Introduction
To maintain body temperature, both energy intake and
blood circulation are critically important. Anti-cancer drugs are
known to induce peripheral neuropathy through nerve hypoperfusion
and reduction of vasa nervorum density (1). In a mouse study, paclitaxel (PTX) and
oxaliplatin induced mechanical allodynia along with peripheral
hypoperfusion (2). Although blood
flow is essential for all cells in the body to obtain nutrients and
oxygen (3), solid tumors often
require a greater blood supply to support their rapid growth;
therefore, angiogenesis is critical for tumor growth (4). In this regard, angiogenesis inhibitors
target tumors based by hampering blood supply, leading to tumor
growth arrest. In contrast, chronic reduction in blood flow and low
oxygen availability may result in tumor hypoxia, which has been
implicated in tumor propagation by adapting cells to nutrient
deprivation, or by facilitating proliferation, local invasion, and
metastatic spread through induction of angiogenesis,
lymphangiogenesis, and/or acquisition of epithelial-to-mesenchymal
phenotypes (5–8).
Chemotherapy reportedly reduces body temperature
(9,10)
and hypothermia is known to suppress immunity by delaying and
prolonging production of proinflammatory cytokines (11,12).
Considering the role of the immune system in tumor surveillance,
such immune suppression by hypothermia may increase risk of tumor
cell progression (13). Indeed,
transplant patients treated with immunosuppressive drugs (14), patients with chronic inflammation and
infection, and elderly persons (15)
are well known to have increased susceptibility to malignancies.
Interestingly, housing temperature reportedly influences results of
preclinical cancer models (16).
Endogenous immune responses were suppressed under standard housing
temperature (20–26°C) compared with thermoneutral temperature
(30–32°C) because the energy needed for intact immune function is
consumed to maintain body temperature (16).
In this study, we investigated effects on metastatic
breast cancer by PTX-induced reductions in body temperature and
peripheral blood flow in a mouse model, and the influence of
thermoneutral temperature on these effects.
Materials and methods
Animals
Female BALB/c mice (7 weeks old; CLEA Japan, Tokyo,
Japan) were used. We used 6–7 animals in each study group, which
were housed 6–7 mice per cage under controlled temperature and
humidity (23±2°C, 50±10%), on a 12-h light/dark cycle. Mice had
access to tap water and food ad libitum. All experiments
were approved and performed according to the guidelines of the
Committee for Animal Experiments at Terumo Corporation and the Care
and Use Committee of Laboratory Animals of the University of
Toyama.
Reagents and cells
PTX (Taxol®; Bristol-Myers Squibb, Tokyo,
Japan), formulated at 6 mg/ml, was dissolved in a vehicle of 13.3%
absolute anhydrous ethanol (Wako Pure Chemical, Osaka, Japan),
13.3% Cremophore® EL (Sigma-Aldrich Japan, Tokyo, Japan)
and 73.4% saline (Otsuka Pharmaceutical Factory, Tokushima, Japan).
We obtained pGL4.50 [luc2P/CMV-RE/Hygro] vector and D-Luciferin
from Promega (Sunnyvale, CA, USA). Mouse mammary carcinoma 4T1
cells that expressed the luciferase gene (4T1-Luc2 cells) were
established and maintained as described before (17).
Measurement of body temperature and
peripheral blood flow
Rectal temperature was measured three times for each
animal, using a rectal probe (RET-3; Physitemp, Clifton, NJ, USA)
and meter (BAT-12R; Physitemp), from which averaged values were
obtained. Body temperatures were measured at approximately 9:00
a.m. Blood flow of ventral tail artery was recorded using laser
Doppler blood flowmeter (ALF21R; ADMEDEC, Tokyo, Japan). Blood flow
was measured three times for about 30 sec. Values for the 10-second
interval between 10 and 20 sec from the starting point were
averaged. Peripheral blood flow was measured at about 10:00 a.m.
Mice were injected with PTX (8 or 12 mg/kg) intraperitoneally
(i.p.) on day 1. Body temperature was measured on day 1 before PTX
injection, and once a day until day 10. Peripheral blood flow was
measured on day 1 before PTX injection, and on days 3, 5, 7 and 10.
All measurements were conducted at 23°C. Mice were divided into
three groups according to their body temperatures on day 1.
Thermoneutral housing
To keep animals at thermoneutral temperature, mice
were housed at 30°C in a cooled incubator (MIR-154; Panasonic,
Osaka, Japan) with 12-h light/dark cycle.
Experimental lung metastasis
model
Six days after 4T1-Luc2 cell inoculation, mice were
injected with D-luciferin (150 mg/kg) intraperitoneally. Twenty min
later, these mice were sacrificed and their lungs were removed.
