Introduction
Malignant mesothelioma (MM) is a highly aggressive
tumor of the mesothelial origin associated with asbestos exposure,
and most commonly develops in the pleura (1–3). MM is
rare, but is increasingly prevalent in many industrialized
countries including Japan even after a ban of asbestos usage
probably because of its long latency period between asbestos
exposure and development of MM (3–5). Thus, the
prevention, diagnosis and therapy of MM are important issues in
occupational medicine.
The diagnosis of MM is usually confirmed by
histological examination of biopsied samples, which are usually
obtained invasive procedures such as thoracoscopic pleural biopsy
(6,7).
These invasive procedures may not be appropriate for mass-screening
to identify MM patients among high-risk population with history of
asbestos-exposure, or cannot be performed for patients with
impaired organ functions. Among less invasive procedures for the
diagnosis, radiographic examinations such as chest roentgenogram
and computed tomography (CT) are most commonly employed, but do not
provide definitive diagnosis of MM. Blood-based tests may be
promising, but the serum mesothelin related protein (SMRP), the
only clinically approved blood-test, may not provide sufficient
diagnostic sensitivity (8,9). Accordingly, a novel blood-based test for
the diagnosis of MM should be established for early diagnosis as
well as improvement of prognosis of MM patients.
Circulating tumor cells (CTCs) are tumor cells that
are shed from the primary tumor and circulate in the peripheral
blood (10). CTCs may be promising
marker as a surrogate of micro-metastasis, but detection of rare
tumor cells contaminated in a vast majority of normal hematological
cells may present a technical challenge (10,11). The
‘CellSearch’ system (Veridex LCC, Raritan, NJ, USA) is an automated
detection system of CTCs using an antibody against an epithelial
marker (EpCAM), which is the only approved system for the clinical
use (only in USA) (12). In a
previous study, we evaluated CTCs with the ‘CellSearch’ in
peripheral blood sampled from patients with diagnosis or suspicion
of MM. The CTC-test provided a significant prognostic value in
discrimination between MM patients and non-MM patients such as
asbestos pleurisy (P=0.036), but the sensitivity was only modest
(32.7%) mainly due to negative or low expression of EpCAM on MM
cells, which may not be effectively captured with an anti-EpCAM
antibody (13). These results clearly
indicate the need for a sensitive system for capture EpCAM-negative
CTCs, and we have developed a high efficient system to capture CTCs
using a microfluidic device ‘CTC-chip’ (14,15). In
the system, CTCs are captured to numerous micro-posts coated with
an antibody against an antigen expressed on target tumor cells, and
the most important advantage is capability of conjugating any
antibody to capture CTCs. In fact, we effectively captured and
isolated EpCAM-negative MM cells (ACC-MESO-4 cells) with the
CTC-chip coated with an antibody against a mesothelial marker
(podoplanin) (15), indicating its
potential capability of capturing a wide variety of CTCs by
conjugating appropriate capture antibodies. In the current study,
we expand and examined capture efficiencies of the ‘universal’
CTC-chip for another MM cell-line (ACC-MESO-1) and with another
capture antibody against another mesothelial marker (mesothelin) to
improve sensitivity in detection of CTCs for clinical application
in the diagnosis of MM.
Materials and methods
Cell lines
Human mesothelioma cell lines, ACC-MESO-1 and
ACC-MESO-4 established in Aichi Cancer Research Center (16) as well as a human lung adenocarcinoma
cell line, PC-9, were purchased from Riken BioResource Center
(Tsukuba, Japan). These cells ware cultured in RPMI-1640 medium
(Wako Pure Chemical Industries, Osaka, Japan) supplemented with 10%
fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C and 5% CO2.
Flow cytometry
Cells were collected and incubated with a primary
antibody, an anti-EpCAM antibory (clone HEA125; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), an anti-podoplanin antibody
(clone E1; Santa Cruz Biotechnology, Inc.), or an anti-mesothelin
antibody (clone K1; Santa Cruz Biotechnology, Inc.). Then, cells
were incubated with a goat anti-mouse IgG antibody conjugated with
FITC (BD Biosciences, San Jose, CA, USA). Flow cytometry analysis
was performed using EC800 Cell Analyser (Sony Biotechnology, Tokyo,
Japan) and FlowJo software (Tree Star, Inc., Ashland,. OR, USA).
