Introduction
In 2014, hepatocellular carcinoma (HCC) was the
second leading cause of cancer and the fifth most common
cancer-associated mortality worldwide (1). Approximately 70–90% of patients with HCC
are associated with hepatitis B virus infection in the highly
endemic Asia-Pacific areas, particularly in China (2). Liver resection and transplantation are
potentially curative treatments in selected patients (3). However, the clinical behavior of HCC may
vary (4). In numerous patients, the
disease manifests an aggressive course with a survival rate of only
months. Other patients may exhibit a comparatively slow clinical
development and survive for >5–10 years following diagnosis. It
is imperative to develop an HCC staging classification to stratify
patients and determine the probability of overall survival (OS)
rate prior to therapy.
Factors, including inflammation-based indices
including neutrophil-to-lymphocyte ratio (NLR) (5), platelet-to-lymphocyte ratio (PLR)
(6), prognostic nutritional index
(PNI) (7) and body mass index (BMI)
(8) and tumor biomarkers including
topoisomerase (Topo) II-α (9) and
Ki67 (10) represent independent
predictors of poor OS rates in patients with HCC and enabled the
refinement of current prognostic models. These novel factors may be
used to determine the OS and development of preventative measures
in those with high risk. The traditional systems, including 7th
Tumor-Node-Metastasis (TNM).system (11) and Cancer of the Liver Italian Program
(CLIP) scoring system (12), may not
be modified based on our understanding of cancer biology and novel
prognostic variables (13).
Various prognostic models incorporating traditional
and newly developed factors have been developed to focus on
early-stage HCC (14), large
(diameter >10 cm) HCC (15) and
multiple HCC (16). Compared with
conventional staging, these systems are limited in terms of
prognostic accuracy in patients treated with curative resection for
solitary HBV-related HCC.
Based on prognostic factors identified previously
(5–10), prognostic risk calculators were
developed to predict prognosis in patients undergoing curative
resection for solitary HBV-related HCC. The accuracy of the
prognostic risk calculator was compared with that of the TNM and
CLIP scoring systems.
Materials and methods
Patients and tumor samples
HCC tumor samples were acquired from the Department
of Patholofy, Fuzhou General Hospital (Fujian, China) between
February 2003 and October 2012. The inclusion criteria were: i)
Single tumor lesion; ii) without any distant metastasis, ascites or
hepatic encephalopathy; iii) 0–1 Eastern Cooperative Oncology Group
score (17) prior to surgery; iv)
pathologically confirmed primary HCC following surgery; v) complete
clinical records and follow-up data; vi) radical resection between
2003 and 2012; and vii) HBV DNA load (IU/ml) ≤104. The
exclusion criteria were: i) Preoperative anticancer treatments; ii)
concomitant positive hepatitis C virus antibody; iii) incomplete
clinical data or tissue biopsy specimens for extra analysis; and
iv) history of inflammatory disease or active concomitant
infection.
The program was approved by the Institutional Ethics
Committee of Fuzhou General Hospital and informed consent was
signed by all the patients.
Clinicopathological and laboratory
examination
The diagnoses of Edmonson grade (18), tumor encapsulation, vascular invasion
and maximal tumor diameter were based on histological examination
of the surgical specimens obtained following liver resection. Blood
samples were measured prior to treatment for platelet count,
α-fetoprotein (AFP), albumin, neutrophil, lymphocyte, white blood
cell count and prothrombin time. The cut-off point was the highest
Youden Index, which was selected as the optimal threshold value
(6).
Immunohistochemical (IHC)
analysis
Formalin-fixed paraffin-embedded sections (4-mm
thick) were heated to 60°C for 2 h, prior to being deparaffinized
with xylene and rehydrated in ethanol through a descending series
of concentrations (100, 100, 85 and 75%). The immunohistochemical
methods described by Hemda Schmilovitz-Weiss et al (19) were used to analyze the expression of
Ki67, using an anti-Ki67 antibody obtained from Fuzhou Maixin
Biotech Co., Ltd. (Fuzhou, China). Antigenic retrieval was
performed by submerging the sections into EDTA antigenic retrieval
buffer and microwaving (100°C, 10 min.). The sections were
incubated with an anti-Ki67 antibody (dilution, 1:200; cat. no.
MAB-0672; Fuzhou Maixin Biotech Co., Ltd.) for 1 h at room
temperature. Following washing, the tissue sections were treated
with a ready-to-use anti-rabbit/mouse secondary antibody (1:50;
cat. no. KIT-9903; Fuzhou Maixin Biotech Co., Ltd.) for 0.5 h at
room temperature. 3,3′-diaminobenzidine was used as the chromogen
(5 min at room temperature). The tissue sections were immersed in
3-amino-9-ethyl carbazole, counterstained with 10% Mayer's
hematoxylin for 5 min at room temperature prior to being dehydrated
and mounted in Crystal Mount. A light microscope (magnifications,
×100 or ×200) was used. The same method was used to analyze the
expression of DNA Topo II-α using an anti-DNA Topo II-α antibody
(dilution, 1:200; cat. no. MAB-0588; Maixin Company, Fuzhou,
China). Semi-quantitative IHC detection was used to calculate Topo
II-α protein level with a 4-point scale (positive tumor cell
counts, graded from 0 to 3: 0=none, 1= ≤25%, 2=25–50 and 3= ≥50%).
HCC tissue samples graded 0 or 1 represented low Topo II-α
expression, whereas those graded 2 or 3 were regarded as a high
Topo II-α expression. Ki67 was scored as a percentage of positively
stained cells: <10%=‘−’; 10–25%=‘+’; 26–50%=‘++’; 51–75%=‘+++’;
and >75%=‘++++’. HCC tissue samples with ‘−’ or ‘+’ Ki67
expression suggested low Ki67 expression; and samples with ‘++’,
‘+++’, or ‘++++’ Ki67 expression represented high Ki67
expression.
