Introduction
Breast cancer is the most frequent carcinoma in
humans with the second-highest mortality rate among women (1). The initiation and progression of breast
cancer is a complicated multi-stage process in which certain
factors interact to disrupt normal cell growth and division. In
addition to the conventional prognostic indicators, including
clinical stage, tumor size and lymph node metastasis, other
biological indicators are being used to assess the prognosis of
patients with breast cancer, guiding clinical treatment (2).
Ki-67 is a nuclear antigen, identified by Gerdes
et al (3) as a nuclear
proliferative marker present in all stages of the cell cycle,
occurring maximally in M phase and absent from G0
(4,5).
Ki-67 has been demonstrated to be present in the early stage of the
breast cancer in previous studies (5–7).
Furthermore, Ki-67, acknowledged as a prognostic marker in breast
cancer, is commonly used to predict the magnitude of
chemotherapeutic benefits in clinical practice (8).
The rapid development of immunohistochemistry (IHC),
has led to it becoming a major supplementary tool for diagnosis and
research in a clinicopathological environment. Since the underlying
technology of IHC encompasses antigen-antibody interactions, a
high-sensitivity and -specificity antibody, in addition to an
imaging system are the key factors of the technique. The
development of semiconductor quantum dots (QDs) will lead to the
creation of an interdisciplinary field comprising bioassays and
bioimaging technologies (9–11). This may also result in substantial
advantages over the use of conventional antibody-based IHC assays,
which rely on organic fluorophores or fluorescent proteins. QDs are
able to emit adjustable light (broad excitation spectrum and narrow
emission spectrum) that may be dyed with a variety of fluorescent
dyes simultaneously under a single excitation wavelength (12). QDs may also be combined with different
target materials. QDs coupled to materials with similar imaging and
treatment function produce diverse biological functional probes
that may be concurrently used for tumor molecular imaging and
targeted therapy (13–15). Previous studies have assessed the
feasibility of using QDs in cancer diagnosis, molecular
classification, treatment and prognosis, and have established a
broad prospect in basic and clinical cancer research (16,17).
In the present study, a novel class of
poly(aspartate)-Na-graft-poly(ethylene glycol)-dodecylamine
(PASP-Na-g-PEG-DDA) was synthesized, which was used to convert the
hydrophobic octadecylamine-coated QD molecules into hydrophilic
forms through surface modification and then chemically conjugate
them to Ki-67 antibodies (QD-Ki-67 probes). Furthermore, the
applicability of the newly synthesized QD-Ki-67 probes was tested
in various breast cancer cell lines by comparing the light
stability between the QD-Ki-67 probes and organic dyes. The in
vitro cytotoxicity of the QD-Ki-67 probes was also
assessed.
Materials and methods
Materials
CuInS2/ZnS hydrophilic QDs
(λex=605 nm) were purchased from Ocean Nanotech LLC
(Springdale, AZ, USA). Water-soluble QDs (QDs encapsulated with
PASP-Na-g-PEG-DDA) were supplied by the Alan G. MacDiarmid
Institute of Jilin University (Changchun, China). The SP6 clone
mouse anti-human Ki-67 monoclonal antibody (cat. no. RMA-0542) was
obtained from Fuzhou Maixin Biotech Co., Ltd. (Fuzhou, China).
Immunoglobulin G (IgG) (from goat serum), bovine serum albumin
(BSA), dimethylsulfoxide (DMSO) and
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) were procured
from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany); DAPI was
acquired from Roche Applied Science (Penzberg, Germany); MTT was
procured from Beyotime Institute of Biotechnology (Nantong, China);
Bisphenol A (BPA), HCl and NaOH were purchased from Sinopharm
Chemical Reagent Co., Ltd. (Shanghai, China) and Shanghai Aladdin
Biochemical Technology Co., Ltd. (Shanghai, China). Human breast
cancer cell line MDA-MB-231 and a normal human mammary
microvascular endothelial cell (HMMEC) line were acquired from the
China-Japan Union Hospital, Jilin University (Changchun, China).
The synthesis of QDs was described previously (18–20).
Bioconjugation of
CuInS2/ZnS QDs with anti-Ki-67
A total of 500 µl antibody (0.1 mg/l SP6 clone mouse
anti-human Ki-67 monoclonal antibody was incubated with 500 µl EDC
and sulfo-N-hydroxysulfosuccinimide (0.1 mmol/l) for 15 min at room
temperature. This concoction was mixed with 500 µl water-soluble
QDs (50 mg) suspended in PBS (pH 7.4). The reaction mixture was
incubated at 4°C for 24 h. The antibody-coupled QDs were washed
with PBS to remove the excess antibody, producing QD-Ki-67 probes.
