive immunotherapy is widely used in
clinical settings, and gentamicin is used extensively in T cell
cultures in vitro as a broad-spectrum antibiotic (1–3). However,
our previous study identified that T cell activity was inhibited by
gentamicin (4). Hemocyanin was
selected as an additive for T cell culture in vitro in order
to maintain the antibacterial environment and enhance the activity
of T cells.
Hemocyanin was identified in mollusks and arthropods
as a respiratory protein (5). In
addition to this primary function, hemocyanin performs multiple
roles in immune defense, functioning similarly to a
phenoloxidase-like enzyme (6), an
antiviral agent (7), an antimicrobial
protein (8) and agglutinin (9). Notably, Min et al (10) identified that 2 hemocyanin fractions
from Litopenaeus vannamei (L. vannamei) exhibited
hemolytic activity, which was likely associated with the diversity
in amino acid sequence and glycosylation of the IgG fractions.
Zheng et al (11) demonstrated
that the hemocyanin from L. vannamei exhibited
antiproliferative properties against HeLa cells in vitro
through mediating the apoptosis mechanism via the mitochondria
triggered pathway. Gesheva et al (12) noted that the hemocyanin from Rapana
thomasiana and Helix pomatia presented marked anticancer
and antiproliferative effects in a murine colon carcinoma model.
Although hemocyanin had been identified as a multifunctional
protein with antimicrobial and anticancer activities, the effects
of hemocyanin from L. vannamei on human T cells is
unclear.
In the present study, hemocyanin, as an
antimicrobial protein, was incorporated into the culturing process
of human T cells; the T cells in all groups were analyzed by
optical microscopy and flow cytometry, and the results indicated
that the application of hemocyanin to T cells cultured in
vitro has a beneficial effect.
Materials and methods
The peripheral blood was donated by healthy male
volunteers (age, 25–35 years) who provided of verbal informed
consent, and the present study was approved by the Animal Welfare
and Research Ethics Committee of the University of South China
(Henan, China). The hemocyanin, which was purified by gel
filtration chromatography on a Sepharose 4B column from L.
vannamei and stored in PBS buffer (0.01 mol/l, pH 7.4), was
donated by Professor Yueling Zhang from Shantou University
(Shantou, China).
T cells cultured in vitro and
microscopic examination
T cells were cultured with 80 U/ml gentamicin
(Yichang Humanwell Pharmaceutical Co., Ltd., Yichang, China), and
were defined as the control group. T cells cultured with 0.05, 0.1
and 0.2 µg/ml hemocyanin were defined as Hem 1, 2 and 3 groups,
respectively.
Lymphocytes were separated and cultured as described
previously (13), with certain
modifications: Peripheral blood with heparin sodium anticoagulant
was centrifuged at 450 × g at room temperature for 5 min. The
plasma was collected and maintained at 4°C subsequent to
inactivation at 56°C for 30 min, then centrifuged at 648 × g at
room temperature for 10 min. The supernatant was collected and
stored at 4°C as autologous plasma. The peripheral blood cell layer
was mixed 1:1 (v/v) with 0.9% saline and used for
Ficoll® density gradient separation (LymphoPrep; PAA
Laboratories GmbH, Cölbe, Germany). Following centrifugation at 648
× g at room temperature for 20 min, the leukocyte layer was
collected in new tubes. Then, the cells were washed twice with 0.9%
saline and centrifuged at 450 × g at room temperature for 7 min.
Subsequently, the lymphocytes were cultured in GT-T551 medium
(Takara Biotechnology Co., Ltd., Dalian, China) with 1,000 U/ml
γ-interferon (Beijing Biocoen Biotechnology Co., Ltd., Beijing,
China) and 10% autologous plasma was added. Following culture for
24 h at 37°C with 5% CO2, 50 µg/ml cluster of
differentiation 3 (CD3) monoclonal antibody (Skoda Biotechnology
Co., Ltd., Beijing, China) and 100 U/ml interleukin-1α (PeproTech
China, Suzhou, China) were added on the first day, and 1,000 U/ml
recombinant human interleukin-2 (SL Pharmaceutical Co., Ltd.,
Beijing, China) and 2% autologous plasma were included in the
medium. The cells were cultured at 37°C and 5% CO2 for 9
days, and the T cells were analyzed by pocH-100i (Sysmex, Kobe,
Japan) and an optical microscope (magnification, ×50) (Olympus
Corporation, Tokyo, Japan).
T cell phenotype analysis
A total of 1 ml cell suspension was collected from
each experimental group. Subsequent to centrifugation at 200 × g at
room temperature for 10 min, the precipitate was resuspended in
0.9% saline and centrifuged again at 200 × g at room temperature
for 10 min. Then, the precipitate was resuspended in 150 µl 0.9%
saline and divided into two groups. The first group was an isotype
control, and 5 µl allophycocyanin (APC) mouse IgG1 (1:10; cat. no.
