Introduction
Oral cancer is one of the most common and invasive
types of cancer, accounting for ~5% of cancer mortalities
worldwide. The management of oral cancer includes radiotherapy,
chemotherapy and surgical excision, with which patients experience
profound adverse effects. These undesired side effects of treatment
occur due to the non-specific action of the therapeutic agents
(1).
Over the previous three decades, advances in drug
delivery systems have reduced the undesired effects of
chemotherapy. Furthermore, the progression of nanotechnology in
cancer chemotherapy has promoted the innovation of diagnostic and
treatment methods. Targeted therapy is the most desired treatment
for oral cancer, aiming for specific site delivery and thereby
lowering the side effects and levels of systemic toxicity. The
delivery of therapeutics through nanodelivery systems consisting of
polymers or lipids have demonstrated increased solubility,
stability and bioavailability, accumulating even inside tumor cells
(2).
Novel drug delivery systems tackle the issues
associated with local oral drug delivery and overcome various
challenges, including lower drug absorption from the oral mucosa
and prompt clearance of the drug from the site of absorption with
saliva and induced mechanical stress, and poor patient compliance
due to unpleasant taste (2). In
addition, the most prominent disadvantage of conventional
chemotherapy used to treat oral cancer is its high systemic
toxicity and poor target specificity at cancer sites (2). Liposomes exhibit marked potential as a
carrier system for therapeutically active agents. Due to their
attributes, including biodegradability, biocompatibility, low
toxicity and the ability to encapsulate hydrophilic and hydrophobic
drugs, liposomes have gained attention in studies investigating
targeted delivery systems for cancer agents. They function by
directly targeting the tumor sites (3), and have been developed as a
multifunctional drug carrier as they are able to prolong systemic
drug circulation, enhance drug accumulation at the target tissue,
increase levels of cellular internalization and provide
organelle-specific drug delivery (4).
These strategies have developed from controlled drug
delivery systems to local controlled drug delivery systems for oral
cancer therapy. Previous developments in drug delivery through the
oral mucosa via bio-adhesive polymers gained notable attention. The
oral cavity offers a unique route for drug delivery, due to its
relatively increased permeability to drugs, drug absorption,
avoidance of hepatic first pass metabolism and enhances
bioavailability (5). The preferred
forms of administration via the oral cavity are adhesive gels,
films, patches and tablets intended for local drug delivery
(6–9).
Oral strips were developed for local application via
the tongue or buccal cavity. Mucoadhesive buccal patches offered
benefits compared with conventional methods (10). Due to the short residence time of oral
gels in oral cavity, buccal patches have advantages of increased
residence time and drug release (5).
For the development of controlled-release mucoadhesive buccal
patches, the time of contact of drug and tumor cells present in
oral mucosa are important aspects for consideration. Though oral
mucosa is more permeable compared with skin, the buccal mucosa
exhibits a limited permeation to drugs. The buccal mucosa exhibits
dual transport mechanisms, including a paracellular transport mode
for hydrophilic compounds and large molecules, and a transcellular
transport mode for lipophilic drugs that typically pass through the
lipid bilayer (11). To overcome the
permeation barrier in oral mucosa, the residence time of
mucoadhesive buccal patches on the buccal mucosa has been increased
to enhance the drug partitioning to the target tissue. Thereby,
such methods may effectively contribute to the development of a
sustained and targeted delivery system for drugs designed for
tissues in the oral cavity (5,12,13).
Previous studies on mucoadhesive polymers
demonstrated that buccal tablets prepared using chitosan exhibited
excellent mucoadhesive properties and a high capacity for drug
permeation through the buccal mucosa (11,14,15).
Mishra et al (16) developed
buccal patches, which consisted of a polymer combination of
hydroxypropyl methylcellulose (HPMC), poly (vinyl alcohol) (PVA)
and sodium carboxymethyl cellulose. These patches were evaluated to
determine their physicochemical properties and in vitro
release profile. The results highlighted the bio-adhesive
performance of PVA and HPMC. Additionally, HPMC and PVA exhibited
an extended release of almost 40% of the drug in 12 h. Abbasi et
al (17) formulated a
doxorubicin-methotrexate (MTX)-loaded nanoparticle to attenuate
oral cancer growth, and evaluated them in an oral squamous cell
carcinoma (OSCC) rat model. Additionally, this study group
identified that the downregulation of matrix metalloproteinase 2
and receptor tyrosine-protein kinase ErbB-2 gene expression in an
OSCC rat model were responsible for the clinical outcomes observed
(17,18). MTX is a commonly used anticancer agent
for various types of cancer, including colon, breast, skin and head
and neck cancer (19–21). Due to the high toxicity of MTX,
various strategies have been attempted to formulate an effective
delivery of MTX with reduced side effects. Dhanikula et al
(22) developed modified
polyester-co-polyether dendrimers that encapsulated MTX and
performed a release study. Furthermore, another study investigated
the controlled release of MTX from intercalated nanocomposites
(23). In the present study, a
controlled-release buccal delivery system for MTX was designed,
where MTX was loaded into a liposome system, increasing the
retention time and release of MTX within the oral cavity, thereby
prolonging the therapeutic effect. The lipid vesicles enhance
anticancer efficiency of MTX and composite chitosan-HPMC-PVA as a
buccal patch, through efficient delivery of MTX at the oral mucosal
membrane. Consequently, a targeted delivery system for MTX was
designed, resulting in site-specific treatment of oral cancer. The
cytotoxicity results of the present study on HSC-3 cells suggest
that apoptosis is the underlying mechanism of action. Mitochondrial
depolarization and pro-oxidant effects were the primary events
observed in the MTX-chitosan-HPMC-PVA composite liposomes that
induced cell apoptosis in HSC-3 cells.
Materials and methods
Materials
Phosphatidylcholine (PC) from soybean lecithin was
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). MTX
was procured from Wako Pure Chemical Industries, Co., Ltd. (Osaka,
Japan). Cholesterol (CL), HPMC, hydroxyethyl cellulose (HEC), PVA,
poly (ethylene glycol) (PEG) and chitosan (CH) were all purchased
from Sigma-Aldrich; Merck KGaA.