The percentage of positive cells and the mean fluorescence
intensity (MFI) was determined by comparison with negative
control.
Preparation of CTC-chip
The polymeric CTC-chip system was used after
two-step coating with an antibody to capture CTCs as described
previously (15). In brief, the chip
was first incubated with a goat anti-mouse IgG antibody
(SouthernBiotech, Birmingham, AL, USA), and then was incubated with
an anti-EpCAM antibody (clone HEA125), an anti-podoplanin antibody,
or an anti-mesothelin antibody (clone K1) to capture tumor cells;
the antibody-coated chip was referred to as ‘EpCAM-chip’,
‘podoplanin-chip’, and ‘mesothelin-chip’, respectively. After
washing with PBS, the chip surface was kept wet.
Sample preparation and evaluation of
cell-capture efficacy
Sample preparation and flow test were performed as
described previously (15). In brief,
500 tumor cells, labeled with CellTrace™ CFSE Cell Proliferation
Kit (Thermo Fisher Scientific, Inc.) and suspended in 1 ml of
phosphate-buffered saline (PBS) containing 5% BSA were applied to
the CTC-chip system.
Images and movies of cells in the chip were
monitored and recorded with a fluorescence microscope CKX41
(Olympus Corporation, Tokyo, Japan) and a digital video camera
(Sony Biotechnology), and determined the actual number of cells
(N-total) that were sent into the chip by counting the number of
cells that passed through the inlet of the chip as well as the
number of captured cells (N-captured) by counting CFSE-labelled
cells remained on the chip. The cell capture efficiency was
represented as N-captured/N-total (14,15). The