555751), 5 µl fluorescein isothiocyanate (FITC) mouse IgG2α (1:10;
cat. no. 555573), 5 µl phycoerythrin (PE) mouse IgG1 (1:10; cat.
no. 555749) and 1 µl PerCP-CyTM5.5 mouse IgG1 (1:50; cat. no.
550795; all from BD Biosciences, Franklin Lakes, NJ, USA) were
added. The second group was the experimental group, and 5 µl FITC
mouse anti-human CD3 (1:10; cat. no. 555339), 5 µl PE mouse
anti-human CD4 (1:10; cat. no. 555347), 1 µl PerCP-CyTM5.5 mouse
anti-human CD8 (1:50; cat. no. 560662) and 5 µl APC mouse
anti-human CD25 (1:10; cat. no. 555434; all from BD Biosciences)
were added. All groups were incubated for 15 min at room
temperature, resuspended in 1 ml 0.9% saline and then centrifuged
again at 200 × g at room temperature for 10 min. Finally, the
precipitate was resuspended in 200 µl 0.9% saline and prepared for
detection with a BD Biosciences Accuri™ C6 Plus flow cytometer (BD
Biosciences). The experiment was repeated in triplicate.
T cell cycle analysis
A total of 1 ml cell suspension was collected from
each group and centrifuged at 200 × g at room temperature for 10
min, and the precipitate was resuspended in 0.9% saline. Following
centrifugation at 200 × g at room temperature for 10 min, the
precipitate was resuspended with 75% frozen ethanol and maintained
at −20°C for 1 h. Then, samples were centrifuged at 200 × g at room
temperature for 10 min. Then, the precipitate was resuspended with
200 µl 0.9% saline, and incubated with 10 µl propidium iodide (PI,
1 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at room
temperature for 30 min. Finally, the cell suspension was detected
using a BD Accuri™ C6 Plus flow cytometer (BD Biosciences) and
analyzed by FlowJo v.7.6.2 software (Tree Star, Inc., Ashland, OR,
USA).
T cell cytotoxicity analysis
HepG2 (American Type Culture Collection, Manassas,
VA, USA) were collected at logarithmic growth phase for use as
target cells, and the concentration of cells was adjusted to
1×105 cells/ml. T cells for use as effector cells were
cultured at 37°C and 5% CO2 for 9 days were resuspended
with GT-T551 medium containing 2% autologous plasma and diluted to
5×106 cells/ml. These cells were divided into three
groups: The effector-target group was 100 µl effector cells and 100
µl target T cells; the effector cell group comprised 100 µl
effector cells and 100 µl GT-T551 medium; the target T cells group
comprised 100 µl target T cells and 100 µl GT-T551 culture medium.
All groups had 5 identical tubes, and were cultured at 37°C and 5%
CO2 for 24 h. A total of 10 µl thiazolyl blue
tetrazolium blue (5 mg/ml; Sigma-Aldrich; Merck KGaA) was added and
cultured at 37°C and 5% CO2 for 4 h. Following
centrifugation at 800 × g at room temperature for 5 min, the
precipitate was dissolved in 100 µl dimethyl sulfoxide
(Sigma-Aldrich; Merck KGaA), agitated for 15 min, and the optical
density (OD) was detected at 490 nm. The killing rate was
calculated as follows:
The killing rate (%) = [1 - (ODeffector-target
T cells well - ODeffector cell well)/ODtarget
T cells well] × 100%.
Statistical analysis
The data are presented as the mean ± standard
deviation. Statistical analyses were performed using SPSS v.17.0
(SPSS, Inc., Chicago, IL, USA). Multiple comparisons between the
groups were performed using one-way analysis of variance and the
Bonferroni method as a post-hoc test. P<0.05 or P<0.01 were
considered to indicate statistically significant differences. All
experiments were repeated ≥3 times.
