Introduction
MUC1, a member of mucin family is overexpressed in
many tumors, but especially in breast cancer. In normal conditions,
MUC1 expression is limited to the apical surface of most ductal
epithelium. In metastatic disease, MUC1 becomes localized
throughout the cell (1). In many
tumor types its expression correlates with poor response to therapy
and poor survival. MUC1 is involved in metastatic progression
through both its extracellular and intracellular domains. It acts
as a cofactor for gene transcription and interacts with proteins in
the extracellular matrix, at the cell membrane, in the cytoplasm,
and in the nucleus (2). MUC1
interacts with four ErbB receptors, but the best characterized is
MUC1-EGFR interaction (3–5). MUC1 associates with adhesion molecules
such as intercellular cell adhesion molecule-1 (ICAM-1) (6,7), members
of Src-family kinases (c-Src, Lyn, Lck) (8–12),
components of Wnt-signaling pathway (13) and MAP kinase pathway, estrogen
receptor α (14), p53 (3), and heat shock protein 90 (HSP90)
(15). MUC1 also activates the
PI3K-Akt pathway and increases the expression of the antiapoptotic
protein by a PI3K-independent mechanism in vitro (16).
The phosphoinositide 3 kinase (PI3K)/Akt/mammalian
target of rapamycin (mTOR) (PAM) pathway is the most frequently
altered pathway in human cancers. Activation of the PAM pathway has
been estimated to be in as frequent as 70% of breast cancers
overall (17). The PI3Ks are a family
of lipid kinases divided into three classes according to the
sequence homology, substrate preference and tissue distribution
(18). Binding of a growth factor or
ligand to its cognate members of the human epidermal growth factor
receptor (HER) family, the insulin and insulin-like growth factor 1
(IGF-1) receptor and others initiates the activation of PI3K, which
phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) to
phosphatidylinositol 3–5-triphosphate (PIP3) and in turn leads to
phosphorylation of Akt (19–21). Akt stimulates cell cycle progression
and proliferation by modulating cell cycle inhibitors, such as p21,
p27kip1 and GSK3, and cell cycle stimulators, such as c-myc and
cyclin D1 (22). Akt also takes part
in programmed cell death through inhibition of both proapoptotic
genes (FasL and Bim) and proteins (BAD and Bax), stimulation of
anti-apoptotic proteins (NF-αK) and degradation of the tumor
suppressor protein p53 (18,23). Phosphorylation of Akt stimulates
protein synthesis and cell growth by activating mTOR, which is a
serine/threonine protein kinase. It is present in two protein
complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2),
which are structurally similar but functionally different (24). mTORC1 leads to cell anabolic growth by
promoting mRNA translocation and protein synthesis and also has
roles in glucose metabolism and lipid synthesis, while mTORC2
organizes the cellular actin cytoskeleton and regulates AKT
phosphorylation (24–26).
The classical Pt-based anticancer drugs such as
cisplatin are useful in the treatment of many tumors. Cisplatin
binds to the major groove of DNA and its cytotoxicity is associated
with inhibition of DNA synthesis and replication by formation of
bifunctional interstrand and intrastrand crosslinks (27,28). In the
recent years, we obtained in our laboratory a series of novel
dinuclear platinum(II) complexes containing berenil and amine
ligands. The compounds display higher antitumor activity than
cisplatin. Berenil [1,3-bis(4′-amidinophenyl) triazene] recognizes
AT rich DNA sequences and it is a strong inhibitor of DNA
topoisomerase II (29,30). Moreover, our complexes bind to the DNA
minor groove and form different types of complex-DNA adducts than
cisplatin (31,32). The dinuclear berenil platinum
complexes with amine ligands are cationic in nature and show
excellent solubility in water. The analysis of the
structure-activity relationship of the dinuclear complexes showed
that berenil provided H-bonding and an electrostatic
pre-association with duplex DNA in the minor groove. The
pre-covalent binding association can be used to control the site of
platination through an increased local concentration at particular
sites on DNA. The berenil platinum complexes differentiate this
from other alkylating agents, which primarily relate to the major
groove of DNA. Structurally novel platinum complexes that bind to
DNA differently than cisplatin may have distinct cytotoxicity and
side effect profiles (33,34).
MUC1 is a type I transmembrane glycoprotein that is
aberrantly overexpressed in various cancer cells especially in
breast cancer cells There are known research works about combined
therapy with monoclonal antibody C595 and docetaxel in several
ovarian cell cancers, but there are no data about combined therapy
based on platinum compounds with anti-MUC1 antibody in breast
cancer cell lines. A novel dinuclear platinum(II) complex (Pt12)
used with anti-MUC1 was compared to its parent drug and cisplatin
with anti-MUC1 in respect to cytotoxicity, DNA, and collagen
biosynthesis in MDA-MB-231 and MCF-7 human breast cancer cells. It
was found that Pt12 with anti-MUC1 was more active inhibitor of DNA
and collagen synthesis as well more cytotoxic agent than Pt12 alone
and cisplatin with anti-MUC1. That was the preliminary study which
gave the basis for further analysis of molecular mechanism of
action.
The aim of the study was to check the mechanism of
action induced by novel berenil complex of platinum(II)-Pt12
together with monoclonal antibody against MUC1 in estrogen receptor
positive breast cancer MCF-7 cells. We analyzed the effect of the
combined treatment on the concentration of selected markers of
apoptosis such as proapoptotic Bax, markers associated with
external (caspase-8) and internal apoptotic pathways (cytochrome
c, caspase-9). The key element of the study was to check the
influence of combined treatment on the concentration of selected
proteins involved in intracellular signal transduction pathways. We
checked the concentration of p53, PI3K and p-Akt.
Materials and methods
Materials
Cisplatin, monoclonal antibody anti-MUC1 GP1.4 were
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Stock cultures of human MCF-7 breast cancer cells were purchased
from the American Type Culture Collection (Manassas, VA, USA).
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum
(FBS) used in a cell culture were products of Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Glutamine, penicillin and
streptomycin were obtained from Quality Biologicals Inc.
(Gaithersburg, MD, USA). ELISA's kits were purchased from USCN Life
Science Inc. (Wuhan, China), BioVendor (Brno, Czech Republic) and
MyBioSource (San Diego, CA, USA). The chemical synthesis and
structure of Pt12 was presented in publication (35).
Cell culture MCF-7
Estrogen receptor positive breast cancer MCF-7 cells
were maintained in DMEM supplemented with 10% FBS, 2 mM glutamine,
50 U/ml penicillin, 50 mg/ml streptomycin at 37°C in a humidified
atmosphere containing 5% CO2.
We have performed the preliminary studies based on
MTT assay for monotherapy and combined treatment. We determined
IC50 values for anti-MUC1 antibody and it was 20 µg/ml.
We reduced the dose of monoclonal antibody to ½ IC50-10
µg/ml. The same experiment was done for Pt12. IC50 value
for Pt12 was 17±2 µM. We have chosen two concentrations: ½
IC50 ± 2 µM (10 µM) and IC50 ± 2 µM (20 µM)
cisplatin was used as a reference in the same doses as our tested
novel compound (Pt12). These results were obtained after 24 h of
incubation with tested compounds and were satisfactory. Furher
studies were done after 24 h of incubation with tested compounds.
Cells were incubated with anti-MUC1 (10 µg/ml), Pt12 (10 µM), Pt12
+ anti-MUC1 (10 µM + 10 µg/ml), cisplatin (10 µM), cisplatin +
anti-MUC1 (10 µM + 10 µg/ml) for 24 h and used to prepare cells
lysates. Briefly, trypsinized cells were washed three times with
cold PBS and centrifuged at 1,000 × g for 5 min at 4°C. The cells
(1×106) were suspended in lysis buffer for whole cell
lysates. After centrifugation the supernatants were frozen
immediately at −70°. The concentration of propapoptotic markers and
proteins involved in intracellular signal transduction pathways was
measured. Cells without addition of compounds were treated as
controls.
Determination of proapoptotic Bax
protein
The high sensitivity assay kit (SEB343Hu; USCN Life
Science Inc.) was used to determine the concentration of
proapoptotic Bax protein in cell lysates. The microtiter plate
provided has been pre-coated with a monoclonal antibody specific to
Bax. Standards and samples were added to the appropriate microtiter
plate wells and incubated for 2 h at 37°C. After the first
incubation step, a biotin-conjugated polyclonal antibody specific
for Bax was pipetted and incubated for 1 h at 37°C. After washing
away any unbound substances, avidin conjugated to horseradish
peroxidase (HRP) was added to each microplate well and incubated.