average and standard error (SE) of capture efficiency were
calculated from results obtained in triplicated experiments. Data
were compared using a non-parametric test (Mann-Whitney U-test for
comparison between 2 groups or Kruskal-Wallis H-test for comparison
among 3 groups). P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed with the SPSS software package (version 21.0; IBM Corp.,
Armonk, NY, USA).
The study was reviewed and approved by the
Institutional Review Board of the University of Occupational and
Environmental Health, Japan.
Results
Expression of EpCAM and podoplanin on
ACC-MESO-1 cells
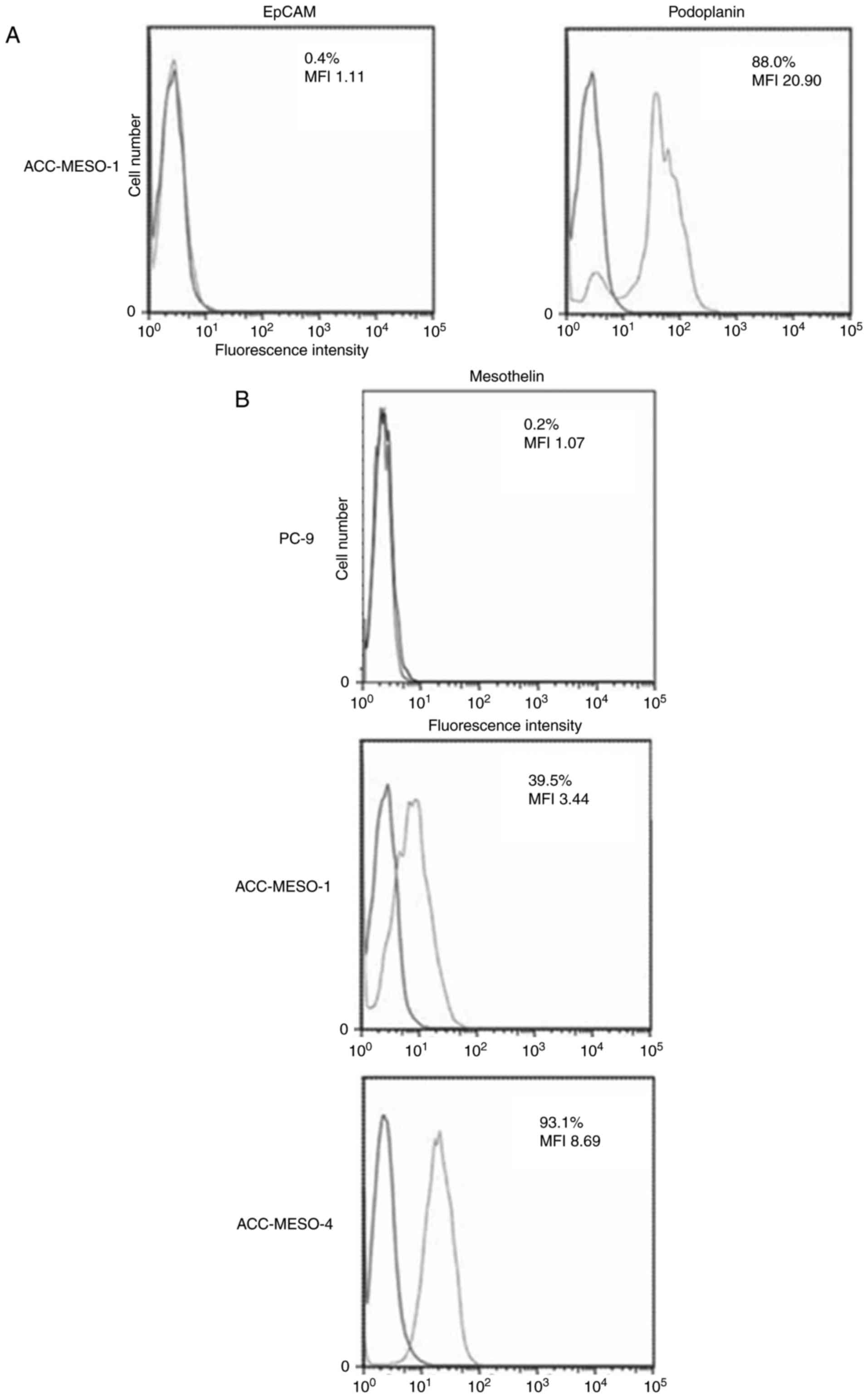
ACC-MESO-1, a human MM cell line, did not express
EpCAM and modestly expressed podoplanin (Fig. 1A).
Expression of Mesothelin on PC-9,
ACC-MESO-1, ACC-MESO-4 cells
PC-9, a human lung adenocarcinoma cell, did not
express mesothelin, and ACC-MESO-1 and MESO-4, human MM cell lines,
weakly expressed mesothelin (Fig.
1B).
Cell capture efficiency
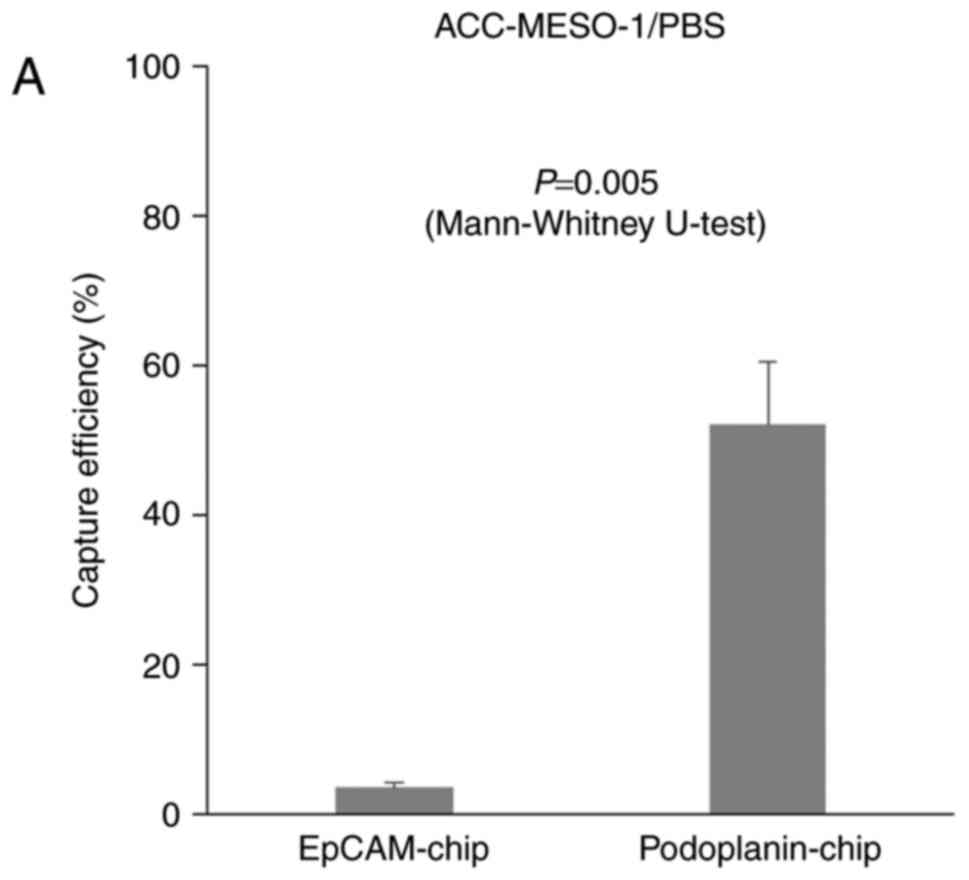
When ACC-MESO-1 cells were spiked in PBS, cells were
not captured by the EpCAM-chip (average capture efficiency, 3.5%);
cells were captured with the podoplanin chip (Fig. 2, Table
I. P=0.005), but the average capture efficiency was only modest
(average capture efficiency, 52.7%) as compared with that (78.3%)
for ACC-MESO-4 obtained in the previous study (15). The average captures efficiency of the
mesothelin-chip for PC-9, ACC-MESO-1, and ACC-MESO-4 were 2.9, 4.3
and 5.4%, respectively (Fig. 3,
Table II).
 | Table I.Capture of ACC-MESO-1 cells spiked in