Cell culture
The human oral squamous cell carcinoma HSC-3 cell
line was purchased from American Type Culture Collection (Manassas,
VA, USA). Cells were cultured in Dulbecco's Modified Eagle's Medium
(DMEM; Sangon Biotech Co., Ltd., Shanghai, China) containing 10%
fetal bovine serum (Sangon Biotech Co., Ltd.), 1% penicillin
streptomycin (10,000 U/ml penicillin and 10 mg/ml streptomycin;
Sangon Biotech Co., Ltd.) and 1% glutamine in tissue culture
flasks, at 37°C, 5% CO2 and 95% humidity.
Preparation of MTX-loaded
liposomes
MTX-loaded liposomes were formulated using the thin
film hydration method using various molar ratios of PC in the
presence of CL. Different molar ratios of PC/CL were mixed with PEG
400 and dissolved in 95% diethyl ether solvent and 99% chloroform
(Sigma-Aldrich; Merck KGaA; 1:1). The solution was transferred to a
round-bottomed flask and the thin lipid layer was obtained by
evaporating the solution in a rotary evaporator (50 rev/min) at
40°C. The lipid film was then lyophilized overnight at 25°C to
remove the remaining organic solvent. This lipid film was
rehydrated with acetate buffer solution (pH 5.5) containing MTX
solutions of various concentrations (0.5 and 1.0%, w/w), and the
resulting solution was agitated at 250 rev/min for 1 h at 25°C to
obtain stable and homogeneous liposomes. Three MTX-entrapped
liposomes (as denoted by ‘M-LP’, ‘M-LF’ and ‘M-LN’) were prepared,
the composition of which described in Table I. These MTX-entrapped liposomes (M-LP,
M-LF, M-LN) exhibited different concentrations of PC and CL
(Table I) and were sonicated in an
ice bath (30 min intervals) and extruded through membrane filters
in five cycles (twice through a nylon filter at 0.45 mm and three
times through a cellulose acetate filter at 0.20 mm) to reduce
particle size in order to be suitable for mucoadhesive delivery
systems (24). The dispersions of
M-LF, M-LN and M-LP were maintained at 4°C (25) and were characterized to obtain the
final optimized MTX-loaded liposomes.
 | Table I.Characterization of MTX-loaded
liposomes. |
Table I.
Characterization of MTX-loaded
liposomes.
| Formulation | PC:CL: PEG 400 | MTX: lipid | Particle size,
nm | PDI | Zeta potential,
mV | Entrapment
efficiency, % |
|---|
| M-LF | 55:40:5 | 1:20 | 105.7±5.5 | 0.13 | 8.1±3.7 | 54.6±3.5 |
| M-LN | 60:35:5 | 1:20 | 111.8±2.8 | 0.34 | 22.4±1.2 | 67.2±1.5 |
| M-LP | 60:35:5 | 1:10 | 137.4±2.6 | 0.31 | 36.0±3.1 | 73.4±1.7 |
Colloidal characterization of
liposomes
The particle size, polydispersity index and zeta
potential of M-LF, M-LN and M-LP were estimated using a Malvern
Zetasizer Nano ZS™ (Malvern Instruments, Malvern, UK). The
liposomes were diluted 20 times with deionized water and evaluated.
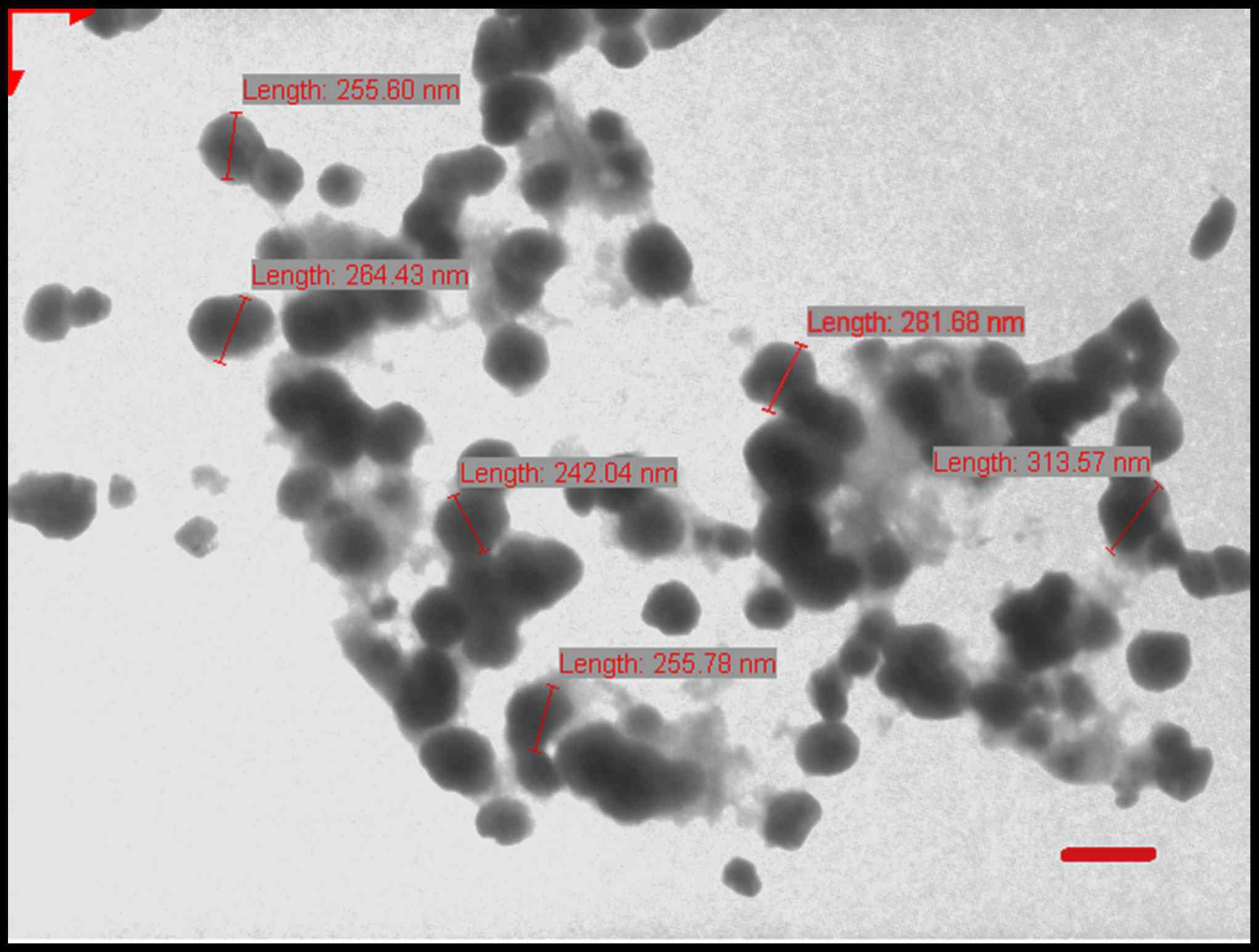
Morphological surface characteristics of the LPs were examined
using transmission electron microscopy (Jeol-100CX; JEOL, Ltd.,
Tokyo, Japan) operating at 100 kV. A drop of sample (5–10 µl) was
positioned on a 300-mesh carbon-coated copper grid to form a thin
liquid film. Excess sample was removed by gently touching the grid
with a filter paper. The negative staining of the films was
performed using 1% (w/v) uranyl acetate at 25°C for 60 sec by
placing a drop of stain to the grid. After 10 sec the excess stain
was removed by touching the edge to a filter paper. The extra
staining solution was cleared with a filter paper and allowed to
air-dry. The stained films were photographed using transmission
electron microscope. All procedures were carried out at 25°C
(Fig. 1).