Follow-up
Patients who underwent hepatectomy between February
2003 and October 2012 were subjected to close clinical observation
(abdominal ultrasound, AFP and liver function test) at 2-to 4-month
intervals. The patients were followed up until January 1, 2015.
Statistical analysis
All statistical analyses were two-sided and
P<0.05 was considered to indicate a statistically significant
difference. Univariate risk ratios and their 95% confidence
intervals (95% CI) were calculated using Cox proportional hazards
regression (HR) models with stepwise selection. Cox multivariate
proportional HR analysis was performed using forward selection
method with all the variables included for their prognostic
significance by univariate analysis with stepwise selection
(P<0.05). Based on the outcomes of the multivariate Cox
proportional hazard model, the prognostic risk calculator was
formulated using R (version 3.2.1; https://www.r-project.org). The effect of the
variables with the highest coefficient (absolute value) was
assigned 100 points. The points were added across independent
variables to obtain the total score, which was converted to
predicted probabilities. The predictive performance of the
prognostic risk calculator was evaluated by concordance index
(C-index) and its calibration using 1,000 bootstrap samples to
decrease the overfit bias. For clinical use of the model, the total
scores of each patient were calculated based on multivariate
analyses. The receiver operating characteristic (ROC) analysis was
used to calculate the optimal cutoff values determined by
maximizing the Youden index (sensitivity + specificity-1). A
Kaplan-Meier curve comparing patients with high risk (score ≥ cut
off point) and low risk (score < cutoff point) was obtained to
show the differences (20). Analyses
were performed using R version 3.2.1 and SPSS version 20.0 (IBM
Corp., Armonk, NY, USA).
Results
Demographic features and
clinicopathological data
The clinical demographics, laboratory and
pathological data and immunohistochemistry of Topo II-α and Ki67
are summarized in Table I. Topo II-α
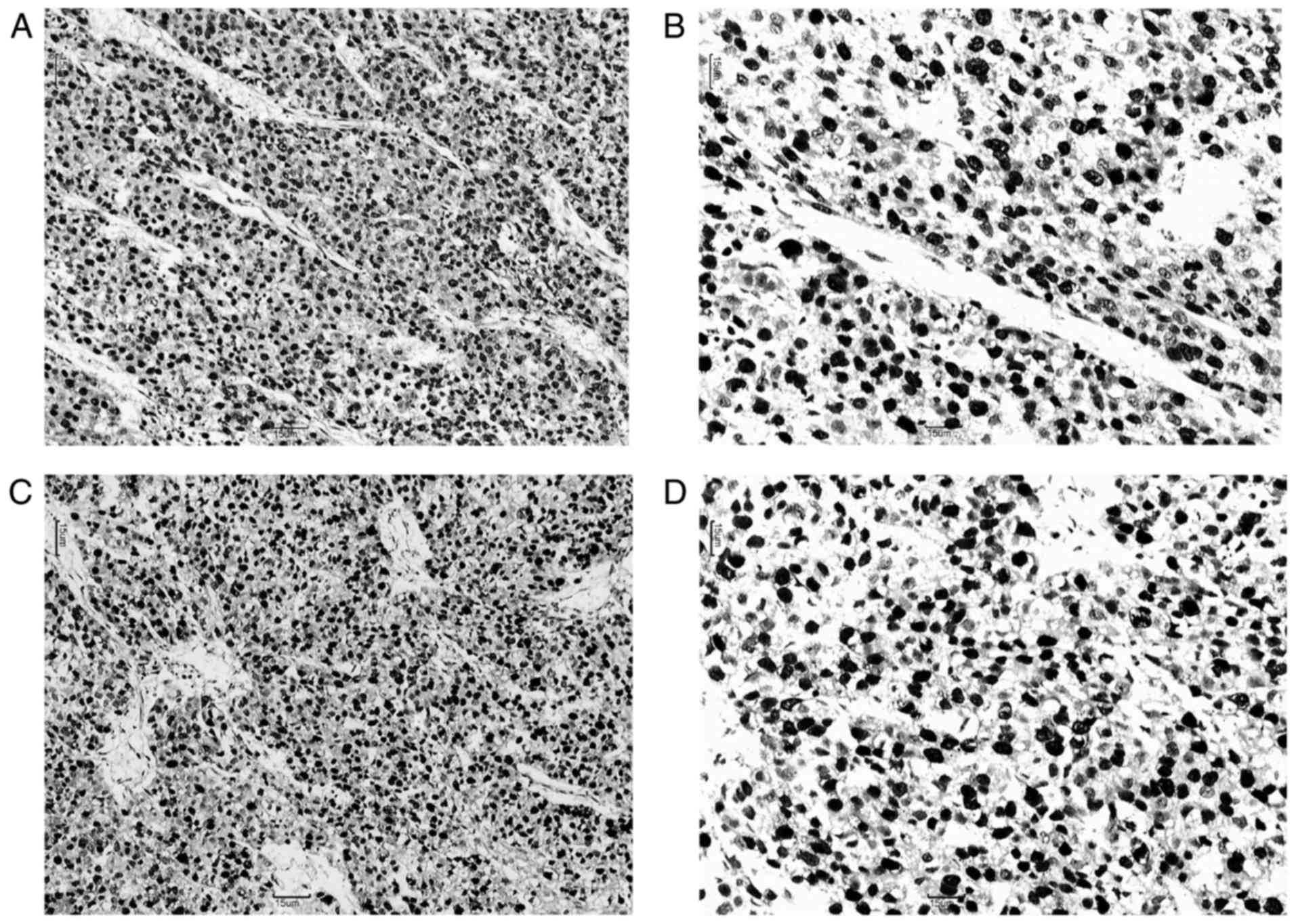
and Ki67 were detected in the nuclei of tumor cells (Fig. 1). The last follow-up of patients in
the present study was on May 31, 2015. The median survival rate of
patients was 69 months (95% CI 53.8–84.2 months). The 1-, 3- and
5-year cumulative survival rates were 86.2, 66.2 and 53.8%,
respectively.
 | Table I.Clinicopathological characteristics of
426 cases of hepatocellular carcinoma. |
Table I.