PBS with EpCAM-chip or Podoplanin-chip. |
Table I.
Capture of ACC-MESO-1 cells spiked in
PBS with EpCAM-chip or Podoplanin-chip.
|
| EpCAM-chip | Podoplanin-chip |
|---|
|
|
|
|
|---|
|
|
|
| Cell capture
efficiency (%) |
|
|
| Cell capture
efficiency (%) |
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Cell | No. of cells
captured | No. of total
cells | Values | Average | Standard error | No. of cells
captured | No. of total
cells | Values | Average | Standard error |
|---|
| ACC-MESO-1 | 21 | 708 | 3.0 |
|
| 513 | 746 | 68.8 |
|
|
|
| 18 | 670 | 2.7 | 3.5 | 0.66 | 347 | 699 | 49.6 | 52.7 | 8.54 |
|
| 37 | 778 | 4.8 |
|
| 204 | 514 | 39.7 |
|
|
 | Table II.Capture from the cell suspension
spiked in PBS with ‘Mesothelin-chip’. |
Table II.
Capture from the cell suspension
spiked in PBS with ‘Mesothelin-chip’.
|
| Mesothelin-chip |
|---|
|
|
|
|---|
|
|
|
| Cell capture
efficiency (%) |
|
|---|
|
|
|
|
|
|
|---|
|
| | | | |
| Variable | No. of cells captured
(N-captured) | No. of total cells
(N-total) | Values | Average | Standard Error
(SE) |
|---|
| PC-9 | 6 | 535 | 1.1 | 2.9 |
|
|
| 17 | 518 | 3.3 |
| 0.95 |
|
| 27 | 632 | 4.3 |
|
|
| ACC-MESO-1 | 37 | 706 | 5.2 | 4.3 |
|
|
| 28 | 614 | 4.6 |
| 0.66 |
|
| 23 | 762 | 3.0 |
|
|
| ACC-MESO-4 | 38 | 591 | 6.4 | 5.4 |
|
|
| 15 | 469 | 3.2 |
| 1.12 |
|
| 50 | 749 | 6.7 |
|
|
When ACC-MESO-1 cells were spiked in PBS, cells were
captured by the podoplanin-chip (average capture efficiency,
52.7%). It was significantly higher than with the EpCAM chip, the
average capture efficiency was 3.5% (Fig.