High-performance liquid chromatography
(HPLC)
MTX-loaded liposomes were quantified using HPLC. The
samples were analyzed on a HPLC system (Waters Alliance, Milford,
MA, USA) using an RP C18 column (250×4.6 mm, particle size 5 µm;
Phenomenex, Torrance, CA, USA) at 25°C. The mobile phase used for
MTX detection and quantification was acetate buffer [acetonitrile
solution (90:10) at flow rate of 1 ml/min and 20 µl injection
volume], and the wavelength for detection was 302 nm using Waters
Tunable UV/Visible Absorbance Detector (Waters Alliance) (26).
Determination of drug entrapment
efficiency
MTX-loaded liposomes were evaluated for percentage
entrapment efficiency (%EE) by vesicle lysis with 0.2% (v/v)
Triton™ X 100 solution, and the lysed suspension was centrifuged at
12,500 × g and 5°C for 15 min. The supernatant was collected for
analysis of the MTX content via the aforementioned HPLC method. The
experiment was performed in triplicate. Experimental and
theoretical percentages of MTX loading were calculated using the
following formula: Percentage entrapment=[amount of drug in
liposomes/total amount of drug added]x100. Based on the highest
encapsulation efficiency with lowest lipid concentration, M-LP was
selected to be loaded into the mucoadhesive films.
Preparation of buccal mucoadhesive
films
A solvent casting method using various polymer
solutions formulated the buccal mucoadhesive films: CH (1% w/v);
HPMC (1% w/v); PVA (1% w/v). The casting solution was a mixture of
three ratios of these polymers left overnight at room temperature
to ensure a clear, bubble-free viscous mixture. The mixture was
cast into a glass Petri dish and allowed to dry at room temperature
(25°C) and 40–45% humidity for 24–48 h until a flexible film was
formed. The films with different compositions were cast as F1 to F7
and their characteristics are presented in Table II. The films were optimized for
physical characteristics including thickness, average weight and
percentage swelling (Table III).
Other parameters including softness, flexibility, translucence and
malleability were also taken into account. The F7 film was
identified to be the most translucent and flexible, with highest
swelling index and average thickness. Thus, the F7 film was
selected to prepare the MTX- and M-LP-loaded mucoadhesive film. The
MTX and M-LP-dispersion was added to the polymer dispersion under
continuous stirring to form M-F7 and M-LP-F7 polymeric suspensions,
respectively. The PEG 400 served as a plasticizer for the films.
The gels formed were maintained in desiccators overnight at room
temperature to ensure a bubble-free and clear casting solution, and
then cast into glass Petri dishes. The cast polymer solution was
allowed to dry at room temperature. Dried M-F7 and M-LP-F7 films
measuring 1 cm2 were packed in aluminum foil, and stored
in desiccators at room temperature (27).
 | Table II.Composition of mucoadhesive buccal
films. |
Table II.
Composition of mucoadhesive buccal
films.
| Formulation | Chitosan, %
w/w | HPMC, % w/w | PVA, % w/w | Physical
characteristics of film |
|---|
| F1 | 99 | 0 | 0 | Stiff and opaque,
malleable |
| F2 | 75 | 24 | 0 | Stiff and opaque,
malleable |
| F3 | 25 | 74 | 0 | Less stiff and
opaque, malleable |
| F4 | 50 | 49 | 0 | Little flexibility
but opaque, malleable |
| F5 | 75 | 0 | 24 | Low flexibility
translucent, malleable |
| F6 | 50 | 0 | 49 | Low flexibility,
translucent, malleable |
| F7 | 25 | 50 | 24 | Soft, flexile,
translucent and malleable |
 | Table III.Physical characteristics of
mucoadhesive films at room temperature. |
Table III.
Physical characteristics of
mucoadhesive films at room temperature.
| Formulation | Thickness ± SD,
mm | Average weight ±
SD, g | Swelling, % |
|---|
| F1 | 0.45±0.023 | 1.063±0.076 | 93.2 |
| F2 | 0.44±0.022 | 0.953±0.126 | 85.4 |
| F3 | 0.36±0.031 | 0.532±0.018 | 62.13 |
| F4 | 0.46±0.01 | 0.678±0.07 | 74.3 |
| F5 | 0.52±0.025 | 0.872±0.03 | 76.5 |
| F6 | 0.55±0.015 | 0.77±0.015 | 94.6 |
| F7 | 0.42±0.037 | 0.681±0.024 | 96.5 |
Pharmaceutical evaluation of mucoadhesive
films
Weight uniformity
Weight uniformity was determined by weighing six
films of each set individually, and the average weight was
determined with the standard deviation.
Film thickness
The thickness of the circular films was measured at
five distinct positions (center and four places at the
circumference) at the surface of each film using a screw gauge, and
the mean value was used as the film thickness.
Determination of the swelling indices
of films in distilled water
The swelling indices of the films were determined:
Films were coated with ethyl cellulose on the lower base so that
sticking of film to dish was avoided. The films were re-weighed
(W1) and allowed to swell in Petri dishes containing 10 ml of
distilled water. The films were incubated at 30°C in distilled
water for 30 min and stored at room temperature. Following this,
the films were re-weighed (W2), and the percentage of swelling was
calculated using the following formula: Swelling index = (W2-W1/W1)
× 100 (28).