Lung luminescence was measured with an imaging system (IVIS
Spectrum; Caliper Life Science, Hopkinton, MA, USA) (17).
Matrigel plug angiogenesis assay
Seven days after injection of 4T1-Luc2
cells/Matrigel matrix, the mice were injected intravenously with
100 µl of 1% Evans blue dye (Sigma-Aldrich Japan). After 30 min,
mice were cardiac-perfused with phosphate-buffered saline (Life
Technologies Japan, Tokyo, Japan) containing 2 mM EDTA (Nacalai
Tesque, Kyoto, Japan) under 3% isoflurane [isoflurane inhalation
solution (Pfizer, Tokyo, Japan)] anesthesia. Matrigel plugs were
removed and incubated with formamide (Wako Pure Chemical, Osaka,
Japan) at 37°C for 48 h to elute the Evans blue dye. The amount of
Evans blue dye was quantified by use of a spectrometer (620 nm)
(18).
Statistical analysis
Data are expressed as mean ± standard error of the
mean (SEM). Data were analyzed with a two-way analysis of variance
followed by post hoc analysis with the Bonferroni's test. In the
metastatic and angiogenesis assay, data were analyzed with one-way
analysis of variance followed by post hoc analysis with Tukey's
test. Statistical analysis was performed using Prism (version 6.07;
GraphPad Software, Inc., La Jolla, CA, USA) (19).
Results
Effect of PTX on body temperature and
peripheral blood flow
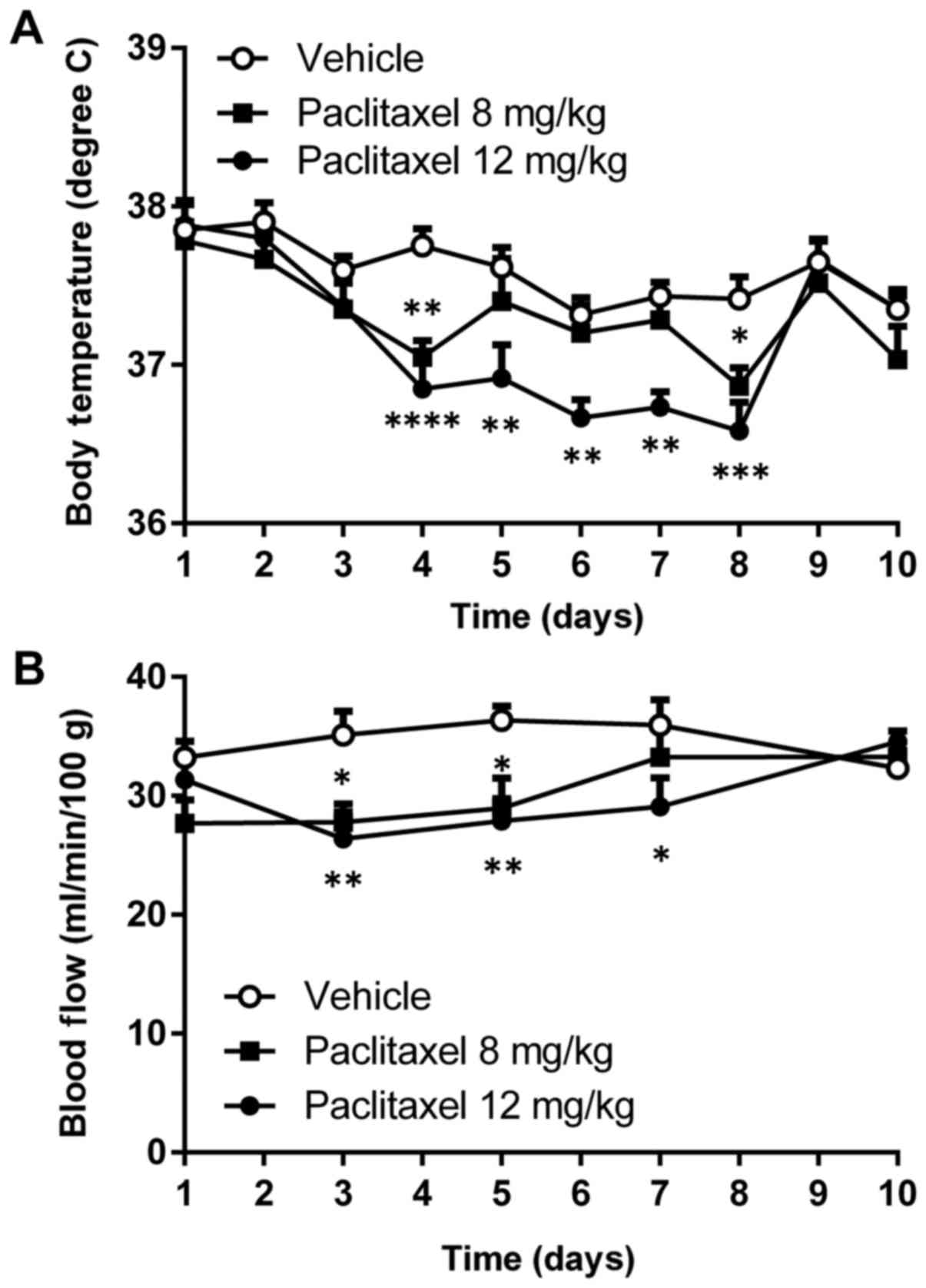
Significant reduction in body temperature was
observed after a single injection of PTX (12 mg/kg) into Balb/c
mice during days 4–8 at 23°C (Fig.