2, Table I. P=0.005, calculated
by Mann-Whitney U-test), but the average capture efficiency was
only modest (average capture efficiency, 52.7%) as compared with
that (78.3%) for ACC-MESO-4 obtained in the previous study
(15). The average captures
efficiency of the mesothelin-chip for PC-9, ACC-MESO-1, and
ACC-MESO-4 were 2.9, 4.3, and 5.4%, respectively (Fig. 3, Table
II. P=0.329, calculated by Kruskal-Wallis H-test).
Discussion
In the present study, we showed that the novel
CTC-chip can capture mesothelioma cells (ACC-MESO-1) when coated
with an antibody against podoplanin that is a specific antigen
expressed on the surface of mesothelial cells (17–19). In
the previous study, we showed that the ‘podoplanin-chip’
effectively captured mesothelioma cells of another cell line,
ACC-MESO-4 (15), indicating that the
novel CTC-chip is a promising modality to detect some kinds of
tumor cells including EpCAM-negative cells due to non-epithelial
origin (e.g., mesothelioma cells) or undergoing
epithelial-mesenchymal transition (EMT). Among a variety of systems
for capture EpCAM-negative CTCs such as size-based or density-based
separation systems (20), we employed
a microfluidic system to capture CTCs called CTC-chip (14,15). The
CTC-chip had been originally developed in USA (21), but the original CTC-chip can capture
only EpCAM-positive CTCs because an anti-EpCAM antibody to capture
CTCs is conjugated and another antibody is not available in the
system. In contrast, the novel CTC-chip can be easily conjugated
with any antibody, and can capture a variety of CTCs regardless of
EpCAM expression status, which referred as a ‘universal’ CTC-chip
(15). In fact, as shown in this
study, the CTC-chip can actually capture EpCAM-negative and
podoplanin-positive mesothelioma cell (ACC-MESO-1) when conjugated
with an anti-podoplanin antibody, and can capture EpCAM-positive
PC-9 cells when conjugated with an anti-EpCAM antibody (15).
The current study showed a modest efficacy of the
podoplanin chip to capture ACC-MESO-1 cells spiked in PBS with an
average capture efficiency of 52.7%, which was somewhat lower than
that for ACC-MESO-4 (average capture rate, 78.3%) obtained in the
previous study (15). In the previous
study, the average capture efficiency of the EpCAM-chip to capture
PC-9 cells with strongest EpCAM expression was 100% and still
higher than that (78.3%) of the podoplnin-chip to capture
ACC-MESO-4 cells with enhanced podoplanin expression, indicating
that the capture efficiency might be influenced antigen-antibody
interaction caused mainly by degree of antigen expression on the
surface of tumor cells (15). The
current study also showed that the capture of ACC-MESO-1 cells with
modest podoplanin expression was less effective by the
‘podoplanin-chip’ (Table III). We
also tried to capture another mesothelioma cell line, MSTO-211H
derived from biphasic mesothelioma patient, with CTC-chip coated
with the anti-podoplanin antibody (clone E1). However it could not
capture well (8.3%, N-captured/N-total; 51/611), because 211H cells
did not express podoplanin (data not shown). We have to try other
MM cells in the future. For clinical samples, it is under
consideration about optimal detection methods.
 | Table III.Capture efficiency of tumor cells
spiked in PBS. |
Table III.