In vitro release of compound from
different film
In vitro release of MTX from M-LP, M-F7 and
M-LP-F7 were assessed using the dialysis bag method at 37±0.5°C.
The formulations M-LP, M-F7 and M-LP-F7 were placed in the dialysis
bag separately and suspended in 20 ml simulated saliva (pH
6.75±0.05) in separate beakers (simulated saliva was prepared by
dissolving 2.38 g Na2HPO4, 0.19 g
KH2PO4 and 8.0 g NaCl in 1,000 ml distilled
water). Each beaker was maintained on a magnetic stirrer rotating
at 250 rpm. After 0.5, 1, 2, 3, 4, 5 and 6 h intervals, a 0.5 ml
sample was taken from each beaker, filtered through a Millipore
filter (0.45 mm) and analyzed using HPLC. A similar volume of fresh
medium was added to each sample immediately following this to
maintain a constant medium volume.
In vitro cell growth-inhibition
assay
The cytotoxicity of MTX, M-LP and M-LP-F7 was
evaluated in HSC-3 cells by an MTT assay. The HSC-3 cells were
cultured in 96-well plates in DMEM media at a density of
29×103 cells/well and were incubated for 24 h at 37°C.
Following this, cells were treated with MTX, M-LP and M-LP-F7 at
equivalent concentrations of MTX (50, 100 and 200 µg/ml) and
incubated at 37°C for an additional 24 h. At the end of the
incubation time, the cells were incubated again at 37°C with MTT
reagent (8 µl, 5 mg/ml) for 2 h, and finally optical density was
read with an ELISA plate reader (Molecular Devices, LLC, Sunnyvale,
CA, USA) at a wavelength of 540 nm. The half maximal inhibitory
concentration (IC50) values were calculated from the
mean absorbance at 490 nm (29).
Cell apoptosis study
Flow cytometric analysis of apoptosis
in HSC-3 cells using Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) assay
The apoptotic effects of MTX and M-LP-F7 was studied
in HSC-3 cells by quantitative discrimination of live cells, early
apoptotic, late apoptotic and necrotic cells using dual staining
with the Annexin V-FITC/PI dye. Briefly, the HSC-3 cells were
treated with MTX and M-LP-F7 having an equivalent concentration of
70 µg/ml MTX and incubated at 37°C for 24 h. Following incubation,
cells were washed and collected in PBS and stained with Annexin
V-FITC/PI according to the manufacturer's protocol (kit supplied by
Sangon Biotech Co., Ltd.) cells were trypsinized (1× trypsin) and
collected via centrifugation at 300 × g at 25°C for 5 min. Cells
were suspended in 400 µl 1× Annexin V solvent. In total, 5 µl
Annexin V-FITC was added to the suspension and incubated for 15 min
at 2–8°C. Furthermore, 10 µl PI was added to the solution and
incubated for 5 min at 2–8°C. Flow cytometric analysis was carried
out immediately following the addition of PI using a FACS Calibur
instrument (BD Biosciences, San Jose, CA, USA). The data were
analyzed using FlowJo software (version 7.6; BD Biosciences).
Determination of mitochondrial
membrane potential (ΔΨm) in HSC-3 cells using flow cytometry
The apoptotic mechanism was investigated in terms of
change in ΔΨm. The JC1 dye was utilized to monitor changes in ΔΨm;
in live cells, JC1 fluoresces red, whereas in dead cells the
florescence changes to green. The cells were cultured in the same
conditions as in the cell apoptosis study. Following 24 h of
treatment with equivalent MTX concentration of 70 µg/ml for MTX and
M-LP-F7, the HSC-3 cells were collected and incubated at 37°C with
1 µl JC1 dye in 500 µl incubation buffer for 1 h, followed by
centrifugation at 300 × g for 10 min at room temperature. The cell
pellet was washed with PBS and reconstituted with 500 µl incubation
buffer and analyzed using flow cytometry (30,31).
Detection of intracellular reactive
oxygen species (ROS) in HSC-3 cells using flow cytometry by
2′,7′-dichlorofluorescin diacetate (DCFDA) assay
The levels of intracellular ROS were evaluated to
determine the effect of M-LP-F7 on ROS-mediated apoptosis in HSC-3
cells. The HSC-3 cells were treated with M-LP-F7 at an equivalent
concentration of MTX (70 µg/ml) for 24 h in order to the determine
extent of ROS production. Cells were extracted in PBS, washed three
times and incubated at 37°C with 5 µM DCFDA for 30 min.
Subsequently, cells were centrifuged at 300 × g for 10 min and
re-suspended in 500 µl PBS prior to flow cytometry analysis.
Hydrogen peroxide was used as the positive control (30,31).
Statistical analysis
All results are expressed as the mean ± standard
deviation (n=3). Differences between formulations were compared
with one-way analysis of variance followed by the Tukey-Kramer
multiple comparisons test, using Graph Pad Prism (version 5;
GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Size, polydispersity index, and zeta
potential of MTX-loaded liposomes (M-LF, M-LN, M-LP)
The average sizes of MTX-loaded liposomes (M-LF,
M-LN, M-LP) varied with the variation in the ratio of lipids. The
mean size of liposomes increased with the ratio of PC.
Additionally, the percentage of MTX used for loading also affected
the liposome size. The size of liposomal formulations ranged
between 105.7 and 137.4 nm (Table I).
For M-LF liposomes with MTX/lipid ratio of 1:20, the vesicle size
was 105.7±5.5 nm, zeta potential was 8.1±3.7 mV with %EE 54.6±3.5.
Increases in the ratio of PC in M-LN and M-LP, resulted in
increases in the size of vesicles to between 111.8±2.8 and
137.4±2.6 nm, respectively. The zeta potential of liposomes also
changed towards more positive values, and %EE was augmented in M-LN
and M-LP.
Characteristics of LP-film
The three polymers, CH, HPMC and PVA, were used for
the development of mucoadhesive buccal films. The casting solution
was a blend of these in various ratios (Table II). The physical attributes of the
films were characterized and the results are summarized in Table III. As the chitosan polymeric
solution was viscous, the developed films were pale yellow colored,
opaque and slightly hard, but varying the ratio of CH along with
HPMC and PVA improved the texture of the film and malleability. The
HEC film formulation was difficult to remove from the casting
surface and possessed a non-homogeneous surface due to the presence
of entrapped air bubbles. The polymeric solutions of films
containing PVA were less viscous and air bubbles were easily
removed, creating a homogeneous film surface. These films were
transparent, malleable and flexible, but were not soft and sharp
edges were formed on folding. The concentration of PVA was
optimized in CH and HPMC polymer combinations in order to obtain
mucoadhesive films which possessed the desired softness and
flexibility.