After another aspiration and washing step, a TMB substrate solution
was added to each well. The enzyme-substrate reaction was
terminated by the addition of a sulfuric acid solution and the
color change was measured at a wavelength of 450 nm. The assay was
performed in duplicate and the concentration of Bax in the samples
was then determined by comparing the OD of the samples to the
standard curve. The range of the standard curve for Bax was:
0.78–50 ng/ml. The minimum detectable dose (MDD) of human Bax was
generally less than 0.32 ng/ml.
Determination of caspase-8 and
caspase-9
Caspase-8 and caspase-9 concentrations in cell
lysates were determined by using an enzyme-linked immunosorbent
assay kit (nos. RBMS2024R and RBMS2025R; BioVendor). Monoclonal
antibodies specific to caspase-8 or caspase-9 were pre-coated onto
a microplate. Standards and samples (100 µl each) were pipetted
into the wells in duplicate and antigen was bound by the
immobilized antibody. The working solution of antibody was also
added to all wells. Then the microplate was incubated for 2 h at
room temperature (RT). After washing away any unbound substances,
an enzyme-linked polyclonal antibody specific for caspase-8 or
caspase-9 (100 µl) was added to each well for 1 h at RT. Following
a wash to remove any unbound antibody enzyme-reagent, a substrate
solution (100 µl) was added to the wells for 15 min; the color
developed in proportion to the amount of antigen bound in the
initial step. Color development was stopped by phosphoric acid, and
the intensity of the color was measured at a wavelength of 450 nm.
The MDD of caspase-8 was 0.1 ng/ml and caspase-9 was: 0.4 ng/ml.
The concentrations of the samples were calculated from the standard
curve and ranged from 0.16 to 10 ng/ml for caspase-8 and 1.6 to 100
ng/ml for caspase-9. The results were presented in nanogram per
milliliter (ng/ml). There was no cross-reactivity with other
caspases.
Determination of cytochrome c and p53
protein
Cytochrome c and p53 concentrations in cell
lysates were determined by using an enzyme-linked immunosorbent
assay kit (nos. RBMS263R and RBMS256R; BioVendor). Anti-human
cytochrome c or anti-human p53 antibody was pre-coated onto
a microplate. Standards and samples were added into appropriate
wells and biotin conjugate was also pipetted to all wells. The
plate was incubated at RT for 2 h. After wash step,
streptavidin-HRP was added into all wells and plate was incubated
for next 1 h in RT. After incubation, unbound streptavidin-HRP was
removed during a wash step and substrate solution was added to the
wells. A colored product was formed in proportion to the amount of
antigen present in the sample or standard. The reaction was
terminated by addition of acid and absorbance was measured at 450
nm. The concentrations of the samples were calculated from the
standard curve. The samples for cytochrome c assessment were
diluted and then multiplied by the dilution factor. The standard
curve ranged from 0.08 to 5 ng/ml for cytochrome c and 0.78
to 50 U/ml for human p53.
Determination of human
phosphotylinosital 3 kinase (PI3K)
Human PI3K concentration in cell lysates was
determined by using an enzyme-linked immunosorbent assay kit
(MBS9303190; MyBioSource). PI3K-specific antibody was pre-coated
onto a microplate. Standards and samples were pipetted into the
wells and any PI3K present was bound by the immobilized antibody.
After removing any unbound substances, a biotin-conjugated
PI3K-specific antibody was added to the wells. After washing,
avidin conjugated HRP was added to the wells. Following a wash to
remove any unbound avidin-enzyme reagent, a substrate solution was
added to the wells and color developed in proportion to the amount
of PI3K bound in the initial step. The color development was
stopped and the intensity of the color was measured. The MDD of
PI3K was typically less than 0.156 ng/ml. The concentrations of the
samples were calculated from the standard curve and ranged from
0.625 to 40 ng/ml. The results were presented in nanogram per
mililiter (ng/ml).
Determination of phospho-Akt
Phospho-Akt concentration in cell lysates was
determined by using an enzyme-linked immunosorbent assay kit
(MBS396298; MyBioSource). An antibody, specific to the non-phospho
and phospho Akt1 protein was immobilized onto the surface of
microtiter wells provided in the kit. Using this ELISA,
cross-reactivity with unphosphorylated Akt1, Akt2, and Akt3 is
minimal. The samples (cell lysate) to be assayed were pipetted into
the wells and allowed to incubate for two h (or overnight for
higher sensitivity), during which time any Akt1 present binds to
the capture antibodies. Unbound material was washed away and a
biotin conjugated anti-phospho Akt1 antibody was added to the wells
and incubated for 2 h at RT. Excess biotin conjugate was removed by
washing and a HRP-conjugated streptavidin was added for 30 min.
Excess HRP conjugate was removed by washing. The HRP catalyzed the
conversion of the chromogenic substrate tetra-methylbenzidine (TMB)
(30 min incubation) from a colorless solution to a blue solution
(or yellow after the addition of stop reagent), the intensity of
which was proportional to the amount of phospho Akt1 protein in the
sample. The coloured reaction product was quantified using a
spectrophotometer. The concentrations were calculated from the
standard curve and ranged from 0.25 to 20 ng/ml.
Caspase-8 enzymatic activity
assay
Caspase-8 activity was measured using FAM-FLICA
Caspase-8 kit (no. 910; ImmunoChemistry Technologies, Bloomington,
MN, USA) according to the manufacturer's instructions. The human
MCF-7 breast cancer cells were treated with the tested compounds
for 24 h, and then harvested and washed with cold buffer PBS. A
total of 5 µl of diluted FLICA reagent and 2 µl of Hoechst 33342
were added to 93 µl of cell suspension and mixed by pipetting. The
cells were incubated for 60 min at 37°C. After incubation, the
cells were washed twice in 400 µl apoptosis wash buffer and
centrifuged at 300 × g. After the last wash, the resuspended cells
in 100 µl apoptosis wash buffer were supplemented with 10 µg/ml PI.
Analysis was performed using the BD FACSCanto II flow cytometer,
and results were analyzed with FACSDiva software (both from BD
Biosciences, San Jose, CA, USA).
Caspase-9 enzymatic activity
assay
Caspase-9 activity was measured using FAM-FLICA
Caspase-9 Kit (no. 913; ImmunoChemistry Technologies) according to
the manufacturer's instructions. The MCF-7 human breast cancer
cells were treated with the tested compounds for 24 h, and then
harvested and washed with cold buffer PBS. 5 µl of diluted FLICA
reagent and 2 µl of Hoechst 33342 were added to 93 µl of cell
suspension and mixed by pipetting. The cells were incubated for 60
min at 37°C. After incubation, the cells were washed twice in 400
µl apoptosis wash buffer and centrifuged at 300 × g. After the last
wash, the resuspended cells in 100 µl apoptosis wash buffer were
supplemented with 10 µg/ml PI. Analysis was performed using the BD
FACSCanto II flow cytometer, and results were analyzed with
FACSDiva software (both from BD Biosciences).
Statistical analysis
Experimental data were presented as means ± standard
deviation since each experiment was repeated three times. One way
Anova (Dunnett's multiple comparisons test) was performed to
demonstrate the difference between single and combined treatments.
A statistically significant difference was defined at P<0.05.
Statistical analysis was performed using GraphPad Prism Version 6.0
(GraphPad Software, Inc., La Jolla, CA, USA).
Results
Impact of Pt12 combined with anti-MUC1
antibody on the intrinsic apoptotic pathway
In our study, we performed ELISA measurements to
check the concentration of selected markers of apoptosis such as:
Proapoptotic Bax, markers associated with external (caspase-8) and
internal apoptotic pathways (cytochrome c, caspase-9).
The breast cancer MCF-7 cells were incubated for 24
h with the tested compounds. We used the compounds alone-anti-MUC1
antibody at two concentrations: 10 and 20 µg/ml, cisplatin and Pt12
at two concentrations: 10 and 20 µM. We checked the combined
effects of anti-MUC1 (10 µg/ml) and cisplatin (10 and 20 µM), and
the anti-MUC1 (10 µg/ml) in combination with Pt12 (10 and 20 µM).
The results were compared with the control cells, cultured without
drugs.