1A). Peripheral blood flow was also reduced by about 25%
compared with untreated mice during days 3–7 after a single
injection of PTX (12 mg/kg; Fig.
1B).
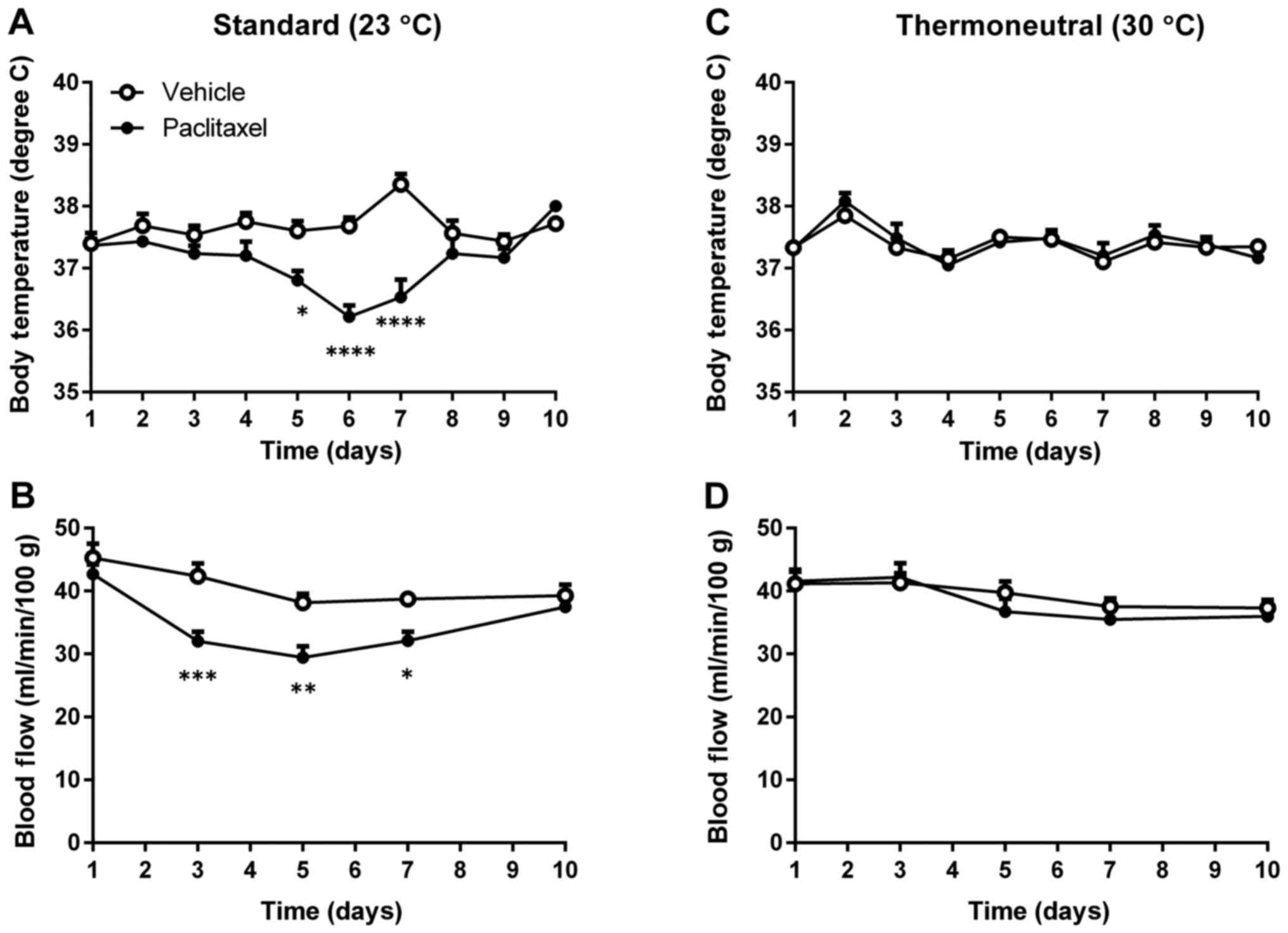
Thermoneutral temperature reversed
body temperature reduction and decreased blood flow induced by
PTX
To find whether thermoneutral temperature could
compensate for reduced body temperature and peripheral blood flow
induced by PTX, mice were housed at either 23°C or 30°C after being
injected with PTX. Although PTX treatment reduced both body
temperature and peripheral blood flow of mice housed at 23°C
(Fig. 2A and B), PTX-treated mice
housed at 30°C showed no reductions in either body temperature or
peripheral blood flow (Fig. 2C and
D). These data indicate that thermoneutral temperature can
compensate for PTX-induced reductions of body temperature and
peripheral blood flow.