The thickness and weight of the formulations varied
from 0.36–0.55 mm and 0.532–1.063 g, respectively. The composition
of the casting solution decided the thickness and weight of the
film formulations. As demonstrated in Table III, the film formulations with a
high concentration of CH exhibited increased weight and thickness
compared with the other two polymers. The thickness of the films
with different concentrations of polymers designated (F1 to F7)
were arranged into the following order:
F1>F5>F2>F6>F4>F7>F3. The weights of the films
were arranged into the following order:
F1>F5>F2>F6>F7>F4>F3.
The percentage swelling index refers to the volume
subsequent to swelling in aqueous liquid under pre-determined
conditions including the weight of the film, temperature and
humidity of the environment. The percentage swelling index of the
films was evaluated by comparing the pre-weight and post weight of
each film, and the effect of swelling on particle release. The
mucoadhesive polymers swelled when exposed to water, and a
consequently a weak network was formed at the bio-adhesive sites,
resulting in bio-adhesion. The percentage swelling of the
mucoadhesive film containing different polymers is summarized in
Table III. The order of the
percentage swelling of films with different concentration of
polymers designated from F1 to F7 was:
F7>F6>F1>F2>F5>F4>F3.
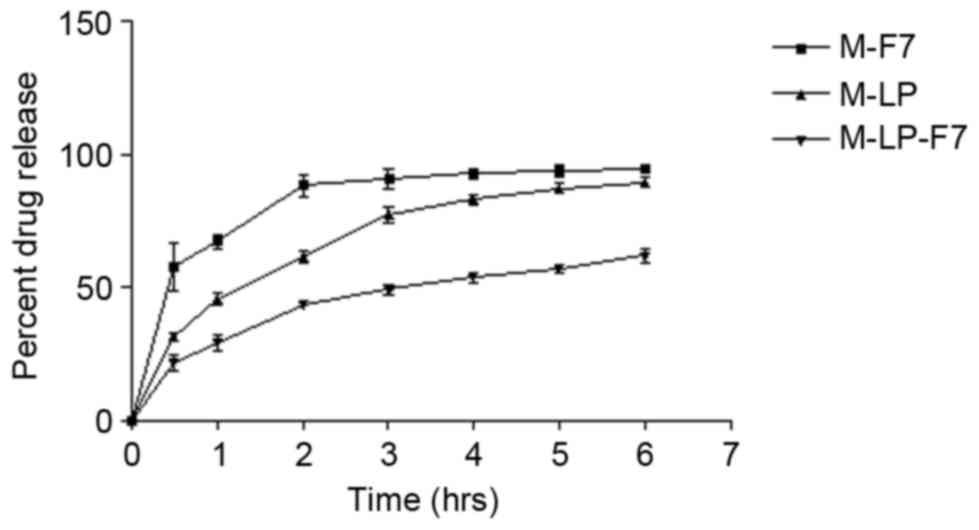
The in vitro release profiles of M-F7, M-LP
and M-LP-F7 were compared in Fig. 2.
As indicated, M-LP-F7 demonstrated a slower release profile
compared with the M-F7 and M-LP films. The sustained release
profile of M-LP-F7 may be attributed to the fact that CH maintained
the integrity of the incorporated vesicles. After 6 h, M-LP-F7
released ~52% of drug, whereas M-F7 and M-LP released 73 and 81%,
respectively.
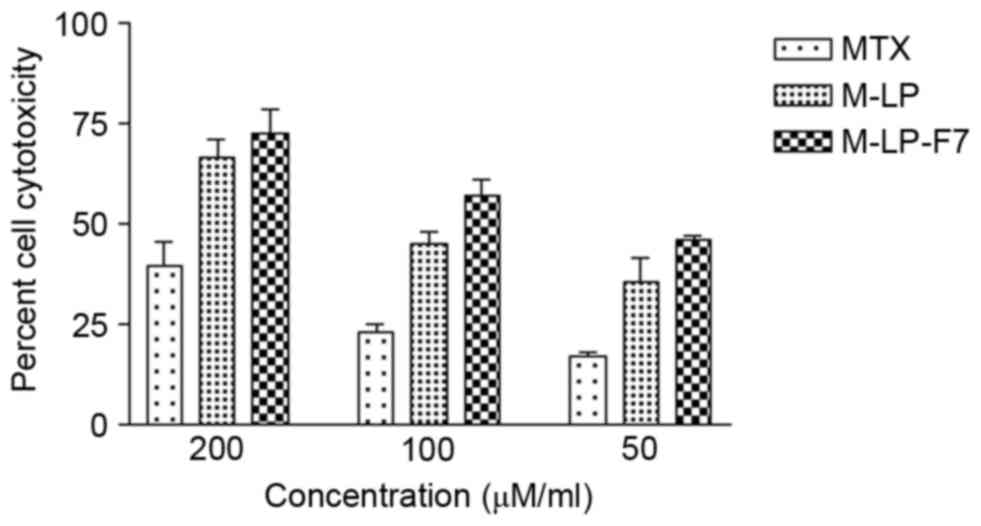
Cell culture assays
In vitro cytotoxicity study of
MTX-loaded liposome-based films
The developed liposomes and liposomes cast in film
formulation were evaluated for cytotoxicity on HSC-3 cells by MTT
assay. MTX, M-LP and M-LP-F7 were incubated with HSC-3 cells at
equivalent concentration of MTX ranging from 50 to 200 µg/ml for 24
h. The IC50 values of MTX, M-LP and M-LP-F7 were
compared, and a significant decrease in the IC50 of MTX
was identified with 75 µg/ml M-LP-F7, as compared with 142 µg/ml
M-LP and 180 µg/ml MTX alone (Fig.
3).