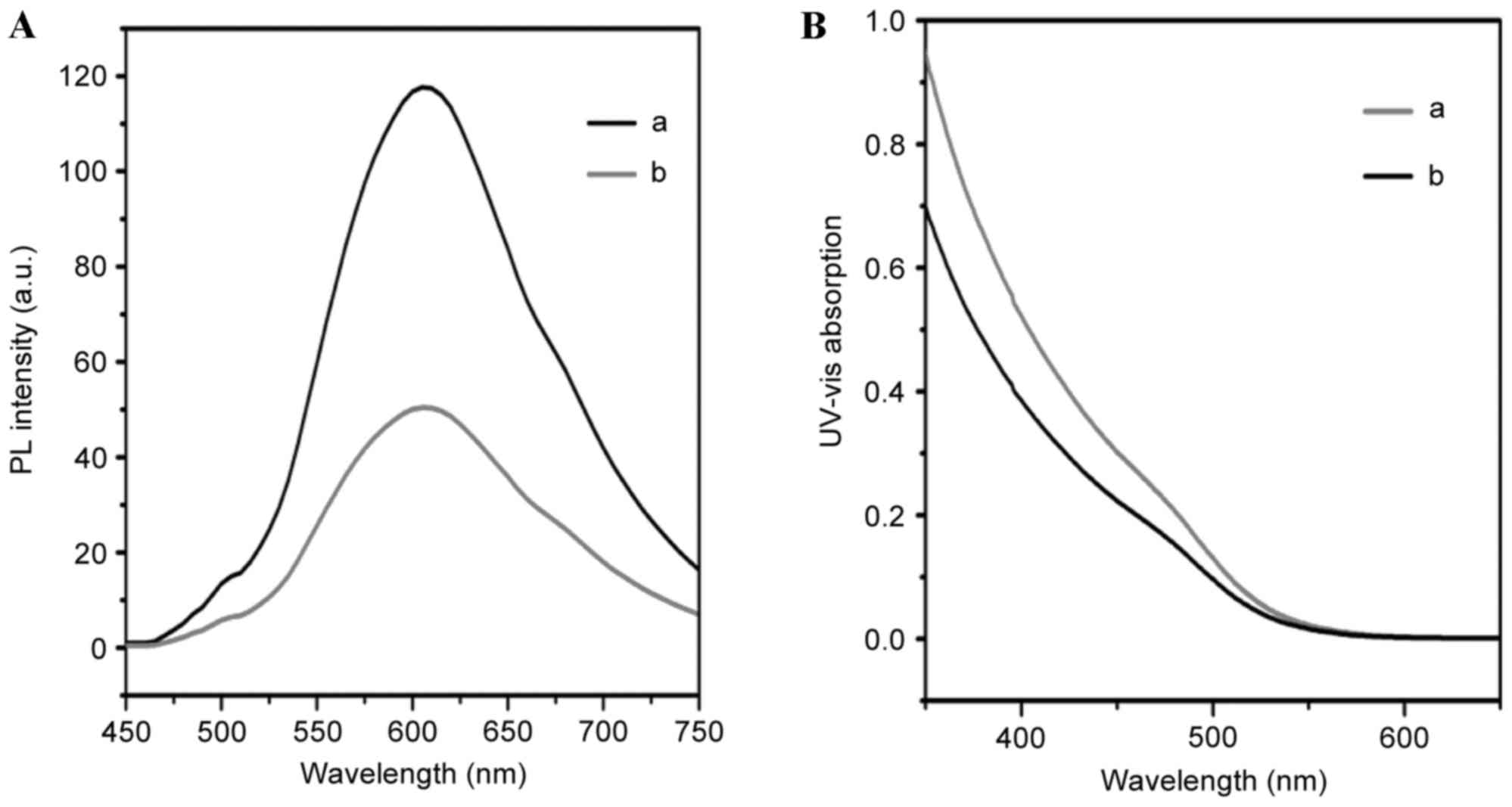
The formation of QD-Ki-67 was varied by photoluminescence (PL) and
UV. The characteristic peak of QDs appeared following Ki-67
antibody conjugation in and the shape of the peak was retained
during the reaction process. The UV-Vis absorption spectrum of the
QD-Ki-67 revealed the characteristic peaks of the QDs.
Cell culture and fixation
Human breast cancer MDA-MB-231 cells and HMMECs
(control) were incubated in 24-well plates (6×104
cells/well), for adherence, in high-glucose Dulbecco's modified
Eagle's medium (Shanghai Solarbio Bioscience & Technology Co.,
Ltd, Shanghai, China) or RPMI-1640 medium (Shanghai Solarbio
Bioscience & Technology Co., Ltd.), respectively, supplemented
with 10% fetal bovine serum (cat. no. 10100147; Gibco; Thermo
Fisher Scientific, Inc.) and 1% (v/v) penicillin/streptomycin. The
two cell lines were incubated in 5% CO2 at 37°C.