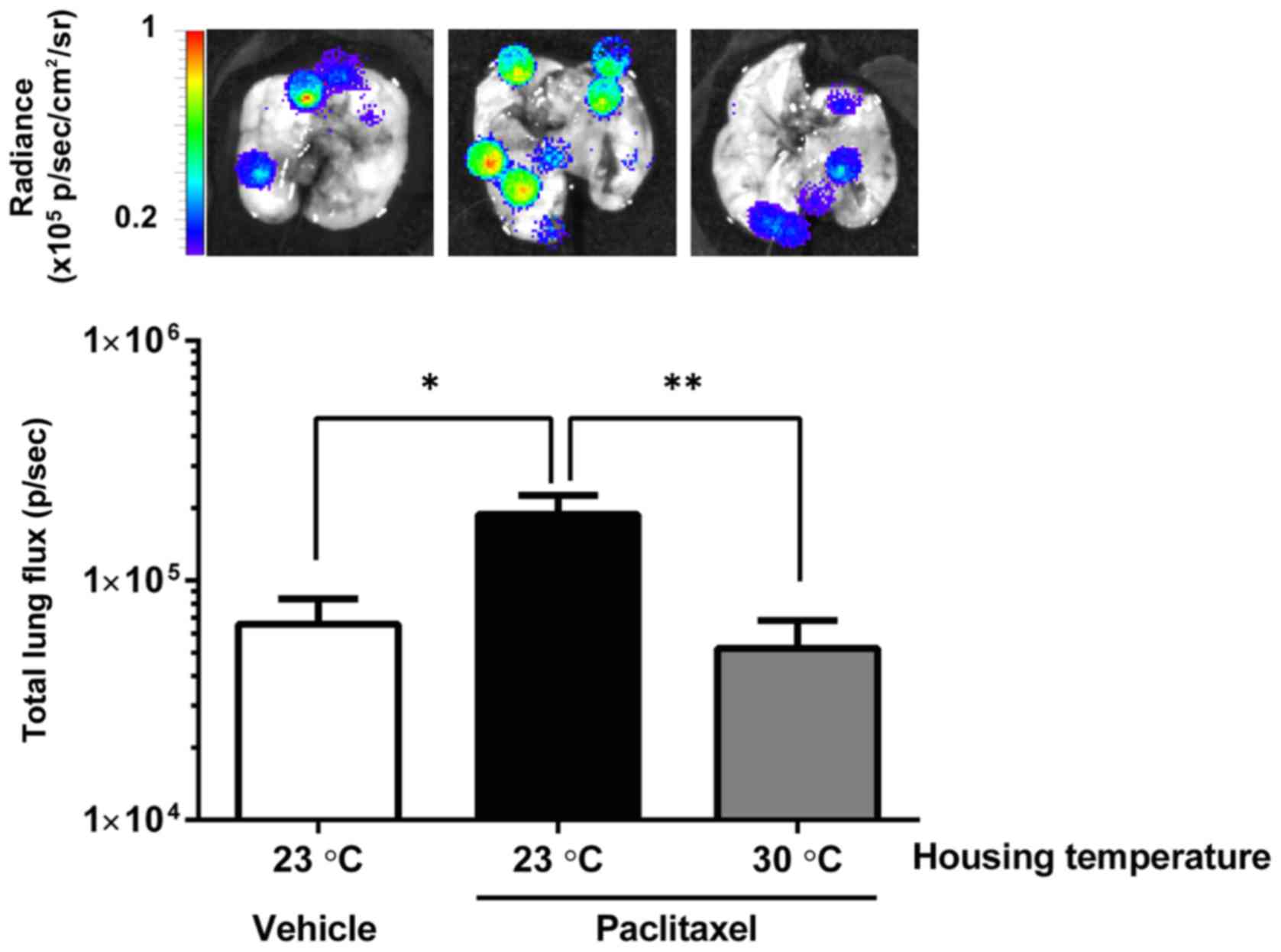
Thermoneutral temperature reverses
PTX's metastasis-promoting effect
We next examined whether body temperature reduction
caused by PTX treatment enhanced in vivo malignant behaviors
such as metastasis or angiogenesis, using murine metastatic mammary
cancer cell line 4T1. Mice were treated with PTX and housed at
either 23°C or 30°C for the next 4 days, and then intravenously
injected with 4T1-Luc2 cells. Upon 4T1-Luc2 cell inoculation, all
mice were housed at 23°C. As shown in Fig. 3, pre-treatment with PTX significantly
increased metastatic lung colonization by 4T1-Luc2 cells among mice
housed at 23°C. In contrast, the mice housed at 30°C did not show
such enhanced metastatic colonization (Fig. 3).