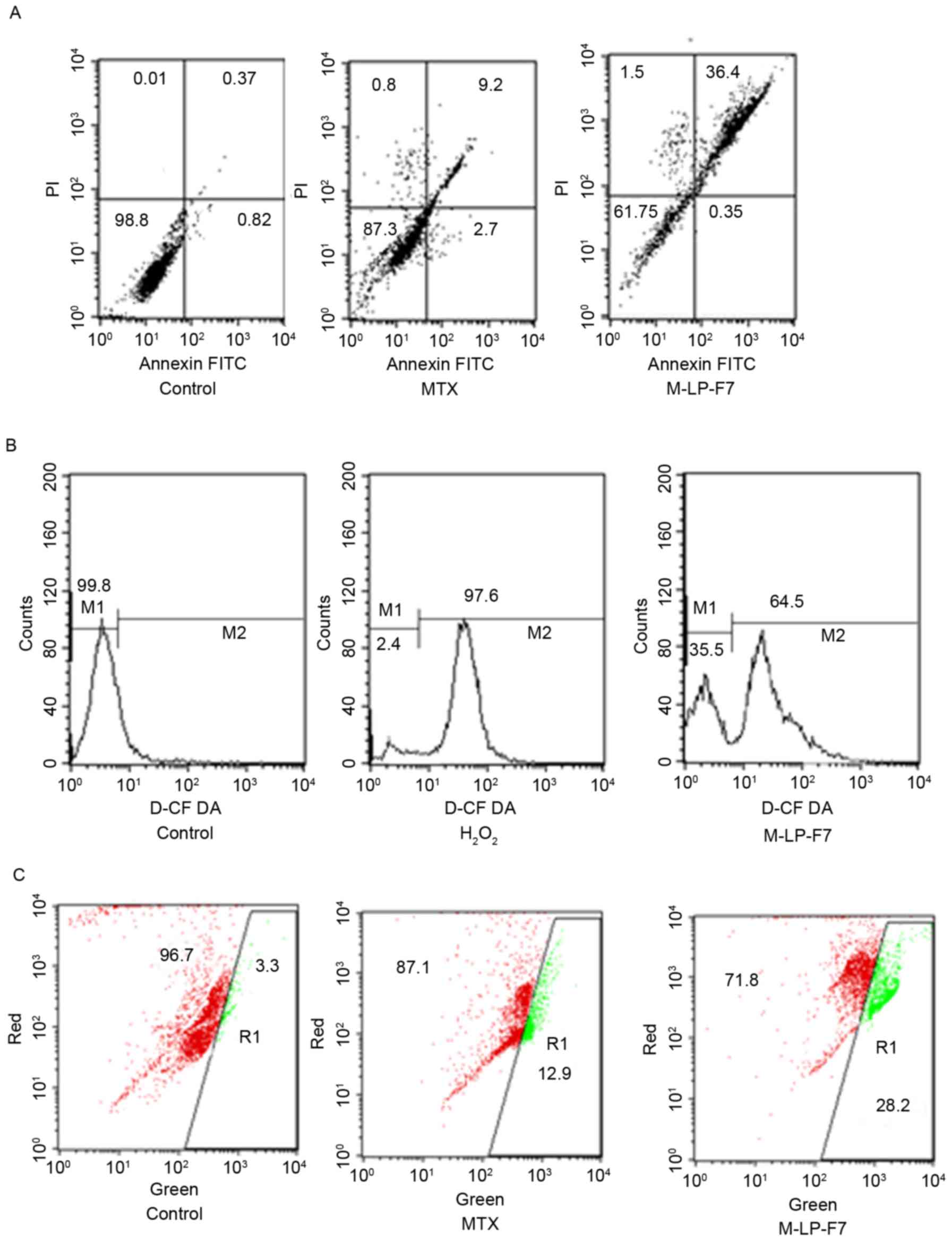
Apoptosis study in HSC-3 cells
The apoptotic effect of MTX, alone and in
formulation, on HSC-3 cancer cells was determined via flow
cytometry in order to detect the total live cell and apoptotic cell
populations. Apoptosis was evaluated using a Annexin V-FITC/PI
assay and dual staining with Annexin V and PI. Fig. 4A demonstrates that the upper right
quadrant represents Annexin V-positive and PI-positive cells. The
M-LP-F7 system increased the rate of HSC-3 cell apoptosis almost
3-fold (Fig. 4A).
Assay of intracellular ROS
Intracellular ROS are responsible for apoptosis in
cancer cells; therefore, raising ROS levels in cancer cells is an
approach for cancer therapy, termed the pro-oxidant effect. The
pro-oxidant effect of M-LP-F7 was measured using a DCFDA assay. The
results demonstrated marked ROS generation by M-LP-F7 compared with
H2O2, with an almost 68% shift in the M2
peak, as demonstrated in Fig. 4B.
Increased ROS levels provided evidence that M-LP-F7 exerts a
pro-oxidant effect in HSC-3 cells.
Mitochondria-dependent intrinsic
pathway as evaluated using a JC1 assay
Mitochondrial depolarization is considered the
intrinsic pathway for apoptosis; therefore, changes in ΔΨm were
evaluated using the florescent probe JC1. The effect of M-LP-F7 in
HSC-3 cells was evaluated in terms of ΔΨm disruption. The results
indicated that the ΔΨm disruption by M-LP-F7 was significant
(P<0.05), and increased 2-fold in comparison with MTX alone
following treatment with an equivalent concentration of MTX (70
µg/ml; Fig. 4C).
Discussion
MTX is a common drug with antifolate characteristics
that is used for cancer chemotherapy. In addition to the
therapeutic effects, it also causes unavoidable adverse effects.
Various strategies have been suggested to reduce the toxic effects
of MTX. Amongst these, the design of lipid-based and polymer-based
carriers has been successful. The present study aimed to develop a
lipid-based local delivery system for oral cancer. To achieve this,
MTX-loaded lipid vesicles were designed and cast in a polymer
solution to develop liposome-laden mucoadhesive films. This
formulation system has various benefits, as the liposomes
contribute to enhancing MTX bioavailability, as well as the
site-specific delivery offered by mucoadhesive patches. The lipid
vesicles were constructed in a size range of between 105.7±5.5 and
137.4±2.6 nm. The size of the vesicles was increased with the
increasing concentration of PC. The zeta potential also varied with
the change in the PC and CL concentration, and size uniformity was
attained at a drug/polymer ratio of 1:10 in the formulation M-LP.
The increased %EE in M-LP and M-LN depended on various factors,
including the method of preparation, vesicle size and lamellarity,
drug physicochemical properties, lipid concentration and drug-lipid
interactions (32–34). M-LP was identified as the optimized
MTX-loaded liposome based on increased %EE with lowest drug/polymer
ratio. M-LP was used for the preparation of liposome-laden buccal
mucoadhesive films.