Clinicopathological characteristics of
426 cases of hepatocellular carcinoma.
|
| Cases (n=426) |
|
|
|---|
|
|
|
|
|
|---|
| Characteristic | n | % | (IQR) median | Mean ± standard
deviation |
|---|
| Sex |
|
|
|
|
| Male | 378 | 88.7 |
|
|
|
Female | 48 | 11.3 |
|
|
| TNM stage |
|
|
|
|
| I/II | 292 | 68.5 |
|
|
| IIIa | 134 | 31.5 |
|
|
| Site |
|
|
|
|
|
Left | 101 | 23.7 |
|
|
|
Right | 325 | 76.3 |
|
|
| Edmondson-Steiner
classification |
|
|
|
|
|
I–II | 83 | 19.5 |
|
|
|
III–IV | 343 | 80.5 |
|
|
| Tumor
encapsulation |
|
|
|
|
|
Absent | 172 | 40.4 |
|
|
|
Present | 254 | 59.6 |
|
|
| Vascular
invasion |
|
|
|
|
|
Absent | 139 | 32.6 |
|
|
|
Present | 287 | 67.4 |
|
|
| Child-Pugh
grade |
|
|
|
|
| A | 259 | 60.8 |
|
|
| B | 167 | 39.2 |
|
|
| Ki67
expression |
|
|
|
|
|
Low | 185 | 43.4 |
|
|
|
High | 241 | 56.6 |
|
|
| Topo II-α
expression |
|
|
|
|
|
Low | 236 | 55.4 |
|
|
|
High | 190 | 44.6 |
|
|
| Age (years) |
|
| 53 (45–61) | 52±12 |
|
≤55 | 205 | 58.1 |
|
|
|
>55 | 148 | 41.9 |
|
|
| Maximal tumor
diameter (cm) |
|
| 5 (2.5–8.0) | 5.75±4.201 |
| ≤5 | 219 | 51.4 |
|
|
|
>5 | 207 | 48.6 |
|
|
| Serum AFP level
(ng/ml) |
|
| 149.0
(8.0–1,000) |
4428.34±16919.153 |
|
≤400 | 215 | 60.9 |
|
|
|
>400 | 138 | 39.1 |
|
|
| NLR |
|
| 2.0 (1.0–3.0) | 3.24±3.810 |
|
≤1.62 | 125 | 29.3 |
|
|
|
>1.62 | 301 | 70.7 |
|
|
| PLR |
|
| 94 (68–133.25) | 110.98±71.996 |
|
≤114.4 | 281 | 66 |
|
|
|
>114.4 | 145 | 34 |
|
|
| PNI |
|
| 50 (46–54) | 50.34±11.441 |
|
≤49.42 | 207 | 48.6 |
|
|
|
>49.42 | 219 | 51.4 |
|
|
| BMI |
|
| 22 (20–25) | 22.65±3.157 |
|
≤23.296 | 256 | 60.1 |
|
|
| >23.296 | 170 | 39.9 |
|
|
| Neutrophil count
(×109/l) |
|
| 4.0 (3.0–6.0) | 5.08±3.941 |
| Lymphocyte count
(×109/l) |
|
| 2.00 (1.0–2.0) | 2.05±2.046 |
| Serum albumin
(g/l) |
|
| 40 (37–44) | 40.23±5.560 |
| Height (m) |
|
| 1.68
(1.64–1.72) | 1.675±0.06 |
| Weight (kg) |
|
| 63 (56–70) | 63.66±10.088 |
| Platelet count
(×109/l) |
|
| 183.5
(135.0–223.0) | 186.31±76.164 |
Univariate and multivariate analyses
of overall survival rates
As illustrated in Table
II, the COX regression univariate analysis indicated that 13
factors were associated with OS. The result demonstrated that the
risk of mortality increased with vascular invasion, TNM stage IIIa,
maximal tumor diameter >5.0 cm, AFP >400 ng/ml, high levels
of Topo II-α and Ki67, NLR >1.62, PLR >114.4 and Child-Pugh
grade B. The risk of mortality decreased with age >55 years,
tumor encapsulation, Edmonson grade I–II, PNI >49.42 and BMI
>23.296. Multivariate analysis was performed to develop a
reduced model using the stopping rule of Akaike's information
criterion with these significant factors.
 | Table II.Univariate/multivariate analyses of
factors associated with survival. |
Table II.
Univariate/multivariate analyses of
factors associated with survival.