Subsequently, the cells were fixed with 4% formaldehyde for 20 min
at 37°C and blocked with 2% (w/v) BSA for 30 min at 37°C, followed
by permeabilization with 0.3% Triton X-100 for 10 min at room
temperature. Cells were washed three times with PBS for 5 min each.
Following washing, the samples were incubated with the QD-Ki-67
complex at 25°C for 1 h.
Imaging of labeled cells
Following fixation and blocking, the two cell
samples were incubated in a confocal Petri dish (37°C, 5%
CO2) for 24 h to allow for adherence to the wells.
Subsequently, 100 µl QD-Ki-67 probes (100 µg/ml) was added to the
dish, which was incubated at 37°C for 1 h. Concurrently, control
cells were treated similarly, but with mock-conjugated QDs (0.2
mg/ml). The cells were washed three times with PBS to prevent the
non-specific binding of QD-Ki-67 probes or QDs. Finally, 1 µg/ml
DAPI was used for nuclear staining at 37°C for 15 min and images
were captured using a laser-scanning confocal microscope with
excitation wavelength of 480 nm at room temperature. The cell
imaging analysis was performed using FV10-ASW 3.1 Viewer software
(Olympus Corporation, Tokyo, Japan).
Photostability comparison
Following fixing and blocking, the MDA-MB-231 cells
were incubated with mouse anti-human Ki-67 monoclonal antibody
(dilution, 1:1,000) at 37°C for 1 h, followed by incubation with
IgG as a secondary antibody (dilution, 1:500) at 37°C for 30 min.
Alternatively, the fixed and blocked MDA-MB-231 cells were
co-incubated with QD-Ki-67 probes for QD-based IHC detection. The
cell nuclei were counterstained with DAPI (1 µg/ml) at 37°C for 15
min. The fluorescence intensity of QDs was monitored and images