Results
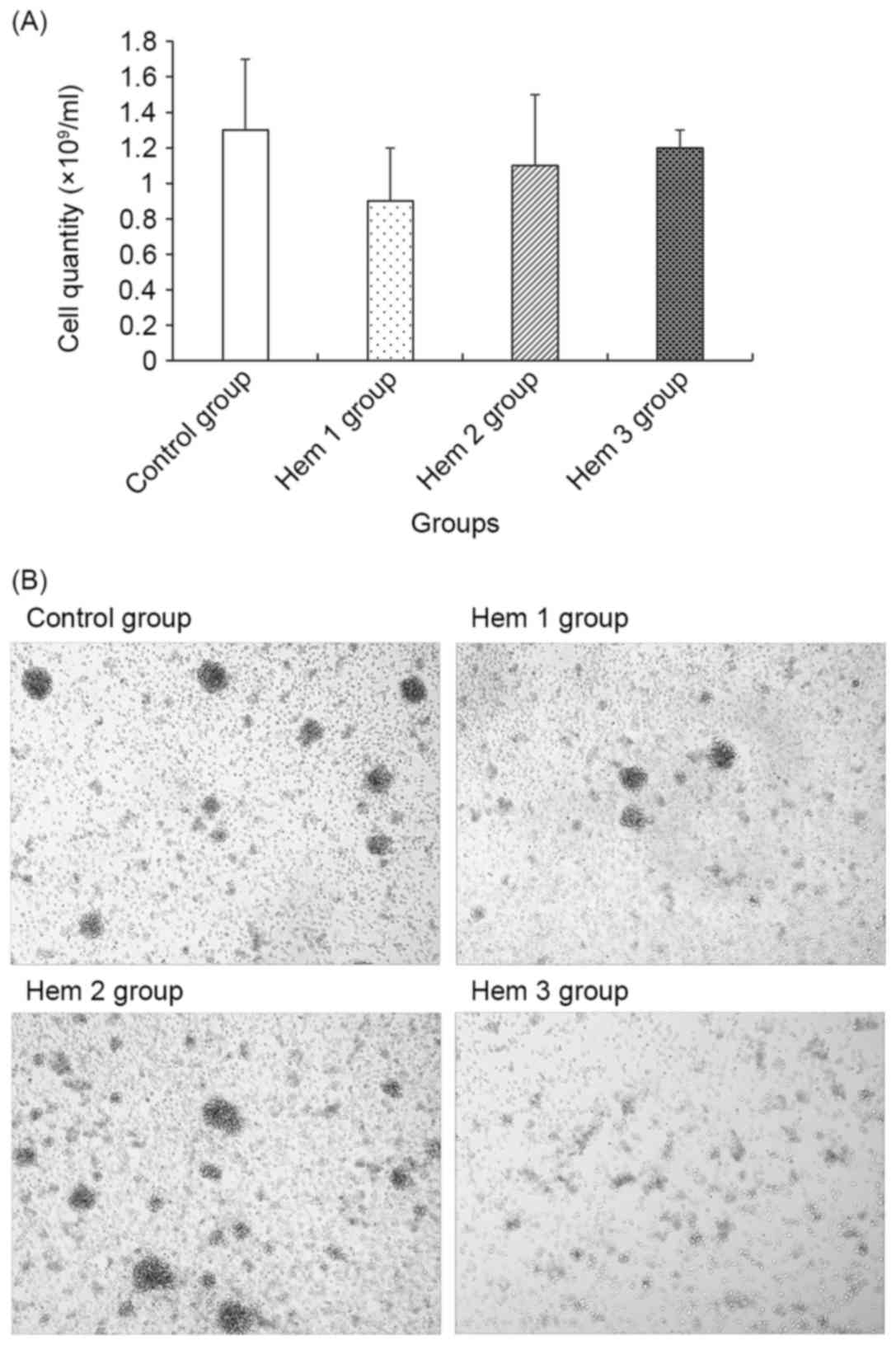
Cell quantity and morphology
assay
As demonstrated in Fig.
1, the cell quantity in the Hem 1, 2 and 3 groups gradually
increased, though all were decreased compared with the control
group. No significant difference between the control group and the
Hem 1, 2 or 3 groups was observed.
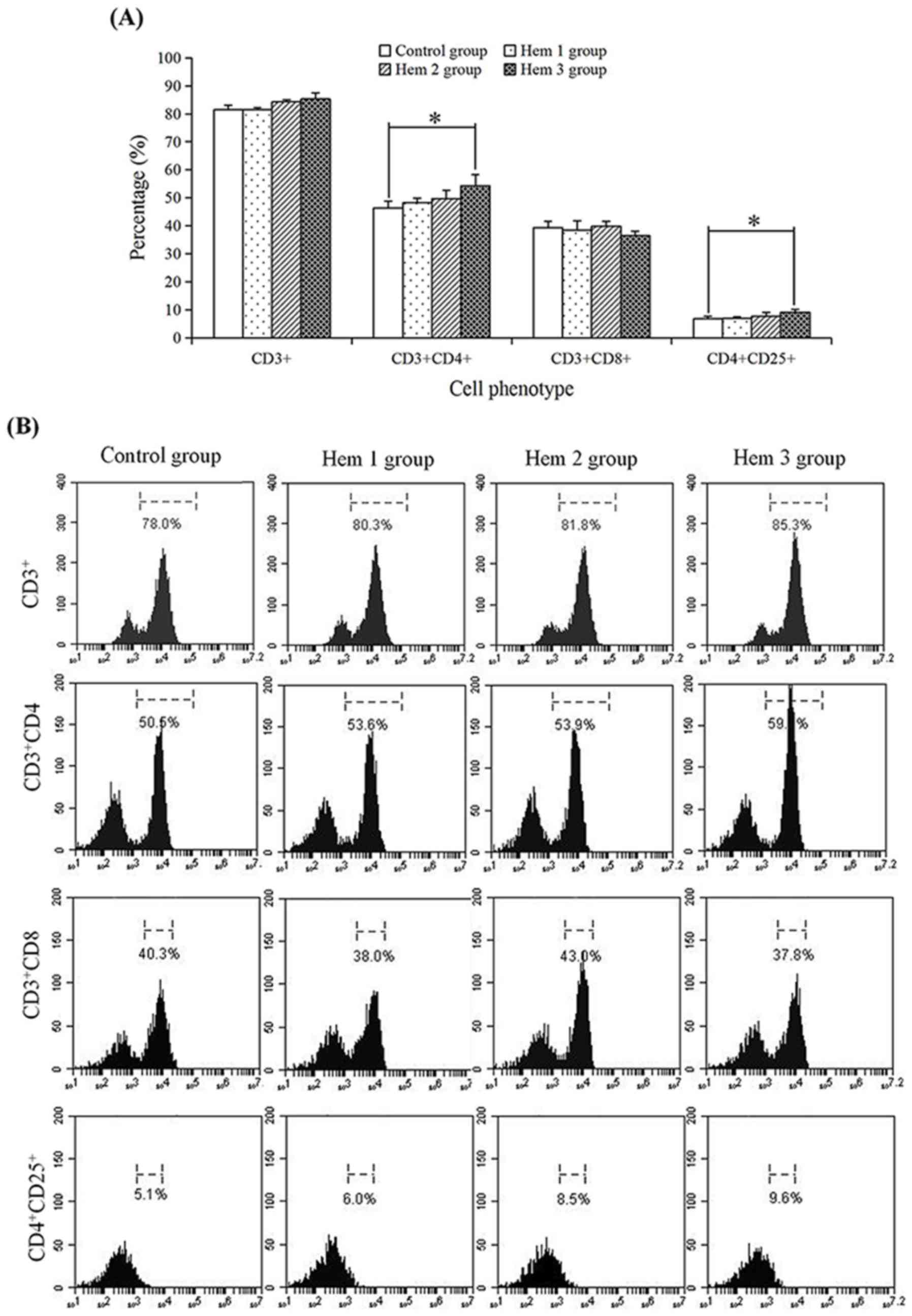
Effect of hemocyanin on the T cell
phenotype
To determine the effect of hemocyanin on T cells
subsets, the proportions of CD3+,
CD3+CD4+, CD3+CD8+ and
CD4+CD25+ T cells were detected by flow
cytometry (Fig. 2). In the control,
Hem 1, 2 and 3 groups, the proportion of CD3+ (81.47,
81.53, 84.23 and 85.30%, respectively),
CD3+CD4+ (46.40, 48.23, 49.73 and 54.33%,
respectively) and CD4+CD25+ (6.77, 6.97, 7.63
and 9.10%) T cells were all increased with an increasing hemocyanin
concentration, but no change was observed in the proportion of
CD3+CD8+ T cells (39.37, 38.50, 39.83 and
36.47%). In addition, the proportions of
CD3+CD4+ and CD4+CD25+
T cells in the Hem 3 group were respectively significantly
increased 1.17-(P=0.02) and 1.34-fold (P=0.03) compared with the