|
| Univariate
analyses | Multivariate
analyses |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
Male | Reference | 0.41–1.13 | 0.138 |
|
|
|
|
Female | 0.68 |
|
|
|
|
|
| Age |
|
|
|
|
|
|
|
≤55 | Reference | 0.69–1.24 | 0.615 |
|
|
|
|
>55 | 0.93 |
|
|
|
|
|
| Tumor
encapsulation |
|
|
|
|
|
|
|
Absent | Reference | 0.31–0.55 | <0.001 | Reference | 0.52–0.96 | 0.025 |
|
Present | 0.41 |
|
| 0.71 |
|
|
| Vascular
invasion |
|
|
|
|
|
|
|
Absent | Reference | 4.81–12 | <0.001 | Reference | 2.18–6.28 | <0.001 |
|
Present | 7.6 |
|
| 3.7 |
|
|
| TNM stage |
|
|
|
|
|
|
|
I–II | Reference | 1.78–3.16 | <0.001 |
|
|
|
|
IIIa | 2.37 |
|
|
|
|
|
| Maximal tumor
diameter (cm) |
|
|
|
|
|
|
|
≤5.0 | Reference | 1.70–3.06 | <0.001 |
|
|
|
|
>5.0 | 2.28 |
|
|
|
|
|
| Edmondson-Steiner
classification |
|
|
|
|
|
|
|
III–IV | Reference | 0.18–0.48 | <0.001 | Reference | 0.32–0.93 | 0.025 |
|
I–II | 0.3 |
|
| 0.54 |
|
|
| Site |
|
|
|
|
|
|
|
Left | Reference | 0.99–2.09 | 0.055 |
|
|
|
|
Right | 1.44 |
|
|
|
|
|
| AFP (ng/ml) |
|
|
|
|
|
|
|
≤400 | Reference | 1.60–2.84 | <0.001 | Reference | 1.09–1.98 | 0.012 |
|
>400 | 2.13 |
|
| 1.47 |
|
|
| Topo II-α |
|
|
|
|
|
|
|
Low | Reference | 1.60–2.86 | <0.001 | Reference | 1.02–1.87 | 0.035 |
|
High | 2.14 |
|
| 1.38 |
|
|
| Ki67 |
|
|
|
|
|
|
|
Low | Reference | 1.56–2.90 | <0.001 |
|
|
|
|
High | 2.13 |
|
|
|
|
|
| NLR |
|
|
|
|
|
|
|
≤1.62 | Reference | 1.78–3.85 | <0.001 | Reference | 1.13–2.53 | 0.011 |
|
>1.62 | 2.62 |
|
| 1.69 |
|
|
| PLR |
|
|
|
|
|
|
|
≤114.4 | Reference | 1.67–2.96 | <0.001 |
|
|
|
|
>114.4 | 2.22 |
|
|
|
|
|
| PNI |
|
|
|
|
|
|
|
≤49.42 | Reference | 0.37–0.66 | <0.001 | Reference | 0.52–0.97 | 0.029 |
|
>49.42 | 0.49 |
|
| 0.71 |
|
|
| BMI |
|
|
|
|
|
|
|
≤23.296 | Reference | 0.53–0.98 | 0.034 |
|
|
|
|
>23.296 | 0.72 |
|
|
|
|
|
| Child-Pugh
grade |
|
|
|
|
|
|
| A | Reference | 4.57–8.63 | <0.001 | Reference | 2.31–4.60 | <0.001 |
| B | 6.28 |
|
| 3.26 |
|
|
The results demonstrated that patients with present
vascular invasion (HR: 3.70; 95% CI: 2.18–6.28), AFP >400 ng/ml
(HR: 1.47; 95% CI: 1.09–1.98), increased levels of Topo II-α (HR:
1.38; 95% CI: 1.02–1.87), NLR >1.62 (HR: 1.69; 95% CI:
1.13–2.53) and Child-Pugh grade B (HR: 3.26; 95% CI: 2.31–4.60)
tended to exhibit decreased survival rates compared with patients
without vascular invasion, AFP ≤400 ng/ml, decreased levels of Topo
II-α, NLR ≤1.62 and Child-Pugh grade A, respectively. Patients with
tumor encapsulation (HR: 0.71; 95% CI: 0.52–0.96), Edmonson grade
I–II (HR: 0.54; 95% CI: 0.32–0.93), PNI >49.42 (HR: 0.71; 95%
CI: 0.52–0.97) tended to live longer compared with the patients
without tumor encapsulation, with Edmonson grades III–IV, or with
PNI ≤49.42, respectively.
Prognostic risk based on multivariate
analyses
Based on multivariate analyses, the following
equation was constructed: Prognostic OS score=26.49 × tumor
encapsulation (present=0; absent=1) + 100.00 × vascular invasion
(absent=0; present=1) + 46.40 × Edmonson grade (I–II=0; III–IV=1) +
29.32 × AFP (≤400=0; >400=1) + 24.72 × Topo II-α (low=0; high=1)
+ 40.18 × NLR (≤1.62=0; >1.62=1) + 25.99 × PNI (>49.42=0;
≤49.42=1) + 90.32 × Child-Pugh grade (A=0; B=1).
The optimum cutoff value of the total score was set
to 234.47 by maximizing the Youden index [sensitivity=0.78;
specificity=0.77; area under the curve (AUC)=0.83] and the sample
was divided into high-risk (>234.47) and low-risk groups
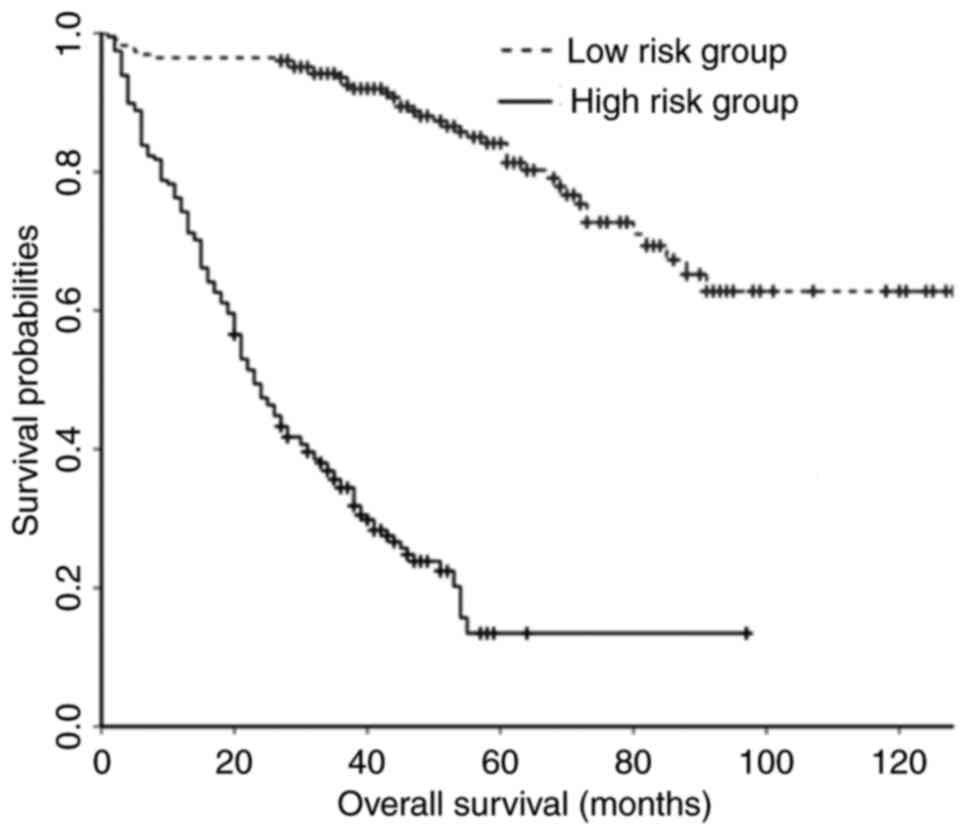
(≤234.47). Kaplan-Meier curves demonstrated that the OS rate of the
low-risk group was increased compared with that of the high-risk
group (Fig. 2).