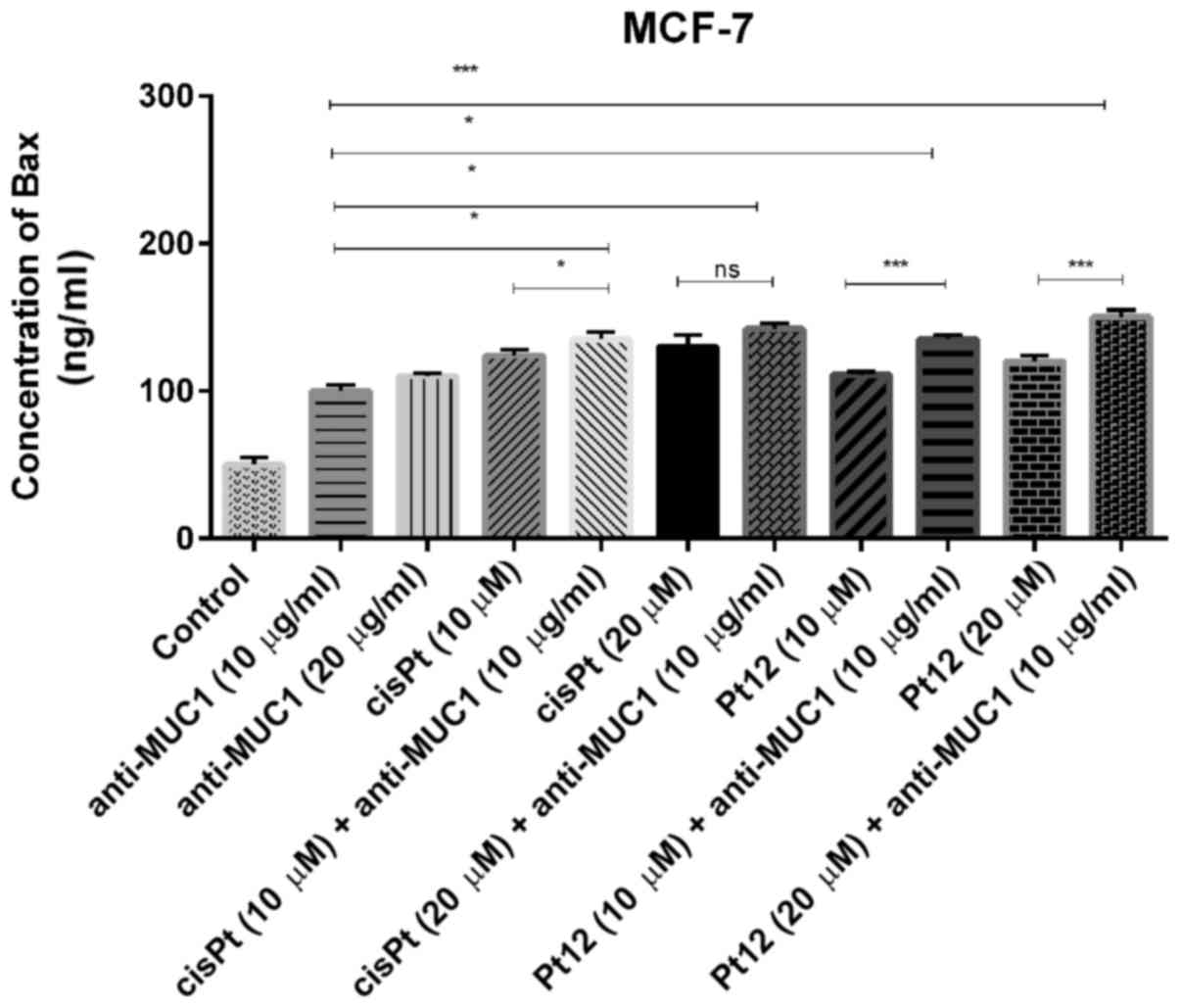
We measured the concentration of proapoptotic Bax
protein (Fig. 1), which is a member
of Bcl-2 family and promotes the release of cytochrome c
from mitochondria to cytosol. All the tested compounds
significantly increased Bax concentration as compared to control.
After 24 h of incubation with agents used in monotherapy, cisplatin
in a dose of 20 µM was a more potent inducer of Bax release. The
effect was a little stronger than that produced by Pt12 and
anti-MUC1 in two doses. The highest concentration of Bax was
observed after combined treatment with Pt12 (20 µM) and anti-MUC1
(10 µg/ml). The concentration of Bax was 150 ng/ml compared to
reference compound cisplatin used with anti-MUC1 in the same doses,
where the level of Bax was 142 ng/ml.
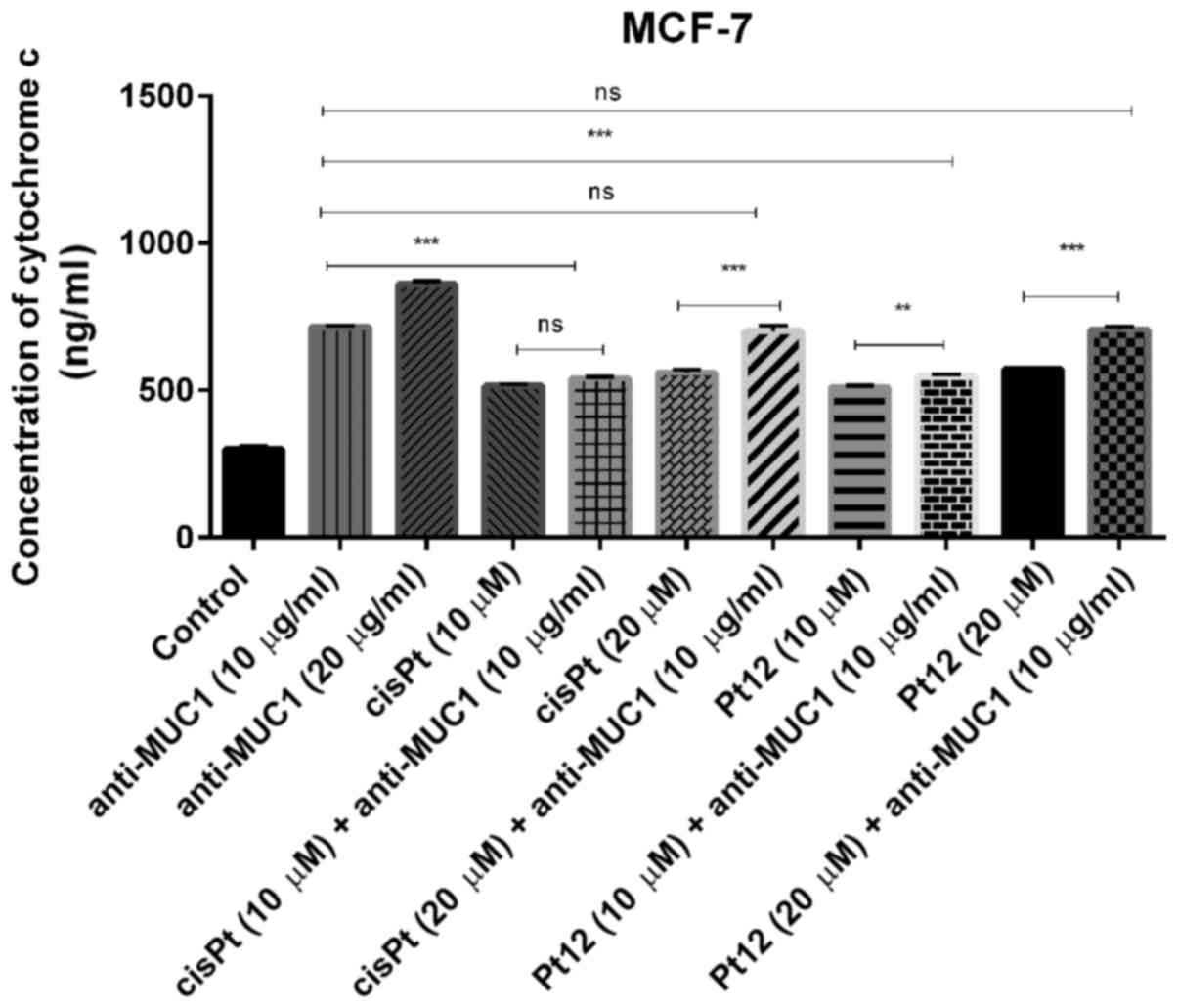
Cytochrome c, a main mediator in intrinsic
apoptotic marker activates the mitochondrial-dependent death in the
cytosol. It binds to the cytosolic Apaf-1 and triggers the
formation of an apoptosome, which recruits pro-caspase-9 to its
caspase recruitment domain (CARD) allowing activation and
proteolysis. The effect of caspase-9 is the activation of executor
caspases-3, −6 and −7 leading to cell death (36). In our study, we proved that all
compounds increased the concentrations of cytochrome c
(Fig. 2) and caspase-9 (Fig. 3) as compared to control. Taking into
account monotherapy, the highest concentration of cytochrome
c (860 ng/ml) was observed after anti-MUC1 treatment in a
dose of 20 µg/ml. Taking into account the combined treatment, the
highest concentration of the analysed marker (707 ng/ml) was
observed after Pt12 (20 µM) with anti-MUC1 (10 µg/ml) in comparison
to the control (300 ng/ml).