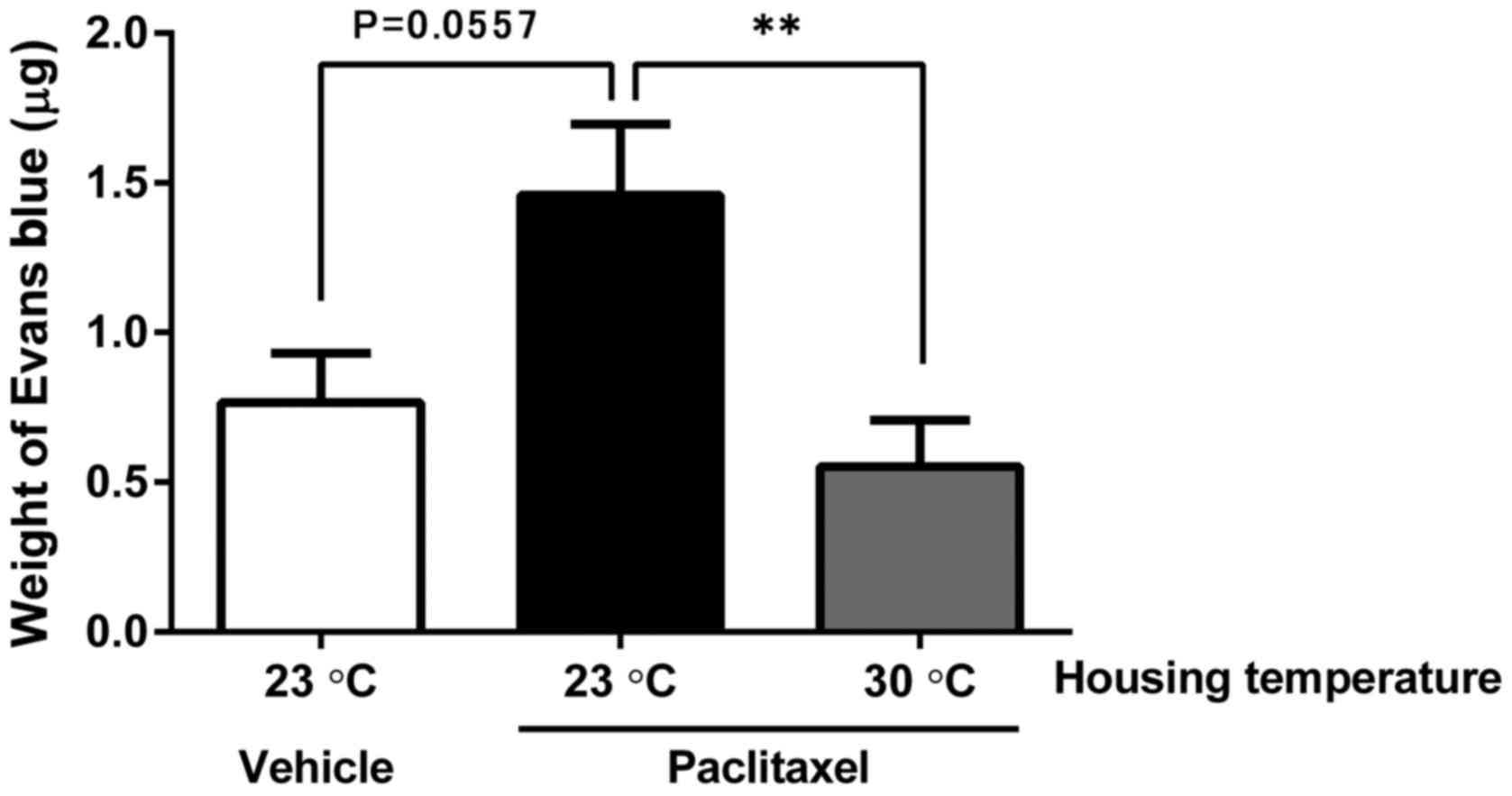
To evaluate angiogenesis, we used a Matrigel plug
assay with 4T1-Luc2 cells under the same treatment and housing
conditions as the metastasis assay. We observed induction of more
angiogenesis in the PTX-treated group compared with the
vehicle-treated group, although the difference was not
statistically significant (P=0.0557). The mice housed at 30°C
showed less angiogenesis in the 4T1-Luc2/Matrigel plug compared
with mice housed at 23°C (Fig. 4).