control group.
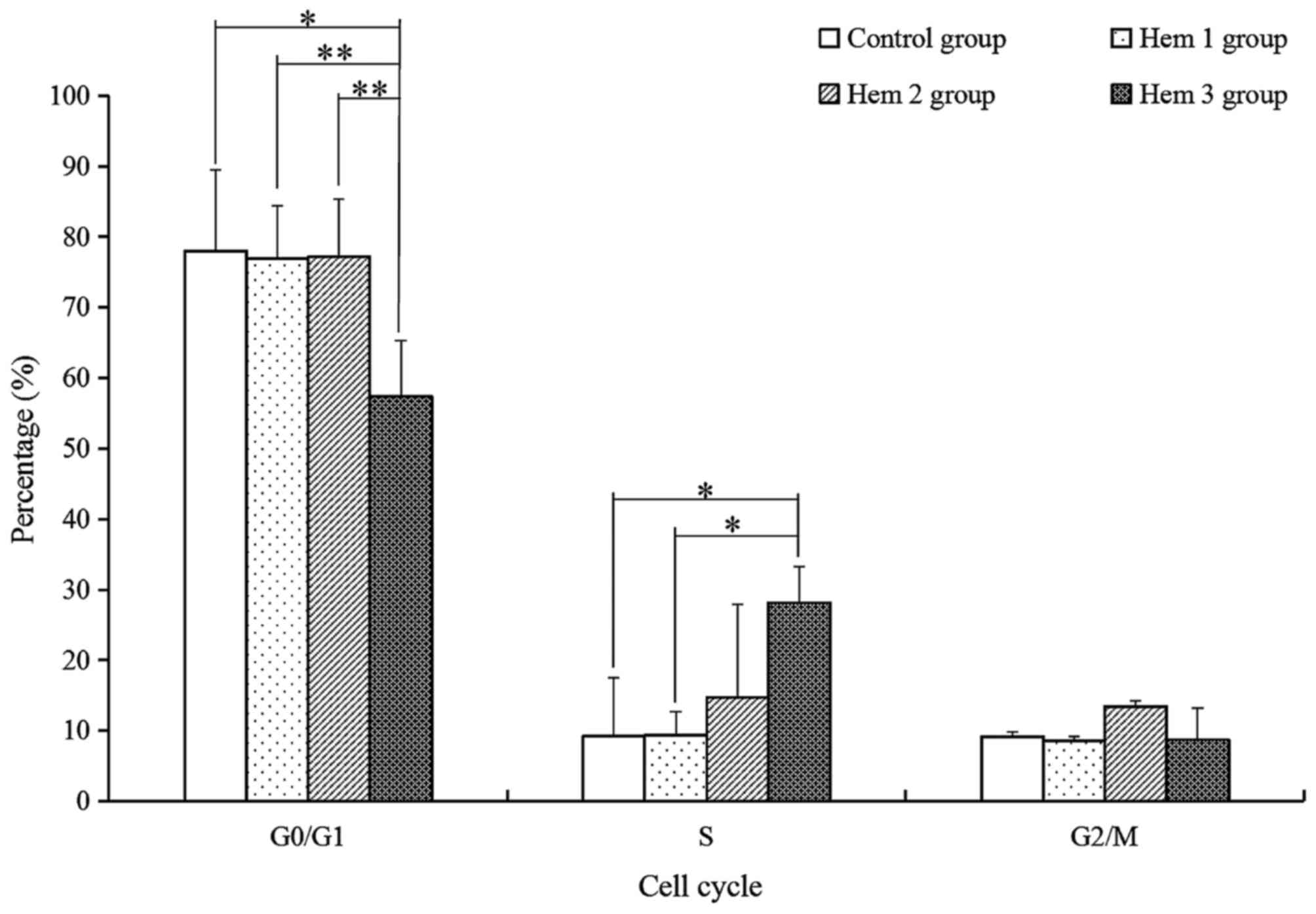
Effect of hemocyanin on T cell cycle
distribution
For the subsequent analysis of the variety of T
cells activity with hemocyanin treatment, the G0/G1, S and G2/M
phase of T cells were analyzed with PI dye and a flow cytometry
assay. As demonstrated in Fig. 3, the
proportion of G0/G1 phase cells in the Hem 3 group were
significantly decreased by 1.36-(P=0.012), 1.34-(P=0.002) and
1.34-(P=0.007) fold compared with the control, Hem 1 and 2 groups,
respectively. The S phase cells of the Hem 3 group were
significantly increased by 3.03-(P=0.027) and 3.01-(P=0.028) fold
compared with the control and Hem 1 groups respectively, and
increased by 1.91-(P=0.143) fold compared with Hem 2 group. No
significant change in the G2/M phase proportion was observed among
all groups.
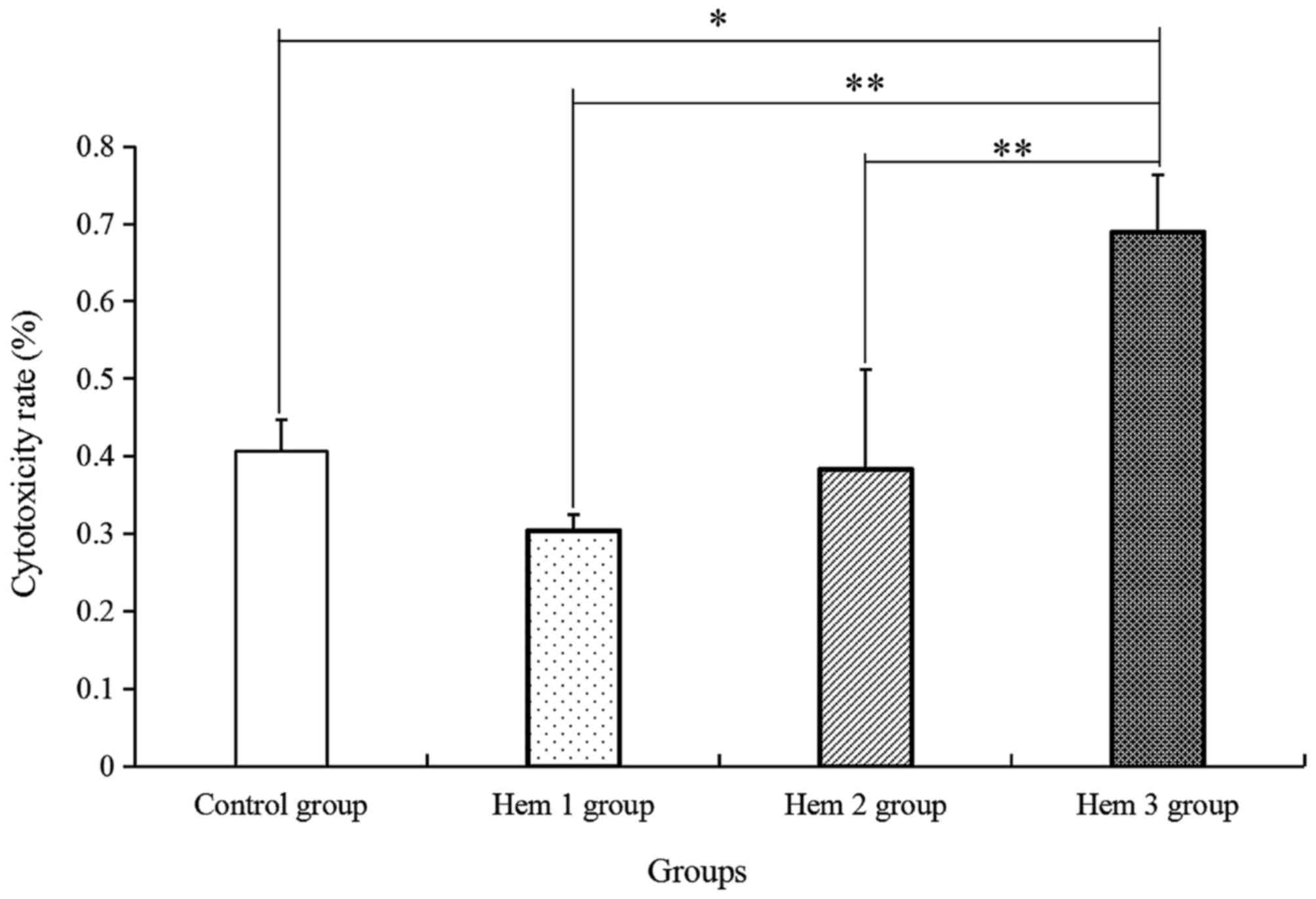
Effect of hemocyanin on T cell
cytotoxicity
Furthermore, the T cell cytotoxicity in HepG2 cells
was verified. As indicated in Fig. 4,
the T cell cytotoxicity in the Hem 1, 2 and 3 groups was gradually
enhanced along with increases in the hemocyanin concentration, as