were captured using a laser-scanning confocal microscope camera at
1-min intervals for 10 min at the excitation wavelength of 480
nm.
Cytotoxicity analysis of
CuInS2/ZnS QDs
The cytotoxicity of the CuInS2/ZnS QDs
was elucidated using the MTT assay. Briefly, HMMECs and MDA-MB-231
cells were cultured in 96-well plates as aforementioned to achieve
a count of 1,000 cells/well. Next, the corresponding medium was
replenished with a range of QD-Ki-67 probe concentrations (0.1,
0.2, 0.3 and 0.4 mg/ml). After 24, 48, and 72 h, 10 µl MTT solution
(5 mg/ml) was mixed in each well prior to incubation for an
additional 4 h. The reaction was quenched by removing the
MTT-containing culture medium and adding 100 µl DMSO. The plate was
agitated for ~10 min and the optical density (OD) was estimated at
490 nm on an ELx800 Absorbance Microplate Reader (BioTek
Instruments, Inc., Winooski, VT, USA).
Statistical analysis
All quantitative values are expressed as the mean ±
standard error of the mean. Statistical analyses were performed
with SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA);
the different groups were compared by one-factor analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Structural characterization of
QD-Ki-67
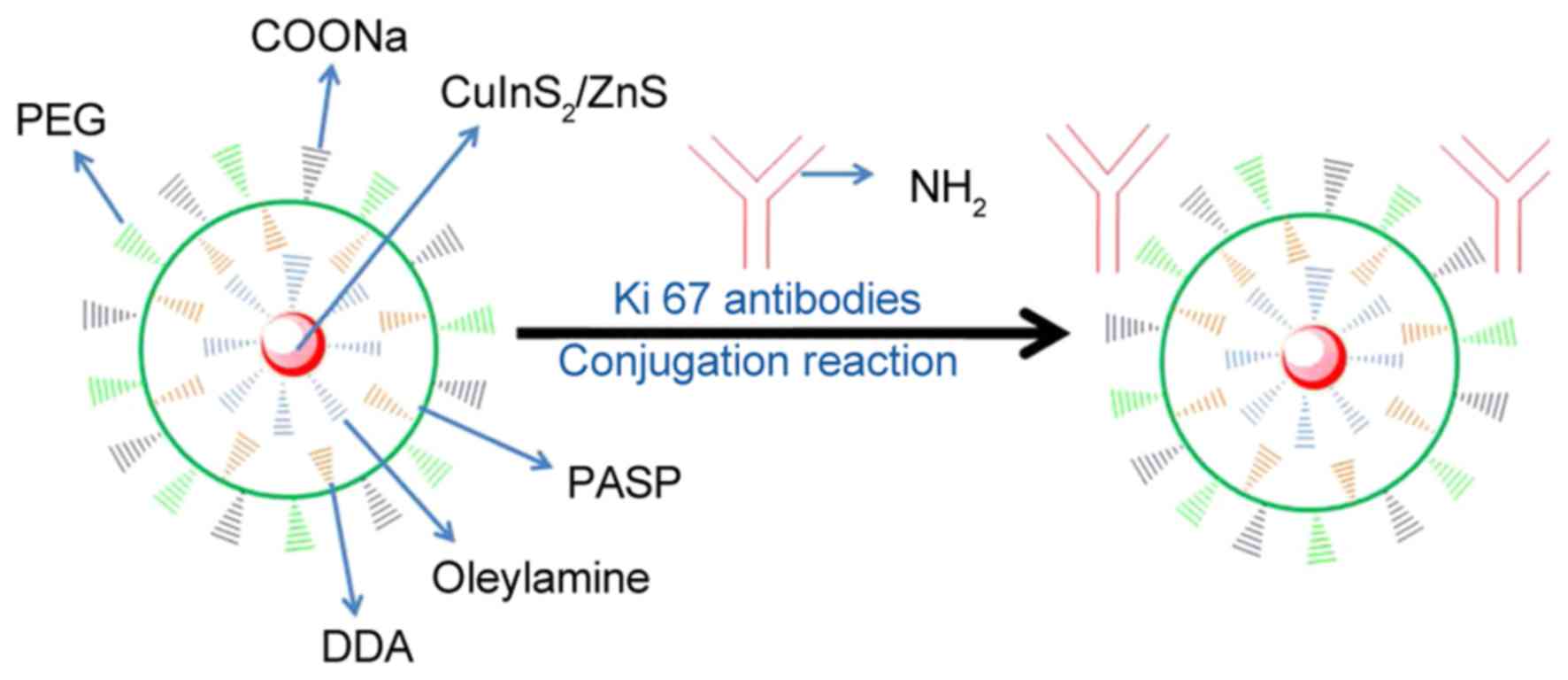
Fig. 1 presents a
schematic diagram of QD-Ki-67 conjugation. Fig. 2 presents the PL and UV confirmation of
the establishment of QD-Ki-67 probes.
Cell imaging
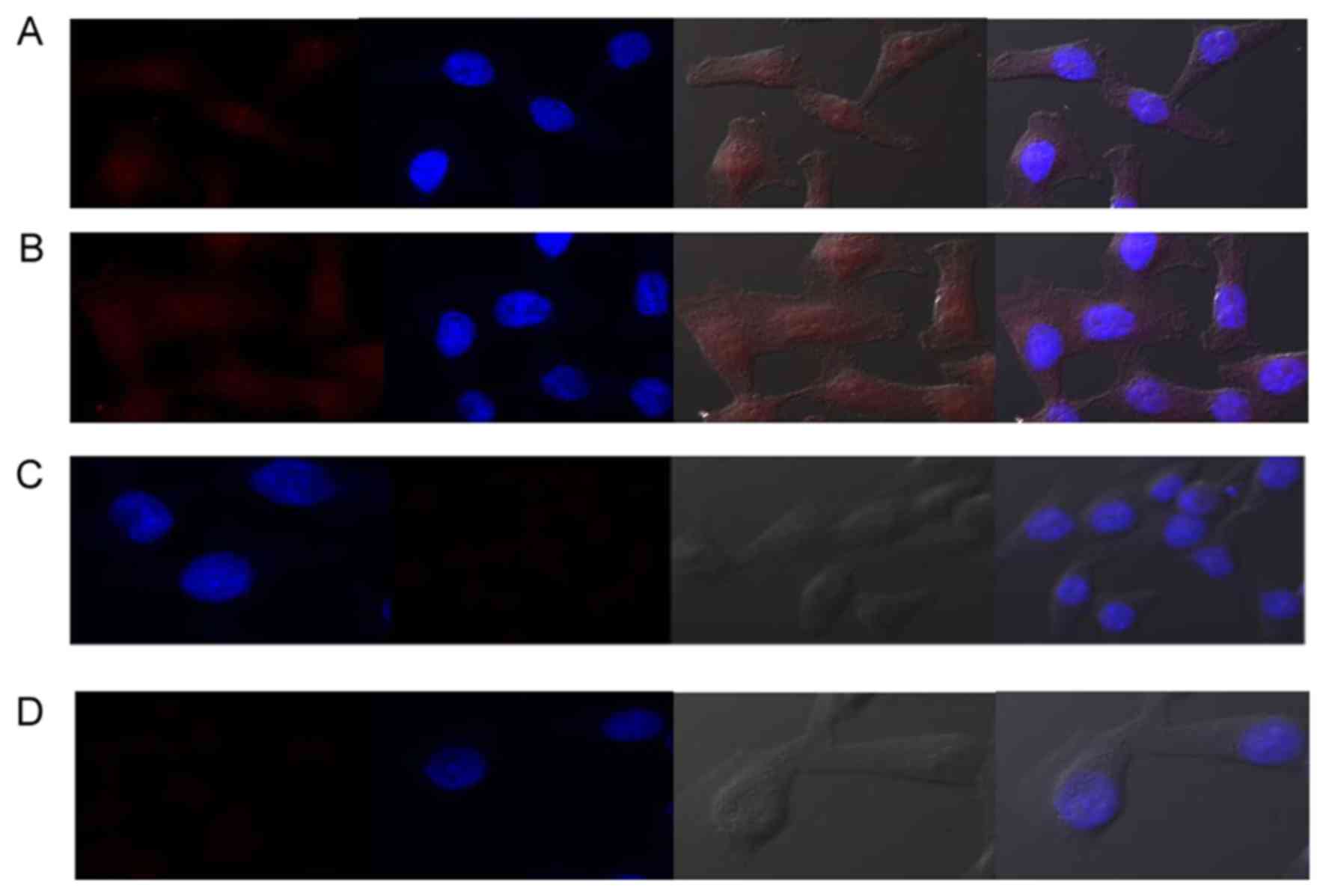
Fig. 3 presents the
fluorescent images of MD-MBA-231 cells and HMMECs labeled with
QD-Ki-67 probes and mock-conjugated with Qdot Blue indicates the
nuclear counterstaining with DAPI.