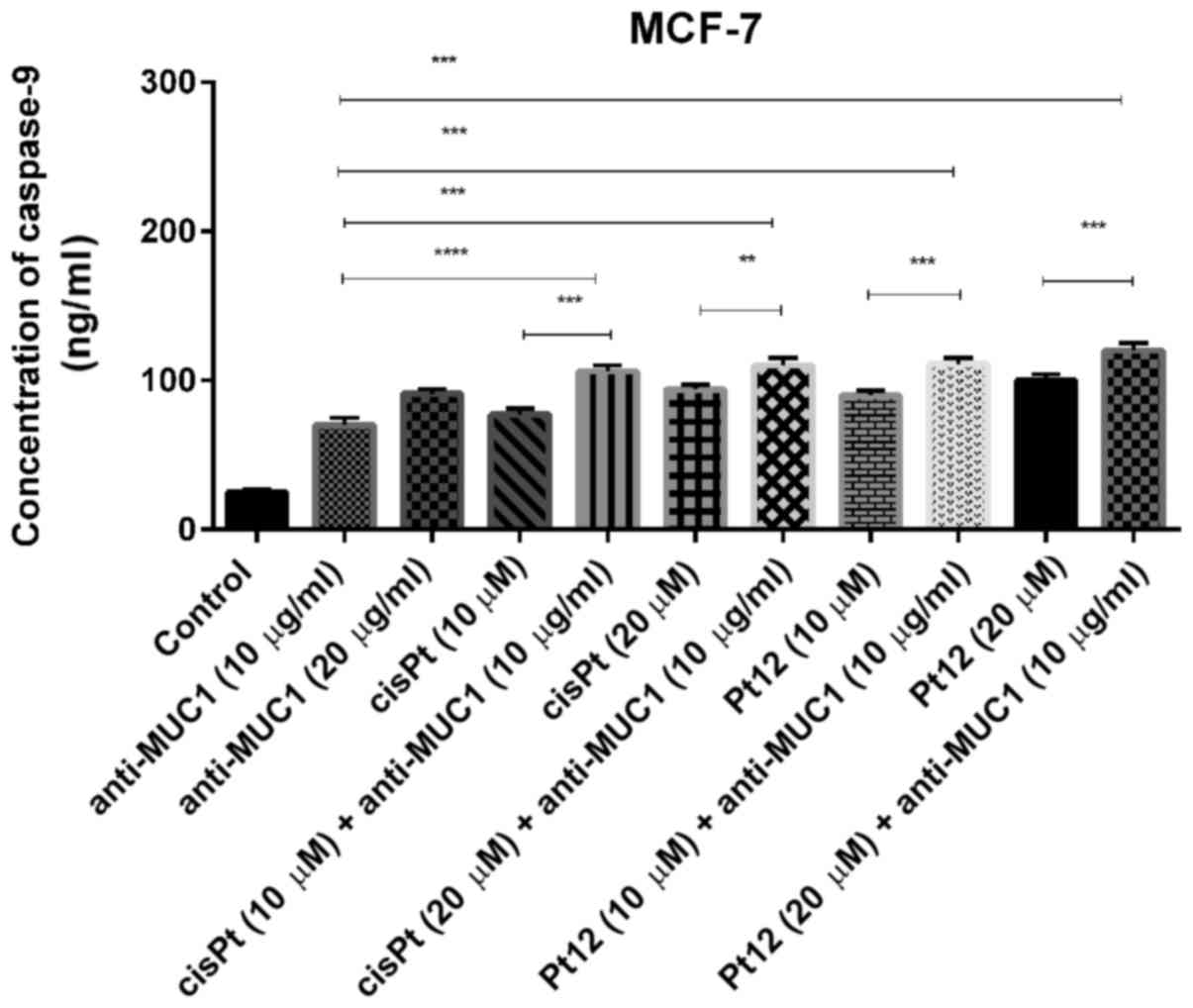
We observed that Pt12 was a stronger inducer of
caspase-9 release compared to cisplatin after 24 h of incubation
with drugs. The concentrations of caspase-9 after treatment with
Pt12 and cisplatin alone were 90 and 77 ng/ml, respectively. The
strongest effect on caspase-9 release was determined after combined
treatment with Pt12 (20 µM) and anti-MUC1 (10 µg/ml). The
concentration of caspase-9 was 120 ng/ml. After incubation with
cisplatin (20 µM) and anti-MUC1 (10 µg/ml), the level of caspase-9
was 94 ng/ml (Fig. 3).
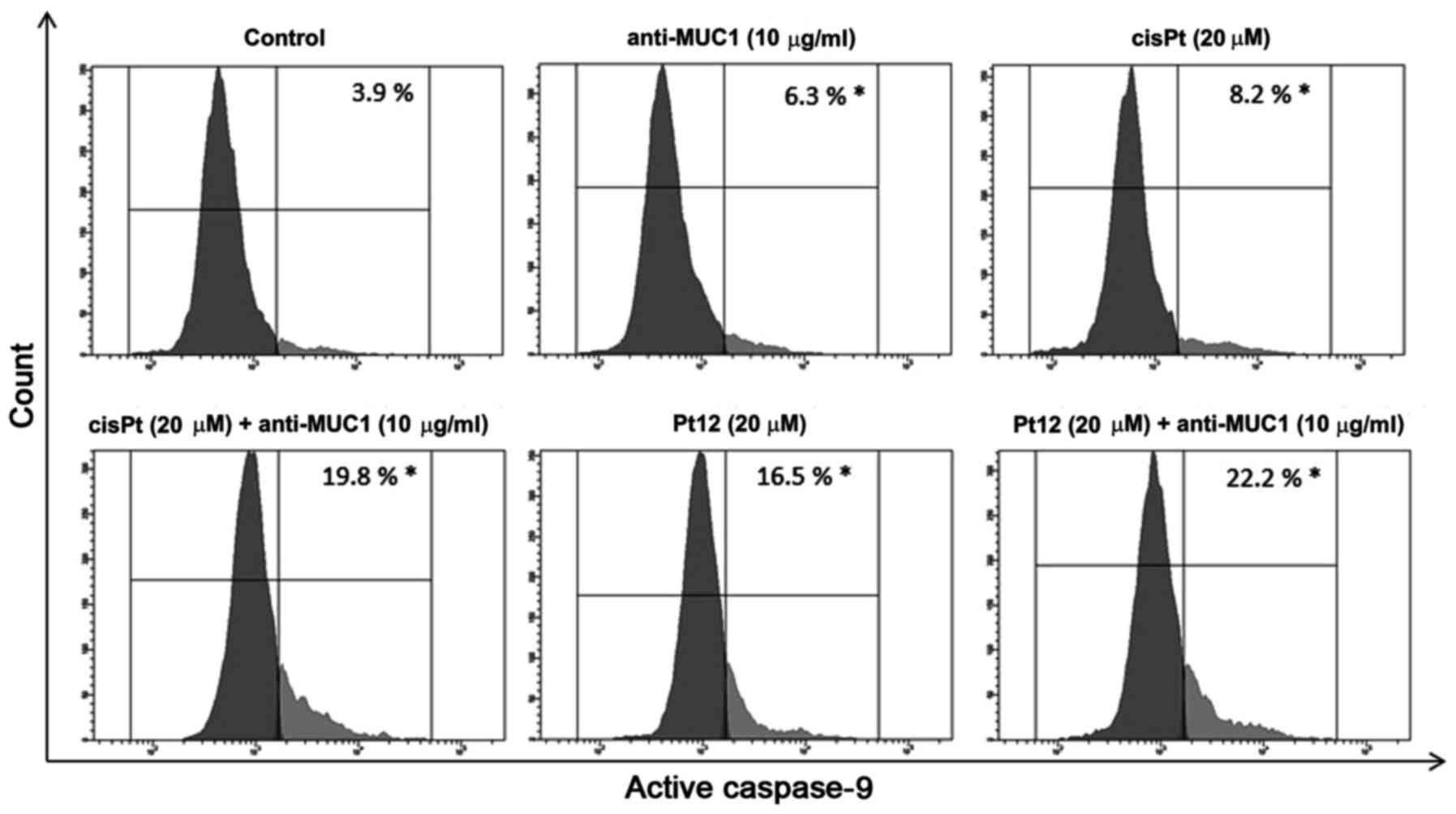
To confirm caspase-9 activation in MCF-7 breast
cancer cells, we treated them with different concentrations of the
compounds tested. Then we lysed the cells and detected the enzyme
activity with flow cytometry. The average values showed that
caspase-9 activity was raised 5.6-fold, respectively in the
anti-MUC1+Pt12 treated cells as compared to the untreated control
cells (Fig. 4). The difference was
statistically significant (P<0.05). The results indicated that
anti-MUC1 together with Pt12 activated the apoptosis initiator
caspase-9 in the MCF-7 cells.