Capture efficiency of tumor cells
spiked in PBS.
| Variable | PC-9 | ACC-MESO-1 | ACC-MESO-4 |
|---|
| EpCAM-chip | 101.1a | 3.5 | 2.3a |
|
Podoplanin-chip | 3.0a | 52.0 | 78.3a |
|
Mesothelin-chip | 2.9 | 4.3 | 5.4 |
In the current study, we employed and examined a
novel antibody, an anti-mesothelin antibody clone K1, to capture MM
cells as mesothelin is one of useful biomarkers of MM (22). However, the ‘mesothelin-chip’ failed
to provide effective capture performance (Fig. 3B), mainly due to lower mesothelin
expression on MM cells (Fig. 1B). We
tried to capture MSTO-211H cells using CTC-chip coated with
anti-mesothelin antibody clone K1. Mesothelin expression of
MSTO-211H cells were measured by flow cytometry and compared with
ACC-MESO-4 cells as positive control. A sample without the primary
antibody was used as negative control. Consequently, the mesothelin
expression of MSTO-211H cells were almost negative (data not
shown), it was in consistent to the previous report (23), and these cells failed to capture
effectively using ‘mesothelin-chip’. The capture efficiency of
MSTO-211H cells was 6.2% (28/453) (data not shown). In addition, we
tried to capture using another anti-mesothelin antibody, clone 5B2
mouse monoclonal antibody. For ACC-MESO-1 or ACC-MESO-4 cells,
capture efficiency were 14.3% (12/84), 7.3% (6/82) respectively
(data not shown). They were hardly captured as well as
anti-mesothelin antibody clone K1 (MESO-1: 3.0–5.2%, MESO-4:
3.2–6.7%). It is need to examine by obtaining other cell lines more
expressed mesothelin or using other mesothelin antibody. In future
studies, other antibodies such as an antibody against CD146, a
recently identified mesothelial marker (24), will be tested to capture MM cells. In
conclusion, the ‘universal’ CTC-chip might be a useful system as
less-invasive blood-based modality in the diagnosis of MM.
Acknowledgements
The authors would like to thank Eri Kawashima for
her technical assistance. This study was supported Grants-in-Aid
for Scientific Research (Grant nos. 26861131, and 16K10697) from
the Japan Society for the Promotion of Science (JSPS) and by the
Grant of The Clinical Research Promotion Foundation 2015.
References
|
1
|
Ismail-Khan R, Robinson LA, Williams CC
Jr, Garrett CR, Bepler G and Simon GR: Malignant pleural
mesothelioma: A comprehensive review. Cancer Control. 13:255–263.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsiouris A and Walesby RK: Malignant
pleural mesothelioma: Current concepts in treatment. Nat Clin Pract
Oncol. 4:344–352. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin RT, Takahashi K, Karjalainen A,
Hoshuyama T, Wilson D, Kameda T, Chan CC, Wen CP, Furuya S, Higashi
T, et al: Ecological association between asbestos-related diseases
and historical asbestos consumption: An international analysis.
Lancet. 369:844–849. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takahashi K and Landrigan PJ: Collequim
Ramazzini: The global health dimensions of asbestos and
asbestos-related diseases. Ann Glob Health. 82:209–213. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tomasson K, Gudmundsson G, Briem H and
Rafnsson V: Malignant mesothelioma incidence by nation-wide cancer
registry: A population-based study. J Occup Med Toxicol. 11:372016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scherpereel A, Astoul P, Baas P, Berghmans
T, Clayson H, de Vuyst P, Dienemann H, Galateau-Salle F, Hennequin
C, Hillerdal G, et al: Guidelines of the European Respiratory
Society and the European Society of Thoracic Surgeons for the
management of malignant pleural mesothelioma. Eur Respir J.
35:479–495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stahel RA, Weder W, Lievens Y and Felip E:
ESMO Guidelines Working Group: Malignant pleural mesothelioma: ESMO
clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 21 Suppl 5:v126–v128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van der Bij S, Schaake E, Koffijberg H,
Burgers JA, de Mol BA and Moons KG: Markers for the non-invasive
diagnosis of mesothelioma: A systematic review. Br J Cancer.