Following the successful fabrication of MTX-loaded
liposomes (M-LP), a polymeric system was utilized for formulating
MTX liposome-loaded buccal films. The hydrophilic polymers HEC,
HPMC and hydrogel CH were used in different combinations and
evaluated to improve the film formulation properties. A CH/HPMC/PVA
polymer composition of 25:50:24 was optimized to develop soft,
flexible and malleable films.
Mucoadhesive polymers, including HPMC, HEC and CH,
have potential in the development of film formulation as they form
a swellable polymeric matrix to control drug release. PEG 400 was
used as a plasticizer and stabilizer. The cellulose-derived
polymers possessed high bio-adhesion properties, which may decrease
formulation leakage and disorderliness. The developed films were
suitable in terms of mechanical properties and malleability, with
good aesthetic and formulation performance (12). Additionally, film bio-adhesion depends
on the swelling behavior of the polymers. As the mucoadhesive
polymers become hydrated, a proper macromolecular mesh-like
biodegradable polymer network formed, which enhanced the
interpenetration of polymers and bio-adhesive strength (35,36). In
the developed film formulations, HPMC films were the most fragile
and easily erodible films due to the highest swelling of the HPMC
polymer (37), whereas CH
strengthened the polymer network and reduced the erosion capacity
of the films, alongside a controlled release of the drug (38). Films with different polymer
concentrations were prepared (F1 to F7) and were characterized
based on physiochemical properties. The F7 film was soft, flexible,
translucent and had weight uniformity. Thus, F7 film was used to
prepare MTX- and M-LP-laden buccal mucoadhesive films.
The cytotoxicity study on HSC-3 cells revealed a
marked decrease in the IC50 of MTX in lipid vesicles
(M-LP), and further in buccal patch M-LP-F7. This may be attributed
to the enhanced permeability of the MTX from M-LP and M-LP-F7 into
the cells. The HSC-3 cell line is highly invasive; therefore, the
present study aimed to detect the mechanism of MTX-induced
apoptosis in HSC3 cells. MTX is active in oral cancer cells, but
exhibits marked limitations regarding cell uptake and site
specificity as it is associated with certain highly toxic effects
(17). This was also evaluated via an
apoptosis assay in HSC 3 cells; M-LP-F7 increased the percentage of
apoptotic cells 3-fold, compared with MTX alone. The results of the
assay also suggested that MTX contributes to the intrinsic
apoptotic pathway through changing ΔΨm, as evident in the JC1
assay, an event that is significantly responsible for cancer cell
apoptosis. M-LP-F7 was responsible for a 2-fold increase in
mitochondrial depolarization. The ΔΨm disruption was associated
with the pro-oxidant effect, which may correspond to ROS
accumulation (39). Therefore,
intracellular ROS levels were also evaluated in order to detect the
expected pro-oxidant mechanism of apoptosis by M-LP-F7. The DCFDA
assay indicated an ~68% peak shift (M2) due to the increased ROS
levels in HSC3 cells caused by M-LP-F7 (containing 70 µg/ml MTX),
in comparison with the positive control
H2O2.
In conclusion, oral mucoadhesive patches for the
delivery of MTX liposomes were produced and evaluated in HSC-3
cells to develop a chemotherapeutic delivery system for oral
cancer. CH-HPMC-PVA-based mucoadhesive buccal patches exhibited
suitable bio-adhesive properties, and prolonged the release of MTX.
Oral mucoadhesive patches for oral cancer may be exploited as an
effective approach to bypass the limitations of site-specific
delivery in oral cancer chemotherapy. Therefore, drug toxicity may
be reduced by lowering the dose required, suppressing toxicity and
adverse effects.
Acknowledgements
The present study was supported by the Zhejiang
Provincial Education Department (grant no. Y201432814).
References
|
1
|
Gavin A, Pham JT, Wang D, Brownlow B and
Elbayoumi TA: Layered nanoemulsions as mucoadhesive buccal systems
for controlled delivery of oral cancer therapeutics. Int J
Nanomedicine. 10:1569–1584. 2015.PubMed/NCBI
|
|
2
|
Calixto G, Bernegossi J, Fonseca-Santos B
and Chorilli M: Nanotechnology-based drug delivery systems for
treatment of oral cancer: A review. Int J Nanomedicine.
9:3719–3735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Strebhardt K and Ullrich A: Paul Ehrlich's
magic bullet concept: 100 years of progress. Nat Rev Cancer.
8:473–480. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deshpande PP, Biswas S and Torchilin VP:
Current trends in the use of liposomes for tumor targeting.
Nanomedicine (London). 8:1509–1528. 2013. View Article : Google Scholar
|
|
5
|
Reddy Chinna P, Chaitanya KS and Rao
Madhusudan Y: A review on bioadhesive buccal drug delivery systems:
Current status of formulation and evaluation methods. DARU.
19:385–403. 2011.PubMed/NCBI
|
|
6
|
Smart JD: Buccal drug delivery. Expert
opin drug deliv. 2:507–517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sudhakar Y, Kuotsu K and Bandyopadhyay AK:
Buccal bioadhesive drug delivery-a promising option for orally less
efficient drugs. J Control Release. 114:15–40. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mizrahi B and Domb AJ: Mucoadhesive
polymers for delivery of drugs to the oral cavity. Recent Pat Drug
Deliv Formul. 2:108–119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shojaei AH: Buccal mucosa as a route for
systemic drug delivery: a review. J Pharm Pharm Sci. 1:15–30.