Impact of Pt12 combined with anti-MUC1
antibody on the extrinsic apoptotic pathway
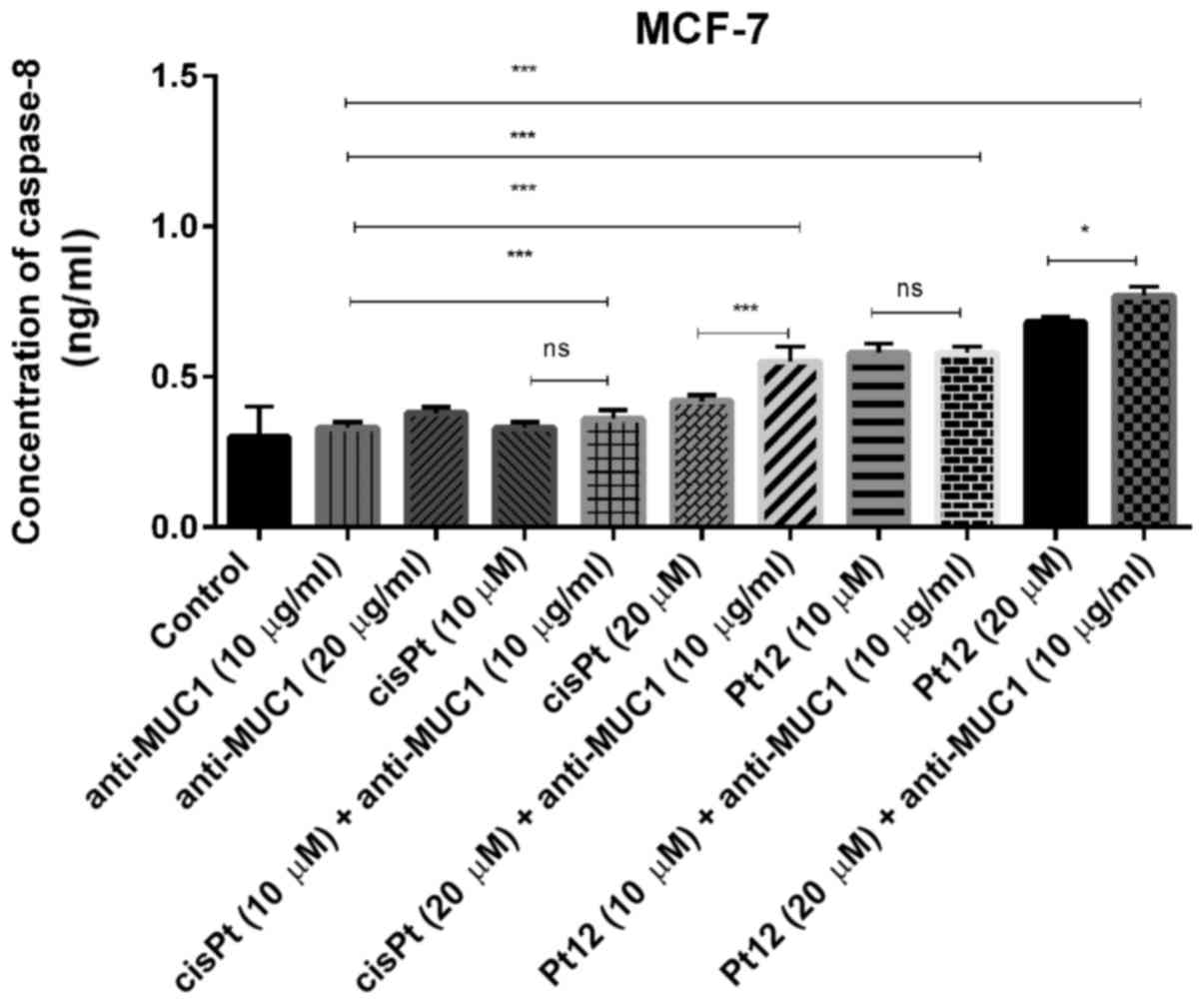
Caspase-8 represents initiator caspases of the
extrinsic apoptotic pathway and its activation results in the
processing of the downstream effector caspases (caspase-3, −6, −7),
which induce cell death. In monotherapy, we observed that only Pt12
in the doses of 10 and 20 µM (0.58 and 0.68 ng/ml) significantly
increased caspase-8 concentration in comparison with two doses of
cisplatin (0.33; 0.42 ng/ml) and anti-MUC1 (0.33 and 0.38 ng/ml).
However, the highest concentration of the apoptotic marker (0.77
ng/ml) was detected after combined treatment with Pt12 (20 µM) and
anti-MUC1 (10 µg/ml). The effect was over twice stronger in
comparison with control (0.3 ng/ml) (Fig.
5).
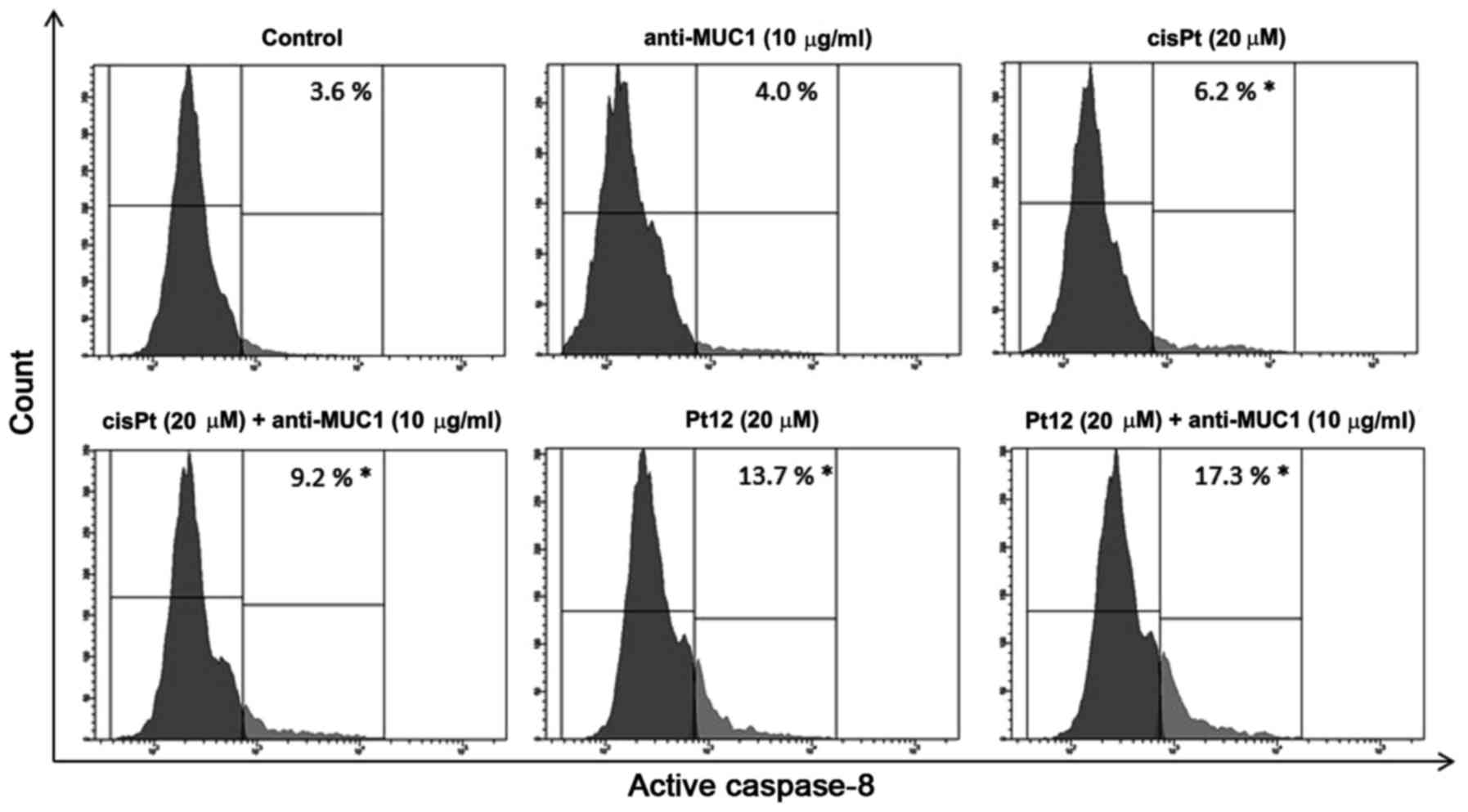
To confirm caspase-8 activation in MCF-7 breast
cancer cells, we lysed the cells and detected the enzyme activity
with flow cytometry. The average values showed that caspase-8
activity was raised 4.8-fold respectively in the anti-MUC1+Pt12
treated cells compared to the untreated control cells (Fig. 6). The differences were statistically
significant (P<0.05). The results indicated that anti-MUC1+Pt12
activated the apoptosis initiator caspase-8 in the MCF-7 cells. The
activation of caspase-8 enzymes suggested the involvement of
combined therapy in the extrinsic apoptotic pathway.