The properties of superior water-solubility,
excitation-dependent emission and high fluorescence quantum yield
render QD-Ki-67 probes as an attractive alternative for live cell
imaging. To substantiate the ability of the probe to combine with
Ki-67, MDA-MB-231 cells (Ki-67-high) and HMMECs (Ki-67-low) cell
lines were incubated with QD-Ki-67 probes, and the results were
obtained using laser-scanning confocal microscopy. All the images
were captured under identical parameters. Fig. 3A indicates that, using QD-Ki-67
probes, the fluorescence signal is homogeneously concentrated in
the nucleus, and the cytoplasmic labeling is minimal in MDA-MB-231
cells. Conversely, mock-conjugated QDs did not demonstrate a
homogeneous concentration in the nucleus (Fig. 3B). Furthermore, no fluorescence signal
was detected in MDA-MB-231 cells (Fig.
3C) or HMMECs (Fig. 3D) for the
blank controls.
QD-Ki-67 probes that entered into the MDA-MB-231
cells displayed multi-colored PL, whereas the shape and viability
of cells were not altered substantially, thereby indicating
successful cell labeling with QD-Ki-67 probes. The present study
indicated that the Ki-67 monoclonal antibody that was conjugated
with the quantum dots maintained the ability to form Ki-67-specific
antigen-antibody complexes. The QD-Ki-67 probes were able to
specifically bind to Ki-67 in the MDA-MB-231 cell nuclei in
vitro. Consecutively, the non-specific binding was minimal.
Therefore, this particular characteristic indicated the ability of
QD-Ki-67 probes to detect of Ki-67 in breast cancer cells.
In vitro cytotoxicity
The toxicity of QDs which contain metal components
such as cadmium ions is a serious concern and several groups have
attempted to identify the primary origins in order to resolve the
associated challenges (21). Certain
studies reported that the coating on the surface of QDs has a
substantial function in its emerging toxicity harbored in the
physiological system (22–25). Evaluating the viability of the cells
is the most common quantitative assay for assessing the toxicity of
any biological material (26,27). In the present study, an MTT assay was
employed for assessing the QD toxicity.
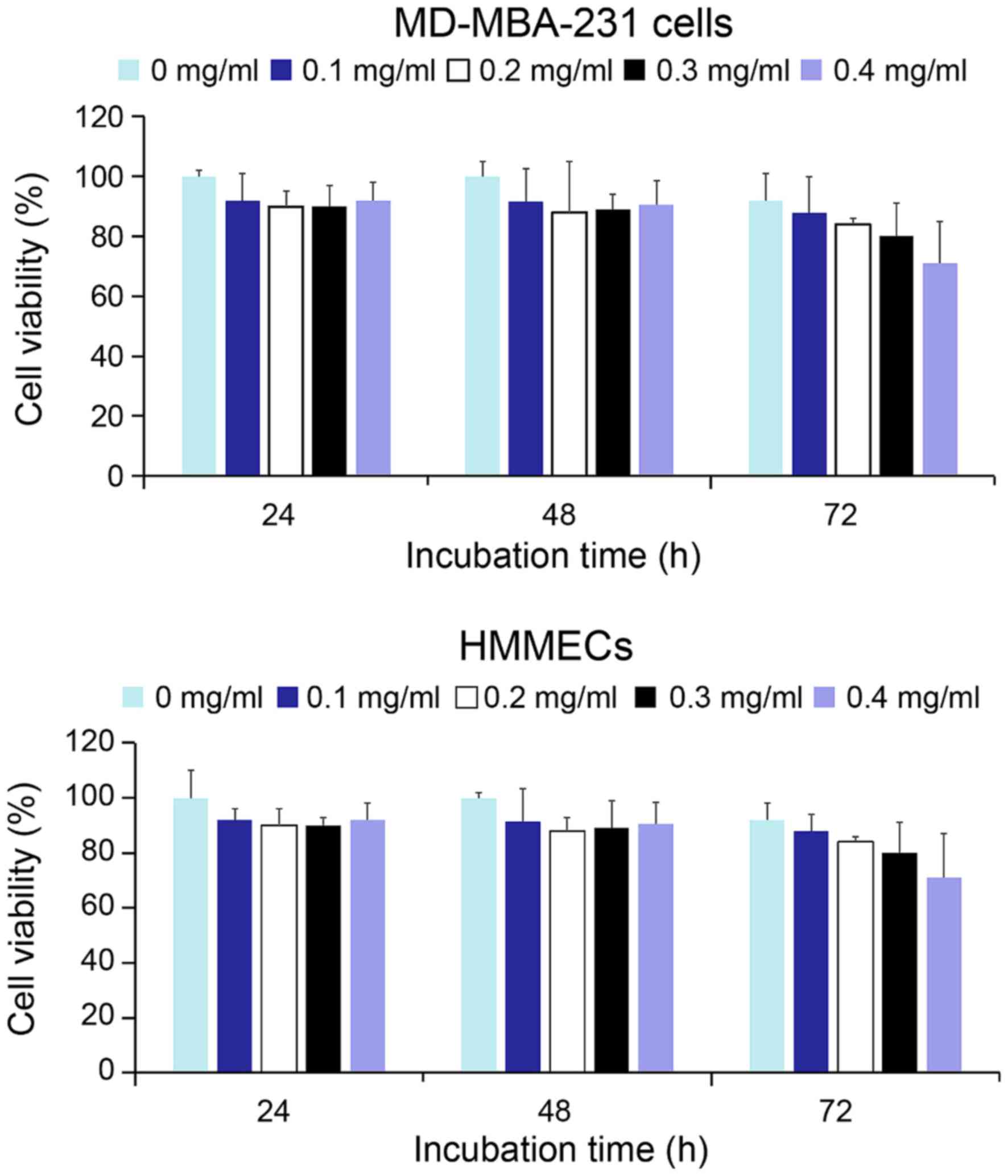
As presented in Fig.
4, the viability of MD-MBA-231 cells and HMMECs treated with a
broad range of concentrations of QD-Ki-67 probes, persisted in
>88% of cells 48 h following treatment. However, at 72 h after
treatment, the viability of HMMECs began to decline at a
concentration of 0.4 mg/ml QD-Ki-67 probe, the highest
concentration. In a previous study by Cho et al (28), 3-mercaptopropionic acid-modified
CdSe/ZnS QDs were demonstrated to be nontoxic to MCF-7 cells
(28). However, this result was
attained from a short experimental period ranging between 12 and 24