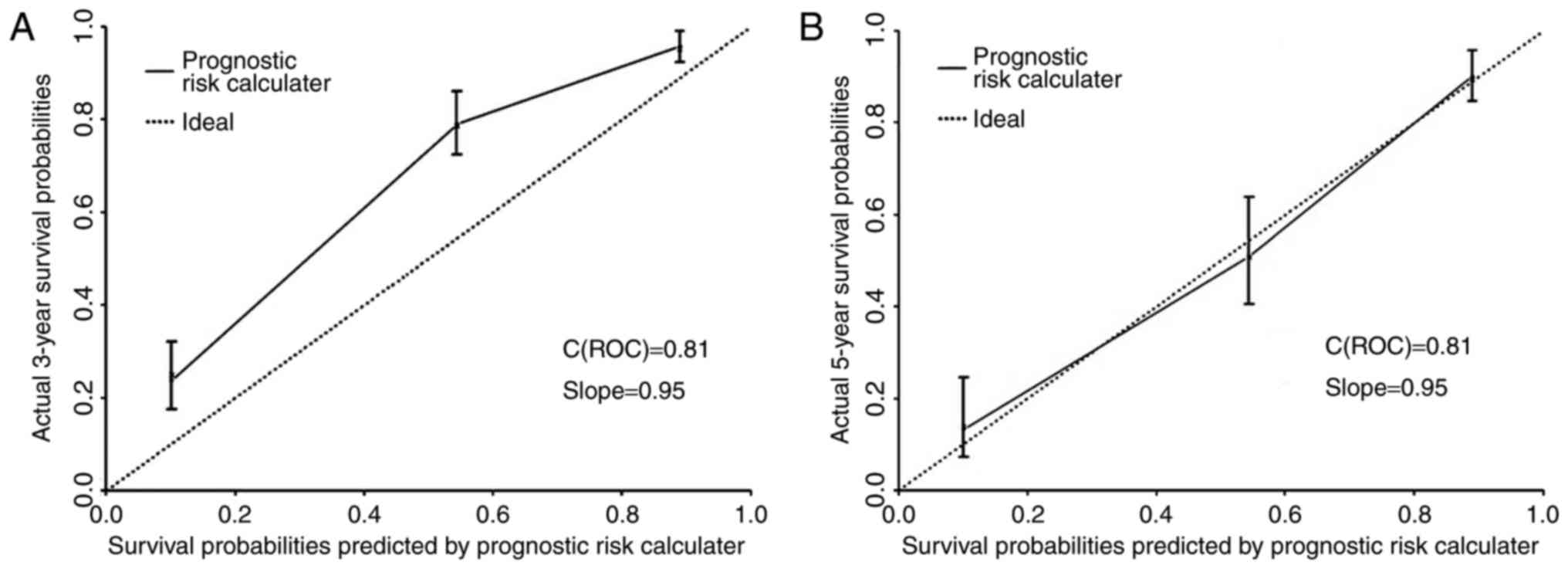
Validation of prognostic accuracy
The prognostic risk calculator of OS rate was built
on the basis of tumor encapsulation, vascular invasion,
Edmondson-Steiner classification, AFP, Topo II-α, NLR, PNI and
Child-Pugh grade with a C-index (prior to adjustment) of 0.81 (95%
CI: 0.78–0.84) and a bootstrap-corrected C-index of 0.81. The
calibration curve giving the survival rate probability in 5 years
following the surgery indicated an optimum consistency between
prediction and actual observation in our model. The curve showing
the prognostic risk calculator of OS rates in 3 years demonstrated
that the predictive effect of our model was relatively weak during
this period (Fig. 3A and B).