Effect of Pt12 combined with anti-MUC1
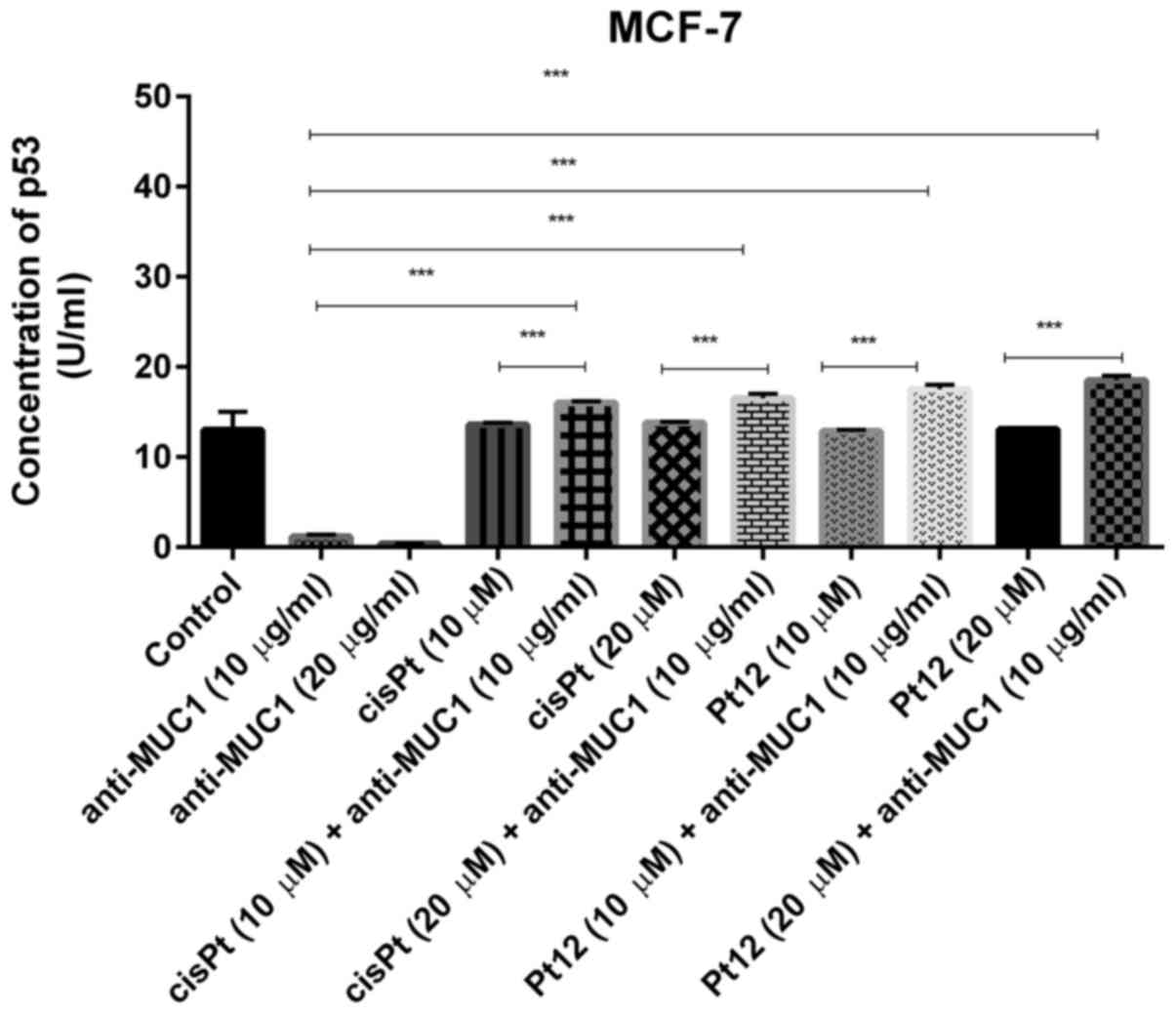
antibody on the p53 concentration
P53 is a major tumor suppressor which upon DNA
damage activates genes involved in either cancer cell growth arrest
or apoptosis (37). We noticed that
anti-MUC1 used in monotherapy in doses of 10 and 20 µg/ml reduced
the concentration of p53 (1.2 and 0.38 U/ml) in cell lysates in
comparison with control (13 U/ml). Pt12 and cisplatin had no
influence on p53 concentration, compared to control. We finally
demonstrated that cisplatin and Pt12 together with anti-MUC1
significantly increased the level of p53 in comparison with
monotherapy (P<0.05) (Fig. 7).
Pt12 combined with anti-MUC1 antibody
reduces the concentration of p-Akt in MCF-7 breast cancer
cells
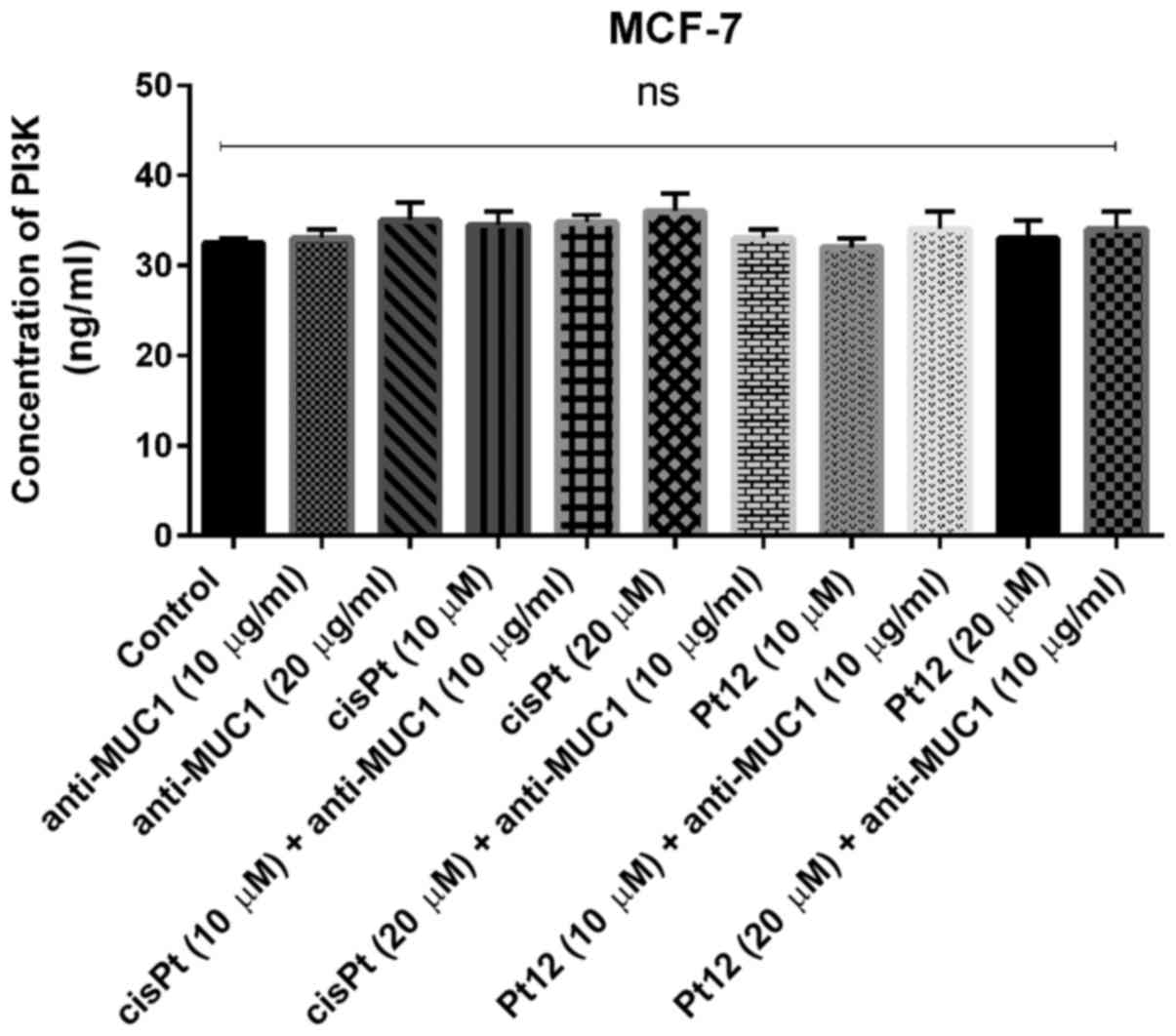
The key element of the study was to check the
influence of combined treatment and monotherapy on the
concentration of PI3K. The analyzed kinase is involved in PAM
pathway, which is the most frequently altered pathway in human
cancers.