h. Furthermore, the model system used by Cho et al (28) was cancer cells, which may exhibit
prolonged viability. The results of the present study were
consistent with those from Qi et al (29), which hypothesized that, over an
extended period, the QDs in the cells ended up in the lysosome and
the degraded QDs subsequently released Cd2+ from the
particle surface of the QDs into the cell. The results of the
present study demonstrated that QD-Ki-67 probes exerted slight
toxic effects on living cells in vitro within 48 h.
Discussion
QDs have enormous potential in breast cancer
research. In the present study, QD-Ki-67 probes entered the
MDA-MB-231 cells, and displayed bright and colorful PL, with no
corresponding change in the shape and viability of cells. Ki-67 is
frequently measured as a static marker of proliferative activity
and by making multiple measurements during treatment, making it a
possible dynamic intermediate marker of treatment efficiency.
Previous studies have demonstrated the prognostic value of Ki-67 in
breast cancer (5,30). To evaluate the routine use and value
of Ki-67 as a prognostic marker, the results of analysis of a large
population-based cohort of a cancer registry indicated that Ki-67
expression is an independent prognostic parameter for disease-free
and overall survival (31). QD-based
fluorescent imaging techniques may quantitatively and
simultaneously detect the expression of Ki-67 in breast cancer
in situ, and sensitively assess the prognostic values of
Ki-67. Jerjees et al (31)
compared the Ki-67 labeling index (Ki-67-LI) and human epidermal
growth factor 2 (HER-2) expression levels to assess their effect on
the biological behavior of luminal breast cancer cells and
prognosis of patients with luminal breast cancer, and identified
that Ki-67-LI and HER-2 were associated with high proliferation and
poor prognosis in estrogen receptor (ER)-positive breast
cancer.
When QDs are used in in vitro or in
vivo experiments, they are present in complex biological
environments that may directly or indirectly affect the colloidal
and optical stability of the QD formulation (32–34). Thus,
the aqueous colloidal stability of QDs is of concern for use in
long-term in vivo imaging applications, including for tumor
detection and labeling. The present study monitored the PL
intensity of the prepared QD formulations in various cell culture
media for >3 days. The change in the PL intensity is used as an
indicator for evaluating the overall colloidal stability of the QD
formulations. Common factors that may affect the PL intensity of QD
dispersion include pH, ions and oxygen concentration (25–27). The
present study optimized the experimental conditions and observed
that QD-Ki-67 probes exhibited substantial water colloid stability.