These results indicate the potential advantage of thermoneutral
housing temperature to prevent the metastasis- and
angiogenesis-promoting effects of PTX.
Discussion
Mammalian body temperature is generally regulated by
a balance between heat loss and generation (20,21).
Several body reactions defend against cold, such as
thermoregulatory behaviors to reduce the loss of heat generated
during basal metabolism, cutaneous vasoconstriction to conserve
heat in the body core, and heat production, including activation of
brown adipose tissue (BAT), and shivering behavior in skeletal
muscle (21–23). In this study, we observed reduced
peripheral blood flow followed by reduced body temperature after
single injections of PTX. These reductions were lasted about 8
days, then recovered. Compared with the vehicle-treated group,
significant blood flow reduction was observed in the low-dose
PTX-treated group, although body temperature was comparable between
the two groups over the same time period (days 5–7). Therefore, we
presume that PTX reduces peripheral blood flow as a compensatory
mechanism for reduction of body temperature. Importantly, supplying
exogenous heat by housing the PTX-treated mice at 30°C compensated
reductions in both body temperature and peripheral blood flow.
These results suggest that impaired heat generation is a mechanism
of PTX-induced body-temperature reduction. Considering that BAT
activity is known to decrease after treatment with taxanes in
breast cancer patients (24),
PTX-induced reduction of body temperature might involve inhibiting
BAT activity. BAT activity is reported to be involved in
psychological stress-induced hyperthermia (25). We observed a slight elevation of body
temperature on day 7 in Fig. 2A. We
don't know the exact reason which caused the elevation of body
temperature. However, the degree of rise in body temperature of
control group was greater than that of PTX-treated group, which
might be because of the inhibiting BAT activity by PTX. In any
case, the exact mechanism through which PTX treatment reduces body
temperature remains to be elucidated.
Thermoneutral temperature refers to the temperature
at which the energy expenditure required to maintain body
temperature is lowest; for mice, this is usually 30–32°C (16,26,27). By
housing mice at thermoneutral temperature, the malignancy-promoting
effects of PTX were suppressed as body temperature and peripheral
blood flow were compensated. Notably, we excluded the possibility
that the reduced lung metastasis by 4T1 cells was a direct
anti-cancer effect of PTX because the 4T1 inoculations in our study
were timed at 4 days after the PTX injections, so that the
administered PTX should have been mostly cleared by then (28). Importantly, tumor formation, growth,
and metastasis of cancer cells are reportedly reduced by keeping
mice at thermoneutral temperature through modulating immune
responses (27). Body temperature
fell by 1–2°C with tumor growth, which exhausted the mice's ability
to maintain normal body temperature at standard housing condition
(27), whereas thermoneutral housing
supplied sufficient heat to allow them to conserve energy needed to
maintain an intact immune system. Indeed, anesthesia-induced
hypothermia (30–32°C) is known to increase lung metastasis from
mammary adenocarcinoma cells by suppressing natural killer cell
activity (29). In obese mice, body
temperature is reportedly 1–2°C lower than in control mice
(30,31); treatment with leptin (an important
regulator of energy balance) suppresses lung metastasis (32) and increases body temperature (33). Taken together, these studies suggest
that reduced body temperature suppresses immune-system cancer
surveillance, and facilitates tumor progression. Thus thermoneutral
temperature may compensate such immune suppression.
To achieve distant metastasis, cancer cells must
enter the circulation and be exposed to the fluid shear stress in
the blood stream, which is influenced by such factors as fluid rate
and viscosity (34). Although
increased fluid shear force can support cancer cells migrating and
binding to the vascular endothelium (35), it also affects tumor cell survival. As
we saw, PTX promotes increased metastatic lung colonization by
4T1-Luc2 cells along with reduced peripheral blood flow. Reduced
fluid shear force might be a mechanism that affects lung metastasis
in PTX-treated mice.