We proved that monotherapy as well as combined
treatment based on Pt12 with anti-MUC1 had no influence on the
concentration of PI3K (Fig. 8). We
also checked the effect of the tested compounds on the
concentration of p-Akt, which takes part in the activation of
target proteins involved in cell survival, proliferation, cell
cycle and invasive potential of cells.
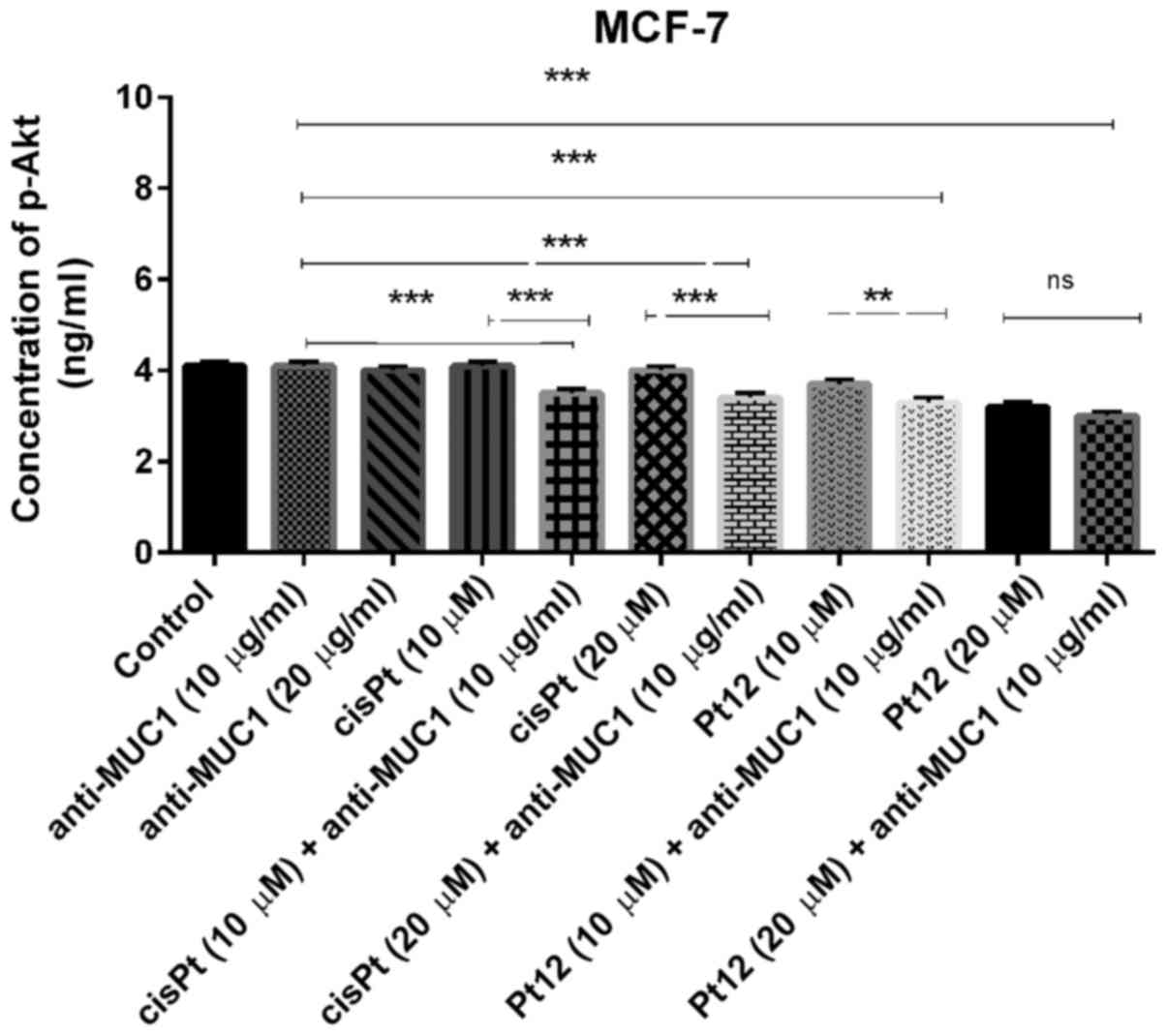
We demonstrated that treatment with compounds such
as anti-MUC1 and cisplatin had no influence on concentration of
p-Akt. The concentration was very similar to control (P>0.05).
The novel berenil complex of platinum(II)-Pt12 significantly
reduced the concentration of the analyzed phosphoprotein; however,
the lowest concentration was detected after combined treatment. The
concentration of p-Akt after treatment with Pt12 and anti-MUC1 was
3 ng/ml (Fig. 9). The combined
treatment based on Pt12 or cisplatin with anti-MUC1 is involved in
p-Akt inhibition. We observed statistically significant differences
between combined treatment and monotherapy (P<0.05).
Discussion
Human cancer is a highly diverse and complex disease
based on multiple etiologies, multiple cell targets and distinct
developmental stages. A key process in the ability of tumor cells
to expand locally is resistance to apoptosis. The suppression of
apoptotic potential can involve the activation of antiapoptotic
factors such as Bcl-2 or loss of expression or mutation of
proapoptotic factors such as p53 (38). However, cancer cell proliferation
might be promoted by the production of growth factors such as PDGF
and TGFα. Modern cancer treatment focuses on molecular defects of
intracellular signal transduction pathways caused by genetic
alterations that drive the oncogenesis. Aberrant expression of the
PI3K-Akt-mTOR pathway is also known to play a critical role in
cancer cell growth, proliferation and angiogenesis (39). Looking for new agents with
proapoptotic potential or targeting PI3K/Akt/mTOR pathway are
possible strategies in breast cancer treatment.
Recently, we confirmed that all the tested compounds
induced apoptosis in MCF-7 and MDA-MB-231 cells, using several
biochemical tests: flow cytometric analysis after Annexin V-FITC
and propidium iodide (PI) staining, mitochondrial membrane
potential and DNA fragmentation. This study confirmed that Pt12
with anti-MUC1 was a more active inhibitor of DNA and collagen
synthesis as well as a more cytotoxic agent than Pt12 alone and
cisplatin with anti-MUC1. Cytotoxicity of Pt12 with anti-MUC1
against breast cancer cells is due to apoptotic cell death as well
as necrotic cell death. That study confirmed that combined
treatment induced apoptosis, although the detailed mechanism was
still unclear (35).
Apoptosis known as a programmed cell death is
executed by a family of proteases called caspases. Caspases play a
crucial role in the mechanism of apoptosis. Initiators (caspase-2,
−8, −9, −10) are responsible for the beginning of apoptotic
pathways and executors (caspase-3, −6 and −7) play central role in
the cleavage of cellular components (36,40).
Recently, we checked the mechanism of Pt12 with anti-MUC1 using
flow cytometry assessment of Annexin V binding assay. It was found
that cytotoxicity of Pt12 with anti-MUC1 against breast cancer
cells is due to apoptotic cell death as well as necrotic cell
death, although detailed characteristics was still unknown
(35). In that study, we demonstrated
higher levels of proapoptotic Bax, cytochrome c and two
initiator caspases such as caspase-8 and caspase-9, which represent
two different apoptotic pathways. Our results proved that Pt12
together with anti-MUC1 induced apoptosis by the extrinsic and
intrinsic cell death pathways.