The results of the present study indicate that the QD-Ki-67 probes
represent a promising alternative to the traditional cellular
labeling agents for the imaging of cells. The in vitro or
in vivo use of QDs has an impact on the physiological
environment, and as a result may directly or indirectly affect the
properties of the formulation, such as colloidal and optical
stability. Thus, the aqueous colloidal stability of QDs
necessitates intensive investigation for usage in the long-term
in vivo applications, including in tumor detection and
labeling. The present study scrutinized the PL intensity of
previous formulations of QDs in different cell culture media over a
period of 72 h. The modified PL intensity aids in the evaluation of
the overall colloidal stability of the QDs. Factors including pH,
ions and oxygen levels may affect the PL intensity of QD dispersion
(24–26). In the present study, the experimental
parameters were optimized and the high water colloid stability of
QD-Ki-67 probes was observed. QD-Ki-67 probes appear to be
promising substitutes for conventional labeling agents for cell
imaging. However, QD toxicity is of grave concern. In comparison
with the existing organic fluorescent dyes, including Alexa Fluor
488 that was used in the present study, QD-ER probes exhibited
excellent optical properties, with high light stability and high
fluorescence quantum yields in particular. The antibody-conjugated
QD probes also exhibited no evident cytotoxicity in live cells.
These advantages indicated that the novel QD-ER probes described in
the present study have the potential for use in in vivo
imaging of cancer tumors; they may be used for cellular imaging and
long-term in vivo observation of tumors.
In conclusion, in the present study a novel
water-soluble compound, QD-Ki-67, was prepared which specifically
combined with the Ki-67 antigen. Furthermore, the efficiency of
detectability of the Ki-67 antigen in MD-MBA-231 cells and HMMECs
was analyzed. Owing to their low cytotoxicity and adequate
biocompatibility, QD-Ki-67 probes were able to permeabilize
MD-MBA-231 cells and HMMECs with only slight changes to the shape
and viability of the cells. Therefore, this novel water-soluble QD
probe exhibits substantial promise for future use in vitro
for the diagnosis of breast cancer.
Acknowledgements
The study was financially supported by The Jilin
Province Key Fund (grant no. 20150204081SF) and The Jilin Province
Development and Reform Commission (grant no. 2015Y035-02).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soerjomataram I, Louwman MW, Ribot JG,
Roukema JA and Coebergh JW: An overview of prognostic factors for
long-term survivors of breast cancer. Breast Cancer Res Treat.
107:309–330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gerdes J, Schwab U, Lemke H and Stein H:
Production of a mouse monoclonal antibody reactive with a human
nuclear antigen associated with cell proliferation. Int J Cancer.
31:13–20. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lopez F, Belloc F, Lacombe F, Dumain P,
Reiffers J, Bernard P and Boisseau MR: Modalities of synthesis of
Ki-67 antigen during the stimulation of lymphocytes. Cytometry.
12:42–49. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yerushalmi R, Woods R, Ravdin PM, Hayes MM
and Gelmon KA: Ki67 in breast cancer: Prognostic and predictive
potential. Lancet Oncol. 11:174–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheang MC, Chia SK, Voduc D, Gao D, Leung
S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al: Ki67
index, HER2 status, and prognosis of patients with luminal B breast
cancer. J Natl Cancer Inst. 101:736–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamamoto S, Ibusuki M, Yamamoto Y, Fu P,
Fujiwara S, Murakami K and Iwase H: Clinical relevance of Ki67 gene
expression analysis using formalin-fixed paraffin-embedded breast
cancer specimens. Breast Cancer. 20:262–270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ: Panel members: Strategies for
subtypes-dealing with the diversity of breast cancer: Highlights of
the St gallen international expert consensus on the primary therapy
of early breast cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alivisatos P: The use of nanocrystals in
biological detection. Nat Biotechnol. 22:47–52. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Michalet X, Pinaud FF, Bentolila LA, Tsay
JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS and Weiss S:
Quantum dots for live cells, in vivo imaging, and diagnostics.
Science. 307:538–544. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alivisatos A, Gu W and Larabell C: Quantum
dots as cellular probes. 7:55–76. 2005.
|
|
12
|
Zrazhevskiy P, Sena M and Gao X: Designing
multifunctional quantum dots for bioimaging, detection, and drug
delivery. Chem Soc Rev. 39:4326–4354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bentolila L, Ebenstein Y and Weiss S:
Quantum dots for in vivo small-animal imaging. J Nucl Med.