1998.PubMed/NCBI
|
|
10
|
Adamczak MI, Hagesaether E, Smistad G and
Hiorth M: An in vitro study of mucoadhesion and biocompatibility of
polymer coated liposomes on HT29-MTX mucus-producing cells. Int J
Pharm. 498:225–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nguyen TX, Huang L, Gauthier M, Yang G and
Wang Q: Recent advances in liposome surface modification for oral
drug delivery. Nanomedicine. 11:1169–1185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boddupalli BM, Mohammed ZN, Nath RA and
Banji D: Mucoadhesive drug delivery system: An overview. J Adv
Pharm Technol Res. 1:381–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shaikh R, Singh Raj TR, Garland MJ,
Woolfson AD and Donnelly RF: Mucoadhesive drug delivery systems. J
Pharm Bioallied Sci. 3:89–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abruzzo A, Cerchiara T, Bigucci F,
Gallucci MC and Luppi B: Mucoadhesive buccal tablets based on
chitosan/gelatin microparticles for delivery of propranolol
hydrochloride. J Pharm Sci. 104:4365–4372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Steinberg D and Friedman M:
Sustained-release drug delivery of antimicrobials in controlling of
supragingival oral biofilms. Expert Opin Drug Deliv. 14:571–581.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mishra SK, Garud N and Singh R:
Development and evaluation of mucoadhesive buccal patches of
flurbiprofen. Acta Pol Pharm. 68:955–964. 2011.PubMed/NCBI
|
|
17
|
Abbasi MM, Monfaredan A, Hamishehkar H and
Jahanban-Esfahlan R: New formulated ‘DOX-MTX-loaded nanoparticles’
down-regulate HER2 gene expression and improve the clinical outcome
in OSCCs model in rat: The effect of IV and oral modalities. Asian
Pac J Cancer Prev. 15:9355–9360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abbasi MM, Jahanban-Esfahlan R, Monfaredan
A, Seidi K, Hamishehkar H and Khiavi MM: Oral and IV dosages of
doxorubicin-methotrexate loaded-nanoparticles inhibit progression
of oral cancer by down-regulation of matrix Methaloproteinase 2
expression in vivo. Asian Pac J Cancer Prev. 15:10705–10711. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cazzaniga ME, Dionisio MR and Riva F:
Metronomic chemotherapy for advanced breast cancer patients. Cancer
Lett. 400:252–258. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lima Costa SA, Gaspar A, Reis S and Durães
L: Multifunctional nanospheres for co-delivery of methotrexate and
mild hyperthermia to colon cancer cells. Mater Sci Eng C Mater Biol
Appl. 75:1420–1426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Merlano M, Benasso M, Cavallari M, Blengio
F and Rosso M: Chemotherapy in head and neck cancer. Eur J Cancer B
Oral Oncol. 30:283–289. 1994. View Article : Google Scholar
|
|
22
|
Dhanikula RS and Hildgen P: Influence of
molecular architecture of polyether-co-polyester dendrimers on the
encapsulation and release of methotrexate. Biomaterials.
28:3140–3152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alexa IF, Pastravanu CG, Ignat M and
Popovici E: A comparative study on long-term MTX controlled release
from intercalated nanocomposites for nanomedicine applications.
Colloids Surf B Biointerfaces. 106:135–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Verma DD, Verma S, Blume G and Fahr A:
Particle size of liposomes influences dermal delivery of substances
into skin. Int J Pharm. 258:141–151. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Srisuk P, Thongnopnua P, Raktanonchai U
and Kanokpanont S: Physico-chemical characteristics of
methotrexate-entrapped oleic acid-containing deformable liposomes
for in vitro transepidermal delivery targeting psoriasis treatment.
Int J Pharm. 427:426–434. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sharma M, Malik R, Verma A, Dwivedi P,
Banoth GS, Pandey N, Sarkar J, Mishra PR and Dwivedi AK: Folic acid
conjugated guar gum nanoparticles for targeting methotrexate to
colon cancer. J Biomed Nanotechnol. 9:96–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Desai KG, Mallery SR, Holpuch AS and
Schwendeman SP: Development and in vitro-in vivo evaluation of
fenretinide-loaded oral mucoadhesive patches for site-specific
chemoprevention of oral cancer. Pharm Res. 28:2599–2609. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Belec L, Tevi-Benissan C, Bianchi A,
Cotigny S, Beumont-Mauviel M, Si-Mohamed A and Malkin JE: In vitro
inactivation of Chlamydia trachomatis and of a panel of DNA (HSV-2,
CMV, adenovirus, BK virus) and RNA (RSV, enterovirus) viruses by
the spermicide benzalkonium chloride. J Antimicrob Chemother.
46:685–693. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumar R, Verma V, Sharma V, Jain A, Singh
V, Sarswat A, Maikhuri JP, Sharma VL and Gupta G: A precisely
substituted benzopyran targets androgen refractory prostate cancer
cells through selective modulation of estrogen receptors. Toxicol
Appl Pharmacol. 283:187–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singh V, Sharma V, Verma V, Pandey D,
Yadav SK, Maikhuri JP and Gupta G: Apigenin manipulates the
ubiquitin-proteasome system to rescue estrogen receptor-β from
degradation and induce apoptosis in prostate cancer cells. Eur J
Nutr. 54:1255–1267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin CC, Yang JS, Chen JT, Fan S, Yu FS,
Yang JL, Lu CC, Kao MC, Huang AC, Lu HF and Chung JG: Berberine
induces apoptosis in human HSC-3 oral cancer cells via simultaneous
activation of the death receptor-mediated and mitochondrial
pathway. Anticancer: res. 27:3371–3378. 2007.PubMed/NCBI
|
|
32
|
Adrian G and Huang L: Entrapment of
proteins in phosphatidylcholine vesicles. Biochemistry.
18:5610–5614. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun B and Chiu DT: Determination of the
encapsulation efficiency of individual vesicles using
single-vesicle photolysis and confocal single-molecule detection.
Analytical chemistry. 77:2770–2776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Akbarzadeh A, Rezaei-Sadabady R, Davaran
S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M and
Nejati-Koshki K: Liposome: Classification, preparation, and
applications. Nanoscale Res Lett. 8:1022013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peppas NA, Moynihan HJ and Lucht LM: The
structure of highly crosslinked poly (2-hydroxyethyl methacrylate)
hydrogels. J Biomed Mater Res. 19:397–411. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peppas NA, Thomas JB and McGinty J:
Molecular aspects of mucoadhesive carrier development for drug
delivery and improved absorption. J Biomater Sci Polym Ed. 20:1–20.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Perioli L, Ambrogi V, Angelici F, Ricci M,
Giovagnoli S, Capuccella M and Rossi C: Development of mucoadhesive
patches for buccal administration of ibuprofen. J Control Release.
99:73–82. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tonglairoum P, Ngawhirunpat T, Rojanarata
T1, Panomsuk S, Kaomongkolgit R and Opanasopit P: Fabrication of
mucoadhesive chitosan coated
polyvinylpyrrolidone/cyclodextrin/clotrimazole sandwich patches for
oral candidiasis. Carbohydr Polym. 132:173–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Suski JM, Lebiedzinska M, Bonora M, Pinton
P, Duszynski J and Wieckowski MR: Relation between mitochondrial
membrane potential and ROS formation. Methods Mol Biol.
810:183–205. 2012. View Article : Google Scholar : PubMed/NCBI
|