compared with that in the control group. Among the four groups, T
cell cytotoxicity in the Hem 3 group was significantly increased by
1.70-, 2.26- and 1.80-fold, compared with in the control, Hem 1 and
2 groups, respectively.
Discussion
Hemocyanin is a metallo-glycoprotein oxygen
transporter, previously indicated to potentially have multiple
functionalities (14). Hemocyanin has
exhibited excellent antimicrobial (15–17),
anti-tumor (18) and immune
enhancement effects (19). In the
present study, hemocyanin was used as an additive in T cell culture
in vitro. The results indicated that the T cells normally
proliferated during culture without gentamicin treatment, and the
cell quantity between the control and experimental groups exhibited
no significant changes (Fig. 1).
Furthermore, the CD3+,
CD3+CD4+, CD3+CD8+ and
CD4+CD25+ phenotypes, which all represent
different T cell subsets, were analyzed by flow cytometry.
CD3+ is the characteristic marker of T cells (20). The CD3+CD4+
phenotype is characteristic of T helper cells, which secret
anti-tumor agents for cytotoxic T lymphocytes (21). T cells with the
CD3+CD8+ phenotype are critical factors in
the immune response to viral infection and cancer (22). CD4+CD25+ T cells
are considered to be regulatory, and to serve a central role in the
prevention of autoimmunity and in the control of immune responses
(23). In the present study, the
quantity of CD3+CD4+ and
CD4+CD25+ T cells in the Hem 3 group were
significantly increased compared with in the control group
(Fig. 2). Therefore, 0.2 µg/ml
hemocyanin may be an effective concentration for use in T cell
culture in vitro.
In addition, the cell cycle distributions of the T
cells were assayed using flow cytometry. Among the four groups
(Fig. 3), the number of G0/G1 phase
cells in the Hem 3 group was significantly decreased compared with
in the control, Hem 1 and 2 groups. However, the number of S phase
cells in the Hem 3 group was significantly elevated compared with
in the control and Hem 1 groups. Chen et al (24) demonstrated that the cell proliferative
activity corresponded with an increase in the number of S phase
cells, therefore, 0.2 µg/ml hemocyanin may be beneficial for T
cells proliferative activity.