50:493–496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He X, Gao J, Gambhir SS and Cheng Z:
Near-infrared fluorescent nanoprobes for cancer molecular imaging:
Status and challenges. Trends Mol Med. 16:574–583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hilderbrand SA and Weissleder R:
Near-infrared fluorescence: Application to in vivo molecular
imaging. Curr Opin Chem Biol. 14:71–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Byers RJ and Hitchman ER: Quantum dots
brighten biological imaging. Prog Histochem Cytochem. 45:201–237.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y and Chen L: Quantum dots, lighting
up the research and development of nanomedicine. Nanomedicine.
7:385–402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang H, Li Y, Sun X, Lv Y, Chen L and
Wang J: Preparation and biological characterization of
pH-responsive PASP-g-PEG-DDA-Hyd-ADR. New J Chem. 37:1623–1629.
2013. View Article : Google Scholar
|
|
19
|
Sun T, Li K, Li Y, Li C, Zhao W, Chen L
and Chang Y: Optimizing conditions for encapsulation of QDs by
varying PEG chain density of amphiphilic centipede-like copolymer
coating and exploration of QDs probes for tumor cell targeting and
tracking. New J Chem. 36:2383–2391. 2012. View Article : Google Scholar
|
|
20
|
Sun X, Li Y, Huang H, Yang B and Wang Y:
Synthesis and application of a targeting diagnosis system via
quantum dots coated by amphiphilic polymer for the detection of
liver cancer cells. Luminescence. 29:831–836. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hardman R: A toxicologic review of quantum
dots: Toxicity depends on physicochemical and environmental
factors. Environ Health Perspect. 114:165–172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu WW, Chang E, Drezek R and Colvin VL:
Water-soluble quantum dots for biomedical applications. Biochem
Biophys Res Commun. 348:781–786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoshino A, Fujioka K, Oku T, Suga M,
Sasaki YF, Ohta T, Yasuhara M, Suzuki K and Yamamota K:
Physicochemical properties and cellular toxicity of nanocrystal
quantum dots depend on their surface modification. Nano Lett.
4:2163–2169. 2004. View Article : Google Scholar
|
|
24
|
Ryman-Rasmussen JP, Riviere JE and
Monteiro-Riviere NA: Surface coatings determine cytotoxicity and
irritation potential of quantum dot nanoparticles in epidermal
keratinocytes. J Invest Dermatol. 127:143–153. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee J, Ji K, Kim J, Park C, Lim KH, Yoon
TH and Choi K: Acute toxicity of two CdSe/ZnSe quantum dots with
different surface coating in Daphnia magna under various light
conditions. Environ Toxicol. 25:593–600. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishiyama M, Tominaga H, Shiga M, Sasamoto
K, Ohkura Y and Ueno K: A combined assay of cell viability and in
vitro cytotoxicity with a highly water-soluble tetrazolium salt,
neutral red and crystal violet. Biol Pharm Bull. 19:1518–1520.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tominaga H, Ishiyama M, Ohseto F, Sasamoto
K, Hamamoto T, Suzuki K and Watanabe M: A water-soluble tetrazolium
salt useful for colorimetric cell viability assay. Anal Commun.
36:47–50. 1999. View
Article : Google Scholar
|
|
28
|
Cho SJ, Maysinger D, Jain M, Röder B,
Hackbarth S and Winnik F: Long-term exposure to CdTe quantum dots
causes functional impairments in live cells. Langmuir.
23:1974–1980. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qi Q, Liu X, Li S, Joshi HC and Ye K:
Synergistic suppression of noscapine and conventional
chemotherapeutics on human glioblastoma cell growth. Acta Pharmacol
Sin. 34:930–938. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Inwald EC, Klinkhammer-Schalke M,
Hofstädter F, Zeman F, Koller M, Gerstenhauer M and Ortmann O:
Ki-67 is a prognostic parameter in breast cancer patients: Results
of a large population-based cohort of a cancer registry. Breast
Cancer Res Treat. 139:539–552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jerjees DA, Alabdullah M, Green AR,
Alshareeda A, Macmillan RD, Ellis IO and Rakha EA: Prognostic and
biological significance of proliferation and HER2 expression in the
luminal class of breast cancer. Breast Cancer Res Treat.
145:317–330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hines DA and Kamat PV: Recent advances in
quantum dot surface chemistry. ACS Appl Mater Interfaces.
6:3041–3057. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Durisic N, Godin AG, Walters D, Grütter P,
Wiseman PW and Heyes CD: Probing the ‘dark’ fraction of core-shell
quantum dots by ensemble and single particle pH-dependent
spectroscopy. ACS Nano. 5:9062–9073. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boldt K, Bruns OT, Gaponik N and
Eychmüller A: Comparative examination of the stability of
semiconductor quantum dots in various biochemical buffers. J Phys
Chem B. 110:1959–1963. 2006. View Article : Google Scholar : PubMed/NCBI
|