104:1325–1333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hollevoet K, Reitsma JB, Creaney J,
Grigoriu BD, Robinson BW, Scherpereel A, Cristaudo A, Pass HI,
Nackaerts K, Portal Rodríguez JA, et al: Serum mesothelin for
diagnosing malignant pleural mesothelioma: An individual patient
data meta-analysis. J Clin Oncol. 30:1541–1549. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanaka F, Yoneda K and Hasegawa S:
Circulating tumor cell (CTC) in lung cancer: Current status and
future perspectives. Lung Cancer (Auckl). 1:77–84. 2010.PubMed/NCBI
|
|
11
|
Tanaka F and Yoneda K: Adjuvant therapy
following surgery in non-small cell lung cancer (NSCLC). Surg
Today. 46:25–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Allard WJ, Matera J, Miller MC, Repollet
M, Connely MC, Rao C, Tibbe AGJ, Uhr JW and Terstappen LW: Tumor
cells circulate in the peripheral blood of all major carcinomas but
not in healthy subjects or patients with nonmalignant diseases.
Clin Cancer Res. 10:6897–6904. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoneda K, Tanaka F, Kondo N, Hashimoto M,
Takuwa T, Matsumoto S, Okumura Y, Tsubota N, Sato A, Tsujimura T,
et al: Circulating tumor cells (CTCs) in malignant pleural
mesothelioma (MPM). Ann Surg Oncol. 21 Suppl 4:S472–S480. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohnaga T, Shimada Y, Moriyama M, Kishi H,
Obata T, Takata K, Okumura T, Nagata T, Muraguchi A and Tsukada K:
Polymeric microfluidic devices exhibiting sufficient capture of
cancer cell line for isolation of circulating tumor cells. Biomed
Microdevices. 15:611–616. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chikaishi Y, Yoneda K, Ohnaga T and Tanaka
F: EpCAM-independent capture of circulating tumor cells with a
‘universal CTC-chip’. Oncol Rep. 37:77–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Usami N, Fukui T, Kondo M, Taniguchi T,
Yokoyama T, Mori S, Yokoi K, Horio Y, Shimokata K, Sekido Y and
Hida T: Establishment and characterization of four malignant
pleural mesothelioma cell lines from Japanese patients. Cancer Sci.
97:387–394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kimura N and Kimura I: Podoplanin as a
marker for mesothelioma. Pathol Int. 55:83–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abe S, Morita Y, Kaneko MK, Hanibuchi M,
Tsujimoto Y, Goto H, Kakiuchi S, Aono Y, Huang J, Sato S, et al: A
novel targeting therapy of malignant mesothelioma using
anti-podoplanin antibody. J Immunol. 190:6239–6249. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ordóñez NG: D2-40 and podoplanin are
highly specific and sensitive immunohistochemical markers of
epithelioid malignant mesothelioma. Hum Pathol. 36:372–380. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Toss A, Mu Z, Fernandez S and
Cristofanilli M: CTC enumeration and characterization: Moving
toward personalized medicine. Ann Transl Med. 2:1082014.PubMed/NCBI
|
|
21
|
Nagrath S, Sequist LV, Maheswaran S, Bell
DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky
A, et al: Isolation of rare circulating tumour cells in cancer
patients by microchip technology. Nature. 450:1235–1239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Husain AN, Colby TV, Ordóñez NG, Krausz T,
Borczuk A, Cagle PT, Chirieac LR, Churg A, Galateau-Salle F, Gibbs
AR, et al: Guidelines for pathologic diagnosis of malignant
mesothelioma: A consensus statement from the international
mesothelioma interest group. Arch Pathol Lab Med. 133:1317–1331.
2009.PubMed/NCBI
|
|
23
|
Scales SJ, Gupta N, Pacheco G, Firestein
R, French DM, Koeppen H, Rangell L, Barry-Hamilton V, Luis E, Chuh
J, et al: An antimesothelin-monomethyl auristatin e conjugate with
potent antitumor activity in ovarian, pancreatic, and mesothelioma
models. Mol Cancer Ther. 13:2630–2640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sato A, Torii I, Okamura Y, Yamamoto T,
Nishigami T, Kataoka TR, Song M, Hasegawa S, Nakano T, Kamei T and
Tsujimura T: Immunocytochemistry of CD146 is useful to discriminate
between malignant pleural mesothelioma and reactive mesothelium.
Mod Pathol. 23:1458–1466. 2010. View Article : Google Scholar : PubMed/NCBI
|