T cell cytotoxicity in HepG2 was analyzed. In the
present study, HepG2 cells were used for T cell cytotoxicity
analysis. Although the HepG2 cell line has been demonstrated to be
misidentified and be derived from hepatoblastoma instead of
hepatocellular carcinoma (25), the
use of HepG2 cells as a model of target cells derived from tumor
tissues is suitable for cytotoxicity analysis of immune cells
cultured in vitro (26–28). Among
the four groups (Fig. 4), the T cell
cytotoxicity in the Hem 1, 2 and 3 groups was gradually increased
with the increased of hemocyanin concentration, and the T cell
cytotoxicity of the Hem 3 group was significantly increased
compared with the control, Hem 1 and 2 groups. Previous studies
have indicated that this killing activity is a major characteristic
of immune cells (29–31). Consequently, the results of the
present study suggest that the concentration of hemocyanin used in
the Hem 3 group may positively contribute to the anti-tumor
activity of T cells.
In conclusion, the present study explored the
possibility that hemocyanin may be used as an additive in T cell
culture in vitro. The results verified that 0.2 µg/ml
hemocyanin may significantly improve T cell proliferative activity
and cytotoxicity, and have potential value in the application of T
cell adoptive immunotherapy.
Acknowledgements
The authors would like to thank Professor Jianhua
Xiao for the revision of the manuscript. This study was supported
by the National Project of the Students in Local Colleges and
Universities Innovative Training Program in 2016 of China (no.
201610555010) and the project of Hunan Province Department of
Education of China (no. 2016-283).
References
|
1
|
Batorov EV, Shevela EY, Tikhonova MA,
Batorova DS, Ushakova GY, Sizikova SA, Sergeevicheva VV, Gilevich
AV, Kryuchkova IV, Ostanin AA and Chernykh ER: Mesenchymal stromal
cells improve early lymphocyte recovery and T cells reconstitution
after autologous hematopoietic stem cell transplantation in
patients with malignant lymphomas. Cell Immunol. 297:80–86. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang J, Li C, Wang Y, Lv H, Guo Y, Dai H,
Wicha MS, Chang AE and Li Q: Cytokine-induced killer (CIK) cells
bound with anti-CD3/anti-CD133 bispecific antibodies target CD133
(high) cancer stem cells in vitro and in vivo. Clin Immunol.
149:156–168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gargett T and Brown MP: Different cytokine
and stimulation conditions influence the expansion and immune
phenotype of third-generation chimeric antigen receptor T cellss
specific for tumor antigen GD2. Cytotherapy. 17:487–495. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao J, Yin W, Chen C and Luo X: Effects of
autologous plasma and gentamicin on immunophenotype and viability
of cytokine-induced killer cells. Xi Bao Yu Fen Zi Mian Yi Xue Za
Zhi. 30:906–908. 2014.(In Chinese). PubMed/NCBI
|
|
5
|
Lu X, Lu H, Guo L, Zhang Z, Zhao X, Zhong
M, Li S and Zhang Y: Cloning and characterization of a novel
hemocyanin variant LvHMCV4 from shrimp Litopenaeus vannamei. Fish
Shellfish Immun. 46:398–405. 2015. View Article : Google Scholar
|
|
6
|
Zhao X, Guo L, Lu X, Lu H, Wang F, Zhong
M, Chen J and Zhang Y: Evidences of abundant hemocyanin variants in
shrimp Litopenaeus vannamei. Mol Immunol. 77:103–112. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang X, Huang C and Qin Q: Antiviral
properties of hemocyanin isolated from shrimp Penaeus monodon.
Antiviral Res. 61:93–99. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang N, Tan NS, Ho B and Ding JL:
Respiratory protein-generated active oxygen species as an
antimicrobial strategy. Nat Immunol. 8:1114–1122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Wang S, Xu A, Chen J, Lin B and
Peng X: Affinity proteomic approach for identification of an
IgA-like protein in Litopenaeus vannamei and study on its
agglutination characterization. J Proteome Res. 5:815–821. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Min S, Yan F, Zhang Y, Ye X, Zhong M, Cao
J, Zou H and Chen J: Characterization of a novel hemolytic activity
of human IgG fractions arising from diversity in protein and
oligosaccharide components. PLoS One. 9:e857112014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng L, Zhao X, Zhang P, Chen C, Liu S,
Huang R, Zhong M, Wei C and Zhang Y: Hemocyanin from shrimp
Litopenaeus vannamei has antiproliferative effect against Hela cell
in vitro. PLoS One. 11:e01518012016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gesheva V, Chausheva S, Mihaylova N,
Manoylov I, Doumanova L, Idakieva K and Tchorbanov A: Anti-cancer
properties of gastropodan hemocyanins in murine model of colon
carcinoma. BMC Immunol. 15:342014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao J, Chen C, Wang Y, Chen X, Chen Z and
Luo X: Influence of autologous dendritic cells on cytokine-induced
killer cell proliferation, cell phenotype and antitumor activity in
vitro. Oncol Lett. 12:2033–2037. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coates CJ and Nairn J: Diverse immune
functions of hemocyanins. Dev Comp Immnol. 45:43–55. 2014.