Hypoxia can also influence tumor cells, as a
stressor that impairs growth or as a trigger for malignant
processes, including angiogenesis. Angiogenesis has a critical
function in tumor metastasis because its permeable and
heterogeneous vasculature facilitates extravasation, circulation,
and relocation of tumor cells (3,4,6). Thus, hypoxia caused by PTX-induced
peripheral blood flow reduction might be a complicating factor in
metastasis.
In this study, we investigated the influence of
reduction of body temperature and peripheral blood flow by PTX on
metastasis and angiogenesis. Further studies will be necessary to
clarify the mechanism how PTX reduces body temperature or the
mechanism how reduced body temperature affects the other systems
such as the immune-system.
In summary, we propose that dysregulation of body
temperature and peripheral blood flow by PTX treatment might
decrease fluid shear force, increase tumor hypoxia, and/or suppress
immunity, thus promoting metastasis or angiogenesis. Maintenance of
body temperature or energy supply for thermogenesis may help
prevent tumor relapse or metastasis after chemotherapy.
Acknowledgements
The authors thank Keisuke Ogura for his technical
support.
References
|
1
|
Kirchmair R, Tietz AB, Panagiotou E,
Walter DH, Silver M, Yoon YS, Schratzberger P, Weber A, Kusano K,
Weinberg DH, et al: Therapeutic angiogenesis inhibits or rescues
chemotherapy-induced peripheral neuropathy: Taxol- and
thalidomide-induced injury of vasa nervorum is ameliorated by VEGF.
Mol Ther. 15:69–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gauchan P, Andoh T, Kato A, Sasaki A and
Kuraishi Y: Effects of the prostaglandin E1 analog limaprost on
mechanical allodynia caused by chemotherapeutic agents in mice. J
Pharmacol Sci. 109:469–472. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paduch R: The role of lymphangiogenesis
and angiogenesis in tumor metastasis. Cell Oncol (Dordr).
39:397–410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gupta MK and Qin RY: Mechanism and its
regulation of tumor-induced angiogenesis. World J Gastroenterol.
9:1144–1155. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vaupel P and Harrison L: Tumor hypoxia:
Causative factors, compensatory mechanisms, and cellular response.
Oncologist. 9 Suppl 5:S4–S9. 2004. View Article : Google Scholar
|
|
6
|
Muz B, de la Puente P, Azab F and Azab AK:
The role of hypoxia in cancer progression, angiogenesis,
metastasis, and resistance to therapy. Hypoxia (Auckl). 3:83–92.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morfoisse F, Renaud E, Hantelys F, Prats
AC and Garmy-Susini B: Role of hypoxia and vascular endothelial
growth factors in lymphangiogenesis. Mol Cell Oncol.
2:e10248212015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao Z, Shang B, Zhang G, Miele L, Sarkar
FH, Wang Z and Zhou Q: Tumor cell-mediated neovascularization and
lymphangiogenesis contrive tumor progression and cancer metastasis.
Biochim Biophys Acta. 1836:273–286. 2013.PubMed/NCBI
|
|
9
|
Guindon J and Hohmann AG: Use of sodium
bicarbonate to promote weight gain, maintain body temperature,
normalize renal functions and minimize mortality in rodents
receiving the chemotherapeutic agent cisplatin. Neurosci Lett.
544:41–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guindon J, Deng L, Fan B, Wager-Miller J
and Hohmann AG: Optimization of a cisplatin model of
chemotherapy-induced peripheral neuropathy in mice: Use of vitamin
C and sodium bicarbonate pretreatments to reduce nephrotoxicity and
improve animal health status. Mol Pain. 10:562014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fairchild KD, Viscardi RM, Hester L, Singh
IS and Hasday JD: Effects of hypothermia and hyperthermia on
cytokine production by cultured human mononuclear phagocytes from
adults and newborns. J Interf cytokine Res. 20:1049–1055. 2000.
View Article : Google Scholar
|
|
12
|
Sahdo B, Evans AL, Arnemo JM, Fröbert O,
Särndahl E and Blanc S: Body temperature during hibernation is
highly correlated with a decrease in circulating innate immune
cells in the brown bear (Ursus arctos): A common feature among
hibernators? Int J Med Sci. 10:508–514. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ribatti D: The concept of immune
surveillance against tumors. The first theories. Oncotarget.
8:7175–7180. 2017.PubMed/NCBI
|
|
14
|
Peto J: Cancer epidemiology in the last
century and the next decade. Nature. 411:390–395. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Melvold RW and Sticca RP: Basic and tumor
immunology: A review. Surg Oncol Clin N Am. 16(711–735):
vii2007.
|
|
16
|
Hylander BL and Repasky EA:
Thermoneutrality, mice, and cancer: A heated opinion. Trends
Cancer. 2:166–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lou C, Takahashi K, Irimura T, Saiki I and
Hayakawa Y: Identification of Hirsutine as an anti-metastatic
phytochemical by targeting NF-κB activation. Int J Oncol.