Comparison of discriminatory
powers
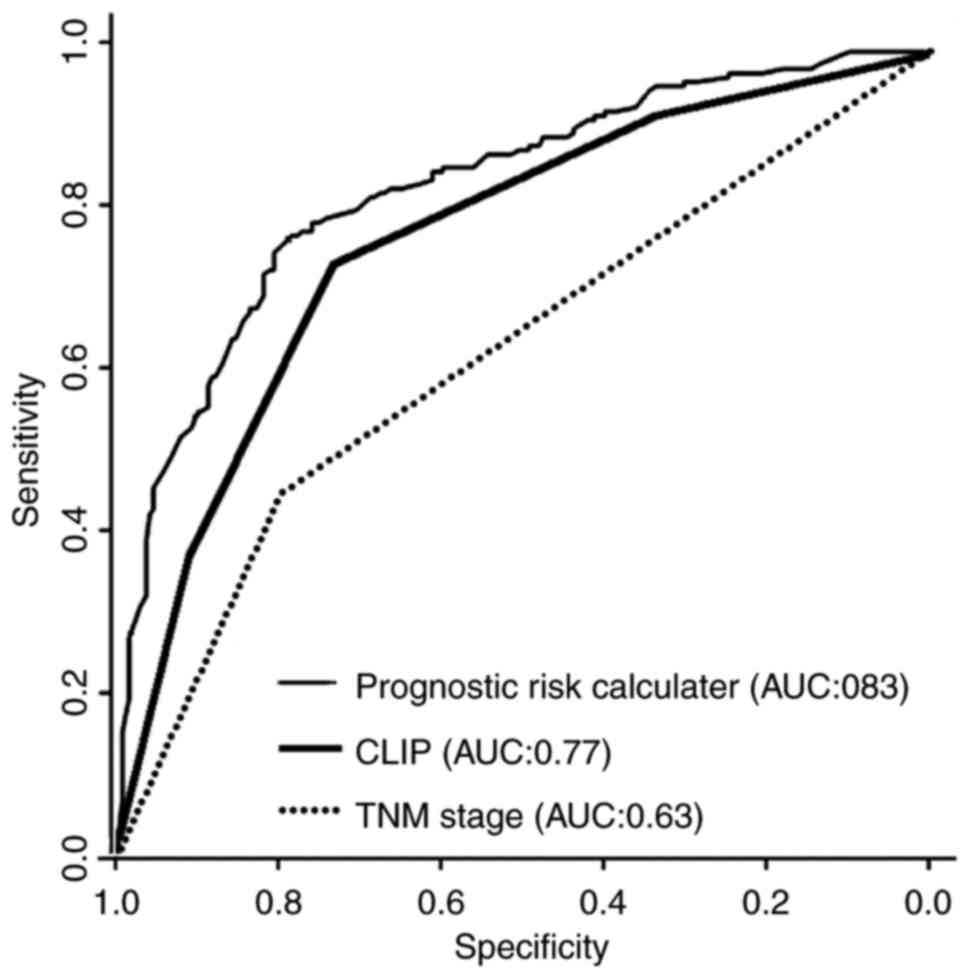
The predictive power of the model from the present
study, the 7th TNM staging system and CLIP were compared by ROC
curve analysis. The model was a significant improvement compared
with the competing models: The AUC was increased compared with that
of the 7th TNM staging system or CLIP (0.83 vs. 0.62–0.77.
P<0.001; Fig. 4).
Discussion
In the present study, a prognostic risk calculator
based on multivariate analysis of patients undergoing curative
resection for solitary HCC was developed. The prognostic model was
built on the basis of tumor encapsulation, vascular invasion,
Edmondson-Steiner classification, AFP, Topo II-α, NLR, PNI and
Child-Pugh grade with a C-index (prior to adjustment) of 0.81 (95%
CI: 0.78–0.84) and a bootstrap-corrected C-index of 0.81. The
calibration plots of the cohorts revealed association between the
predicted and the actual survival rates.
The American Joint Committee on Cancer (AJCC) TNM
system and CLIP are widely used staging systems for HCC. The
traditional TNM staging system concentrates on the presentation of
the neoplasm mostly, without adequately reflecting the biological
characteristics of HCC (11). In a
prospective study of 195 patients reported by Cillo et al
(21), CLIP was associated with
improved prognostic ability compared with the AJCC/TNM 2002 system
in operative patients. The multi-dimensional model presented in the
present study incorporates not only each individual pre-treatment
data including liver function (Child-Pugh grade) and laboratory
parameters (AFP, NLR and PNI), but also data from pathological
reports confirmed following surgery, including tumor encapsulation,
tumor staging (vascular invasion and TNM stage), Edmonson-Steiner
grade and tumor biomarkers (Topo II-α). A comparison of this
prognostic risk calculator with the CLIP or 7th TNM staging system
demonstrated that the new ROC curve was associated with increased
sensitivity and specificity for predicting OS.
Previous studies have demonstrated that tumor
vascular invasion is correlated with poor prognosis (22). Portal vein tumor thrombus and
microvascular invasion are important risk factors for long-term
survival rates of HCC (23,24). The multivariate analysis performed in
the present study indicated that the HR of vascular invasion for OS
was 3.70 and was the highest of all independent risk factors.
A clear margin is difficult when a solitary HCC
lacks a capsule surrounding the tumor. Advanced surgical risks and
poorer prognosis following liver resection were observed in such
patients. Therefore, it was important to consider the complete
tumor capsule for a solitary HCC for surgical safety and long-term
survival rate. As expected, tumor capsule was an independent
prognostic factor in the present study.
Oishi et al (25) revealed that differentiation and
angiogenic activity, proliferation, tumor size, vascular invasion
and AFP ratio were negatively associated. However, Edmonson-Steiner
grade was not included in any previous staging system and
represents a key variable in the model in the present study.
Liver function is affected by comorbidities,
including cirrhosis, HBV and HCV, and is therefore considered to
predict patient outcomes [CLIP, BCLC (Barcelona Clinic Liver
Cancer) and JIS (Japan Integrated Staging Score)]. In the present
study, Child-Pugh grade B (HR: 3.26; 95% CI: 2.31–4.60) represented
a negative prognostic factor for survival rate following radical
hepatectomy and was associated with a poorer survival rate.