View Article : Google Scholar
|
|
15
|
Zanjani NT, Miranda-Saksena M, Valtchev P,
Diefenbach RJ, Hueston L, Diefenbach E, Sairi F, Gomes VG,
Cunningham AL and Dehghani F: Abalone hemocyanin blocks the entry
of HSV-1 into cells: A potential new antiviral strategy. Antimicrob
Agents Chemother. 60:1003–1012. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wen Y, Zhan S, Huang H, Zhong M, Chen J,
You C, Wang F and Zhang Y: Identification and characterization of
an 18.4 kDa antimicrobial truncation from shrimp Litopenaeus
vannamei hemocyanin upon Vibrio parahaemolyticus infection. Fish
Shellfish Immun. 56:450–458. 2016. View Article : Google Scholar
|
|
17
|
Destoumieux-Garzón D, Saulnier D, Garnier
J, Jouffrey C, Bulet P and Bachère E: Crustacean immunity:
Antifungal peptides are generated from the C terminus of shrimp
hemocyanin in response to microbial challenge. J Biol Chem.
276:47070–47077. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Antonova O, Dolashka P, Toncheva D,
Rammensee HG, Floetenmeyer M and Stevanovic S: In vitro
antiproliferative effect of Helix aspersa hemocyanin on multiple
malignan T cells lines. Z Naturforsch C. 69:325–334. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo D, Wang H, Zeng D, Li X, Fan X and Li
Y: Vaccine potential of hemocyanin from Oncomelania hupensis
against Schistosoma Japonicum. Parasitol Int. 60:242–246. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Q, Liu XY, Zhang T, Zhang XF, Zhao
L, Long F, Liu ZK and Wang EH: The dual-functional capability of
cytokine-induced killer cells and application in tumor immunology.
Hum Immunol. 76:385–391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen J, Wei Y, He J, Cui G, Zhu Y, Lu C,
Ding Y, Xue R, Bai L, Uede T, et al: Natural killer T cells play a
necessary role in modulating of immune-mediated liver injury by gut
microbiota. Sci Rep. 4:72592014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Salaun B, Yamamoto T, Badran B,
Tsunetsugu-Yokota Y, Roux A, Baitsch L, Rouas R, Fayyad-Kazan H,
Baumgaertner P, Devevre E, et al: Differentiation associated
regulation of microRNA expression in vivo in human CD8+
T cells subsets. J Transl Med. 9:442011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan R, Xiang Y, Yang L, Liu Y, Chen P,
Wang L, Feng W, Yin K, Fu M, Xu Y and Wu J: Impaired NK cells'
activity and increased numbers of CD4+CD25+
regulatory T cells in multidrug-resistant Mycobacterium
tuberculosis patients. Tuberculosis (Edinb). 98:13–20. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen C, Wang ML, Jin C, Chen HJ, Li SH, Li
SY, Dou XF, Jia JQ and Gui ZZ: Cordyceps militaris polysaccharide
triggers apoptosis and G0/G1 cell arrest in cancer cells. J
Asia-Pac Entomol. 18:433–438. 2015. View Article : Google Scholar
|
|
25
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: HepG2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
26
|
Huang J, Li C, Wang Y, Lv H, Guo Y, Dai H,
Wicha MS, Chang AE and Li Q: Cytokine-induced killer (CIK) cells
bound with anti-CD3/anti-CD133 bispecific antibodies target
CD133(high) cancer stem cells in vitro and in vivo. Clin Immunol.
149:156–168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
El Ansary M, Mogawer S, Elhamid SA,
Alwakil S, Aboelkasem F, Sabaawy HE and Abdelhalim O: Immunotherapy
by autologous dendritic cell vaccine in patients with advanced HCC.
J Cancer Res Clin Oncol. 139:39–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu H, Li J, Wang F, Gao Y, Luo Y, Wang P,
Li C and Zhu Z: Comparative study of different procedures for the
separation of peripheral blood mononuclear cells in
cytokine-induced killer cell immunotherapy for hepatocarcinoma.
Tumour Biol. 36:2299–2307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qu HQ, Zhou XS, Zhou XL and Wang J: Effect
of DC-CIK cell on the proliferation, apoptosis and differentiation
of leukemia cells. Asian Pac J Trop Med. 7:659–662. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abdelrahman MM, Fawzy IO, Bassiouni AA,
Gomaa AI, Esmat G, Waked I and Abdelaziz AI: Enhancing NK cell
cytotoxicity by miR-182 in hepatocellular carcinoma. Hum Imuunol.
77:667–673. 2016. View Article : Google Scholar
|
|
31
|
Xue CM, Chen C, Xu J and Chen LR:
Influence of some traditional Chinese medicines (TCMS) on
cytokine-induced killer cells proliferation and anti-tumor features
in vitro. Int J Res Ayurveda Pharm. 4:228–232. 2013. View Article : Google Scholar
|