45:2085–2091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suehiro J, Hamakubo T, Kodama T, Aird WC
and Minami T: Vascular endothelial growth factor activation of
endothelial cells is mediated by early growth response-3. Blood.
115:2520–2532. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin WL, Wu SK, Chiang CF, Hsu YH, Lin TH,
Liou HC, Fu WM and Lin WL: Short-time focused ultrasound
hyperthermia enhances liposomal doxorubicin delivery and antitumor
efficacy for brain metastasis of breast cancer. Int J Nanomedicine.
9:4485–4494. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alawi KM, Aubdool AA, Liang L, Wilde E,
Vepa A, Psefteli MP, Brain SD and Keeble JE: The sympathetic
nervous system is controlled by transient receptor potential
vanilloid 1 in the regulation of body temperature. FASEB J.
29:4285–4298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feketa VV and Marrelli SP: Induction of
therapeutic hypothermia by pharmacological modulation of
temperature-sensitive TRP channels: Theoretical framework and
practical considerations. Temperature (Austin). 2:244–257. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morrison SF: Central control of body
temperature. F1000Res. 5:pii: F1000. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ootsuka Y and Tanaka M: Control of
cutaneous blood flow by central nervous system. Temperature
(Austin). 2:392–405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gadea E, Thivat E, Merlin C, Paulon R,
Kwiatkowski F, Chadeyras JB, Coudert B, Boirie Y, Morio B and
Durando X: Brown adipose tissue activity in relation to weight gain
during chemotherapy in breast cancer patients: A pilot study. Nutr
Cancer. 66:1092–1096. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kataoka N, Hioki H, Kaneko T and Nakamura
K: Psychological stress activates a dorsomedial
hypothalamus-medullary raphe circuit driving brown adipose tissue
thermogenesis and hyperthermia. Cell Metab. 20:346–358. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lodhi IJ and Semenkovich CF: Why we should
put clothes on mice. Cell Metab. 9:111–112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kokolus KM, Capitano ML, Lee CT, Eng JW,
Waight JD, Hylander BL, Sexton S, Hong CC, Gordon CJ, Abrams SI and
Repasky EA: Baseline tumor growth and immune control in laboratory
mice are significantly influenced by subthermoneutral housing
temperature. Proc Natl Acad Sci USA. 110:20176–20181. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma YL, Ye J, Zhang PX, Xia XJ and Liu YL:
Comparative study on pharmacokinetics and tissue distribution of a
novel microemulsion based on the paclitaxel/L-OH lipid complex and
paclitaxel injection in cremophor. Yao Xue Xue Bao. 48:1698–1704.
2013.(In Chinese). PubMed/NCBI
|
|
29
|
Ben-Eliyahu S, Shakhar G, Rosenne E,
Levinson Y and Beilin B: Hypothermia in barbiturate-anesthetized
rats suppresses natural killer cell activity and compromises
resistance to tumor metastasis: A role for adrenergic mechanisms.
Anesthesiology. 91:732–740. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jürgens HS, Schürmann A, Kluge R, Ortmann
S, Klaus S, Joost HG and Tschöp MH: Hyperphagia, lower body
temperature, and reduced running wheel activity precede development
of morbid obesity in New Zealand obese mice. Physiol Genomics.
25:234–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Klaus S, Münzberg H, Trüloff C and
Heldmaier G: Physiology of transgenic mice with brown fat ablation:
Obesity is due to lowered body temperature. Am J Physiol.
274:R287–R293. 1998.PubMed/NCBI
|
|
32
|
Mori A, Sakurai H, Choo MK, Obi R, Koizumi
K, Yoshida C, Shimada Y and Saiki I: Severe pulmonary metastasis in
obese and diabetic mice. Int J Cancer. 119:2760–2767. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luheshi GN, Gardner JD, Rushforth DA,
Loudon AS and Rothwell NJ: Leptin actions on food intake and body
temperature are mediated by IL-1. Proc Natl Acad Sci USA.
96:7047–7052. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kon K, Maeda N and Shiga T: Erythrocyte
deformation in shear flow: Influences of internal viscosity,
membrane stiffness, and hematocrit. Blood. 69:727–734.
1987.PubMed/NCBI
|
|
35
|
Mitchell MJ and King MR: Fluid shear
stress sensitizes cancer cells to receptor-mediated apoptosis via
trimeric death receptors. New J Phys. 15:150082013. View Article : Google Scholar
|