Cumulative evidence suggests that host inflammatory
reaction serves a significant function in carcinogenesis via
sustained proliferative signaling, angiogenesis and by promoting
invasion and metastasis (26). The
prognostic risk calculator proposed in the present study comprises
comprehensive laboratory indices including inflammation-based
indices (NLR and PNI), which were previously demonstrated to be
independent risk factors for HCC prognosis. Emerging evidence
suggests that NLR has prognostic value for patients with HCC
(5). Pinato et al (7) demonstrated that PNI is an independent
predictor of poor overall survival rate in patients with HCC at
different stages and liver functional status. Recently, Fu et
al (27) built a nomogram based
on inflammatory biomarkers for resectable HCC. However, the
nomogram only contained NLR not PNI. The present study demonstrated
that PNI and NLR were independent predictors of OS. Multiple
clinical trials have demonstrated the prognostic value of other
laboratory markers of systemic inflammation including C-reactive
protein and modified Glasgow prognostic score in cancer populations
(16,28,29).
However, in numerous hospitals, particularly those with limited
medical resources, the level of serum C-reactive protein is not
regularly evaluated due to the need for advanced equipment. NLR and
PNI are easily determined from comprehensive blood testing and
represent appropriate laboratory markers to predict survival
rates.
With advances in understanding of cancer biology,
the function of biomarkers in predicting survival rates has
garnered increased attention. The present study provided additional
data confirming the reliability of the results. Tumor cell
proliferation status is an important parameter that reflects tumor
biology and directly affects the prognosis and efficiency of
treatment (9,10). Topo II-α (9) is a commonly used proliferation marker
that serves a function in DNA replication and chromosomal
segregation by unwinding the DNA double helix. It is crucial for
the active survival of cells and represents a common biomarker and
target for multiple anticancer agents (30,31).
Determination of Topo II-α expression facilitates the prognosis of
overall survival rates of patients and their reaction to therapy
(32). The prognostic value of Topo
II-α has been discussed in different studies (33,34). In
our survival rate models, Topo II-α was a key variable with an HR
of 1.38 for OS. Ki67 is a traditional proliferation marker found
within the cell nucleus (35) and is
associated with poor prognosis in HCC (10). Univariate but not multivariate
analysis indicated that Ki67 was associated with OS in the cohort
of the present study.
The use of laboratory indices and tumor biomarkers
as adjuncts to the tumor staging system enables the formulation of
a personalized therapeutic strategy. Nevertheless, multiple
limitations to our prognostic risk calculation exist. IHC analysis
is not routinely used globally. Furthermore, since the patients
comprised predominantly an HBV-prevalent Chinese population at a
single institution and represented single-tumor patients, the
outcomes are unreliable. External validation in a larger patient
population is required. Additional studies with data derived from
multiple centers will be necessary to investigate differences in
outcomes.
To conclude, the prognostic models in the present
study are widely available, user-friendly, low-cost and accurate
for patients undergoing curative resection for solitary HCC. This
is the first clinical evaluation of multiple parameters
incorporating individual liver function, tumor stage, inflammatory
indices and biomarkers, to the best of the authors' knowledge.
These risk equations can be used for patient counseling and
management, in addition to prognostic evaluation.
Acknowledgements
The authors would like to thank Professor Yu Yinghao
(The Fuzong Clinical College of Fujian Medical University) for his
technical assistance. The present study was supported by the Key
Project of Natural Science Foundation of Fujian Province for Lizhi
Lv (grant no. 2014Y0034).
References
|
1
|
Stewart BW and Wild CP: World cancer
report 2014International agency for research on cancer. World
Health Organization; 2014, View Article : Google Scholar
|
|
2
|
Amarapurkar D, Han KH, Chan HL and Ueno Y:
Asia-Pacific Working Party on Prevention of Hepatocellular
Carcinoma: Application of surveillance programs for hepatocellular
carcinoma in the Asia-Pacific region. J Gastroenterol Hepatol.
24:955–961. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Earl TM and Chapman WC: Hepatocellular
carcinoma: Resection versus transplantation. Semin Liver Dis.
33:282–292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan ST, Lo Mau C, Poon RT, Yeun C, Liu
Leung C, Yuen WK, Lam Ming C, Ng KK and Chan Ching S: Continuous
improvement of survival outcomes of resection of hepatocellular
carcinoma: A 20-year experience. Ann Surg. 253:745–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okamura Y, Sugiura T, Ito T, Yamamoto Y,
Ashida R, Mori K and Uesaka K: Neutrophil to lymphocyte ratio as an
indicator of the malignant behaviour of hepatocellular carcinoma.
Br J Surg. 103:891–898. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Chen ZH, Xing YF, Wang TT, Wu DH,
Wen JY, Chen J, Lin Q, Dong M, Wei L, et al: Platelet-to-lymphocyte
ratio acts as a prognostic factor for patients with advanced
hepatocellular carcinoma. Tumour Biol. 36:2263–2269. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pinato D, North BV and Sharma R: A novel,
externally validated inflammation-based prognostic algorithm in
hepatocellular carcinoma: The prognostic nutritional index (PNI).
Br J Cancer. 106:1439–1445. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee YL, Li WC, Tsai TH, Chiang HY and Ting
CT: Body mass index and cholesterol level predict surgical outcome
in patients with hepatocellular carcinoma in taiwan-a cohort study.
Oncotarget. 7:22948–22959. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
El Zawahry H, Nasar H, Mourad M, Zaky R
and Samie AA: P-107Topoisomerase II Alpha (TopoIIα) As A Prognostic
Biomarker In Hepatocellular Carcinoma. Ann Oncol. 26:iv302015.
View Article : Google Scholar
|
|
10
|
Luo Y, Ren F, Liu Y, Shi Z, Tan Z, Xiong
H, Dang Y and Chen G: Clinicopathological and prognostic
significance of high Ki-67 labeling index in hepatocellular
carcinoma patients: A meta-analysis. Int J Clin Exp Med.
8:10235–10247. 2015.PubMed/NCBI
|
|
11
|
Easson EC: TNM classification of malignant
tumours. Br J Cancer. 30:101–102. 1974. View Article : Google Scholar
|
|
12
|
Llovet JM and Bruix J: Prospective
validation of the cancer of the liver italian program (CLIP) score:
A new prognostic system for patients with cirrhosis and
hepatocellular carcinoma. Hepatology. 32:678–680. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sobin LH: TNM: Evolution and relation to
other prognostic factors. Semin Surg Oncol. 21:3–7. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shim JH, Jun MJ, Han S, Lee YJ, Lee SG,
Kim KM, Lim YS and Lee HC: Prognostic nomograms for prediction of
recurrence and survival after curative liver resection for
hepatocellular carcinoma. Ann Surg. 261:939–946. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Xia Y, Li J, Wu D, Wan X, Wang K, Wu
M, Liu J, Lau WY and Shen F: Prognostic nomograms for pre-and
postoperative predictions of long-term survival for patients who
underwent liver resection for huge hepatocellular carcinoma. J Am
Coll Surg. 221:962–974.e4. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang P, Qiu J, Li J, Wu D, Wan X, Lau WY,
Yuan Y and Shen F: Nomograms for pre-and postoperative prediction
of long-term survival for patients who underwent hepatectomy for
multiple hepatocellular carcinomas. Ann Surg. 263:778–786. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Repetto L, Fratino L, Audisio R A,
Venturino A, Gianni W, Vercelli M, Parodi S, Lago Dal D, Gioia F,
Monfardini S, et al: Comprehensive geriatric assessment adds
information to Eastern cooperative oncology group performance
status in elderly cancer patients: An Italian group for geriatric
oncology study. J Clin Oncol. 20:494–502. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou L, Rui JA, Zhou WX, Wang SB, Chen SG
and Qu Q: Edmondson-Steiner grade: A crucial predictor of
recurrence and survival in hepatocellular carcinoma without
microvascular invasio. Pathol Res Pract. 213:824–830. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmilovitz-Weiss H, Tobar A, Halpern M,
Levy I, Shabtai E and Ben-Ari Z: Tissue expression of squamous
cellular carcinoma antigen and Ki67 in hepatocellular
carcinoma-correlation with prognosis: A historical prospective
study. Diagn Pathol. 6:1212011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kinoshita A, Onoda H, Imai N, Iwaku A,
Oishi M, Fushiya N, Koike K, Nishino H and Tajiri H: Comparison of
the prognostic value of inflammation-based prognostic scores in
patients with hepatocellular carcinoma. Br J Cancer. 107:988–993.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cillo U, Vitale A, Grigoletto F, Farinati
F, Brolese A, Zanus G, Neri D, Boccagni P, Srsen N, D'Amico F, et
al: Prospective validation of the Barcelona clinic liver cancer
staging system. J Hepatol. 44:723–731. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thuluvath PJ: Vascular invasion is the
most important predictor of survival in HCC, but how do we find it?
J Clin Gastroenterol. 43:101–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang L, Xu J, Ou D, Wu W and Zeng Z:
Hepatectomy for huge hepatocellular carcinoma: Single institute's
experience. World J Surg. 37:2189–2196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lim KC, Chow PK, Allen JC, Chia GS, Lim M,
Cheow PC, Chung AY, Ooi LL and Tan SB: Microvascular invasion is a
better predictor of tumor recurrence and overall survival following
surgical resection for hepatocellular carcinoma compared to the
Milan criteria. Ann Surg. 254:108–113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oishi K, Itamoto T, Amano H, Fukuda S,
Ohdan H, Tashiro H, Shimamoto F and Asahara T: Clinicopathologic
features of poorly differentiated hepatocellular carcinoma. J Surg
Oncol. 95:311–316. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu YP, Ni XC, Yi Y, Cai XY, He HW, Wang
JX, Lu ZF, Han X, Cao Y, Zhou J, et al: A novel and validated
inflammation-based score (IBS) predicts survival in patients with
hepatocellular carcinoma following curative surgical resection: A
STROBE-compliant article. Medicine (Baltimore). 95:e27842016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Proctor MJ, Morrison DS, Talwar D, Balmer
SM, Fletcher CD, O'Reilly DS, Foulis AK, Horgan PG and McMillan DC:
A comparison of inflammation-based prognostic scores in patients
with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer.
47:2633–2641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kinoshita A, Onoda H, Imai N, Nishino H
and Tajiri H: C-reactive protein as a prognostic marker in patients
with hepatocellular carcinoma. Hepatogastroenterology. 62:966–970.
2015.PubMed/NCBI
|
|
30
|
Watt PM and Hickson ID: Structure and
function of type II DNA topoisomerases. Biochem J. 303:681–695.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wendorff TJ, Schmidt BH, Heslop P, Austin
CA and Berger JM: The structure of DNA-bound human topoisomerase II
alpha: Conformational mechanisms for coordinating inter-subunit
interactions with DNA cleavage. J Mol Biol. 424:109–124. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ali Y and Hamid Abd S: Human topoisomerase
II alpha as a prognostic biomarker in cancer chemotherapy. Tumour
Biol. 37:47–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Panvichian R, Tantiwetrueangdet A,
Angkathunyakul N and Leelaudomlipi S: TOP2A amplification and
overexpression in hepatocellular carcinoma tissues. Biomed Res Int.
2015:3816022015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Watanuki A, Ohwada S, Fukusato T, Makita
F, Yamada T, Kikuchi A and Morishita Y: Prognostic significance of
DNA topoisomerase IIalpha expression in human hepatocellular
carcinoma. Anticancer Res. 22:1113–1119. 2001.
|
|
35
|
Scholzen T and Gerdes J: The Ki-67
protein: From the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|