Some authors have shown that MUC1 interacts with p53
and leads to the activation of p21, a main player in the prevention
of apoptosis and abrogation of the transcription of Bax (41). Other researchers indicate that
increased expression of p53 is responsible for the blockade of cell
cycle and contribute to the induction of apoptosis (42). We demonstrated that only combined
treatment with both chemotherapeutic agents (cisplatin and Pt12)
significantly increased the concentration of p53. The results
obtained after the incubation with cisplatin or Pt12 together with
anti-MUC1 antibody proved that the role of p53 in apoptosis
induction is uncontested. After the incubation with cisplatin or
Pt12 alone, we observed that the concentration of p53 is similar in
comparison with control. However, anti-MUC1 antibody reduced the
concentration of the protein tested as compared to control.
Monotherapy led to the induction of apoptosis in another way,
independently of p53 protein. Abeysinghe et al (43) proved that the novel iron chelator,
tachypyridine [N,N',N'-tris (2-pyridylmethyl)-cis,
cis-1,3,5-triaminocyclohexane] initiates an apoptotic mode of cell
death that does not require functional p53. Some human tumors
contain a functionally defective p53, that reduces sensitivity to
commonly used chemotherapeutic drugs, such as cisplatin, so
induction of apoptosis independently of p53 may be an advantage in
anticancer treatment (43).
The literature data show a link between MUC1 and
PI3K/Akt pathway. MUC1 acts as an oncoprotein through the PI3K-Akt
pathway and promotes the growth and survival of cancer cells.
Kosugi et al (44) have
demonstrated that MUC1 C-terminal subunit is involved in the
regulation of glucose uptake and lactate production in
MUC1-C-induced transformation of rat fibroblasts and in human
breast cancer cells. The results also proved that the MUC1
cytoplasmic domain interacts directly with PKM2 and regulates PKM2
activity. MUC1 stimulates glycolysis through phosphoinositide 3
kinase and serine/threonine kinase (PI3K-Akt) (44). Other studies have demonstrated that
MUC1-C activates the PI3K->Akt pathway, which in turn stimulates
the activity of the glycolytic enzymes, hexokinase and
phosphofructose kinase (16). Raina
et al (45) have shown that
MUC1 C-terminal subunit (MUC1-C) cytoplasmic domain associates with
PI3K p85 in non small lung cancer cells. The inhibition of MUC1-C
with cell-penetrating peptides blocks this interaction with PI3K
p85 and suppresses constitutive phosphorylation of Akt and its
downstream effector, mTOR. They tested MUC1-C peptide inhibitor
GO-203, which was responsible for downregulation of PI3K→ Akt
signaling and inhibition of growth cancer cells (45). Woo et al (46) also showed the relationship between the
activation of the PI3K-Akt by MUC1 and angiogenesis mediated by
VEGF. The present results show the inhibition of p-Akt after
treatment with Pt12 and anti-MUC1 antibody. The concentration of
PI3K was unchanged in comparison with control sample, thus
suggesting that Akt is inhibited independently of PI3K.
The present study has some limitations associated
with lack of usage of more than one cell line, but we showed that
combined treatment is a promising strategy in anticancer treatment
and represents the alternative to monotherapy. All compounds used
alone (Pt12, cisplatin, anti-MUC1 antibody) increased the
concentration of proapoptotic Bax, cytochrome c and
caspase-9 in comparison with control, thus suggesting that they
activate the mitochondrial apoptotic pathway. Pt12 alone
significantly increased the concentration of caspase-8 responsible
for the initiation of the extrinsic apoptotic pathway. However, the
strongest effect was observed after Pt12 combined with anti-MUC1
antibody. These two compounds added together strongly induced
apoptosis in MCF-7 breast cancer cells by external and internal
apoptotic pathways. We also demonstrated that combined treatment
based on Pt12 and anti-MUC1 antibody significantly reduced p-Akt
concentration.
The obtained results should be confirmed by more
experimental methods such as WB, qPCR, cell cycle analysis, TUNEL
etc and in vivo studies are also required.
In summary, the present study revealed that combined
treatment based on Pt12 and anti-MUC1 antibody with its
proapoptotic activity, p-Akt inhibitory properties might be a
promising strategy in breast cancer treatment.
Acknowledgements
This study was supported by research grant
N/ST/MN/17/001/2229 from Medical University of Bialystok
(Bialystok, Poland).
References
|
1
|
Rahn JJ, Dabbagh L, Pasdar M and Hugh JC:
The importance of MUC1 cellular localization in patients with
breast carcinoma: An immunohistologic study of 71 patients and
review of the literature. Cancer. 91:1973–1982. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Horm TM and Schroeder JA: MUC1 and
metastatic cancer. Expression, function and therapeutic targeting.
Cell Adh Migr. 7:187–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh PK and Hollingsworth MA: Cell
surface-associated mucins in signal transduction. Trends Cell Biol.
16:467–476. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schroeder JA, Thompson MC, Gardner MM and
Gendler SJ: Transgenic MUC1 interacts with epidermal growth factor
receptor and correlates with mitogen activated protein kinase
activation in the mouse mammary gland. J Biol Chem.
276:13057–13064. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pochampalli MR, el Bejjani RM and
Schroeder JA: MUC1 is a novel regulator of ErbB1 receptor
trafficking. Oncogene. 26:1693–1701. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rahn JJ, Chow JW, Horne GJ, Mah BK,
Emerman JT, Hoffman P and Hugh JC: MUC1 mediates transendothelial
migration in vitro by ligating endothelial cell ICAM-1. Clin Exp
Metastasis. 22:475–483. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Regimbald LH, Pilarski LM, Longenecker BM,
Reddish MA, Zimmermann G and Hugh JC: The breast mucin MUCI as a
novel adhesion ligand for endothelial intercellular adhesion
molecule 1 in breast cancer. Cancer Res. 56:4244–4249.
1996.PubMed/NCBI
|
|
8
|
Li Q, Ren J and Kufe D: Interaction of
human MUC1 and beta-catenin is regulated by Lck and ZAP-70 in
activated Jurkat T cells. Biochem Biophys Res Commun. 315:471–476.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Kuwahara H, Ren J, Wen G and Kufe D:
The c-Src tyrosine kinase regulates signaling of the human DF3/MUC1
carcinoma-associated antigen with GSK3 beta and beta-catenin. J
Biol Chem. 276:6061–6064. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Chen W, Ren J, Yu WH, Li Q, Yoshida
K and Kufe D: DF3/MUC1 signaling in multiple myeloma cells is
regulated by interleukin-7. Cancer Biol Ther. 2:187–193. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mukherjee P, Tinder TL, Basu GD and
Gendler SJ: MUC1 (CD227) interacts with lck tyrosine kinase in
Jurkat lymphoma cells and normal T cells. J Leukoc Biol. 77:90–99.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Al MA and Gendler SJ: Muc1 affects c-Src
signaling in PyV MT-induced mammary tumorigenesis. Oncogene.
24:5799–5808. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamamoto M, Bharti A, Li Y and Kufe D:
Interaction of the DF3/MUC1 breast carcinoma-associated antigen and
beta-catenin in cell adhesion. J Biol Chem. 272:12492–12494. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei X, Xu H and Kufe D: MUC1 oncoprotein
stabilizes and activates estrogen receptor alpha. Mol Cell.
21:295–305. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren J, Bharti A, Raina D, Chen W, Ahmad R
and Kufe D: MUC1 oncoprotein is targeted to mitochondria by
heregulin-induced activation of c-Src and the molecular chaperone
HSP90. Oncogene. 25:20–31. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raina D, Kharbanda S and Kufe D: The MUC1
oncoprotein activates the antiapoptotic phosphoinositide
3-kinase/Akt and Bcl-xL pathways in rat 3Y1 fibroblasts. J Biol
Chem. 279:20607–20612. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JJ, Loh K and Yap YS: PI3K/Akt/mTOR
inhibitors in breast cancer. Cancer Biol Med. 12:342–354.
2015.PubMed/NCBI
|
|
18
|
Castaneda CA, Cortes-Funes H, Gomez HL and
Ciruelos EM: The phosphatidyl inositol 3-kinase/AKT signaling
pathway in breast cancer. Cancer Metastasis Rev. 29:751–759. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chitnis MM, Yuen JS, Protheroe AS, Pollak
M and Macaulay VM: The type 1 insulin-like growth factor receptor
pathway. Clin Cancer Res. 14:6364–6370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baselga J: Targeting the
phosphoinositide-3 (PI3) kinase pathway in breast cancer.
Oncologist. 16 Suppl 1:S12–S19. 2011. View Article : Google Scholar
|
|
22
|
Ito K, Bernardi R and Pandolfi PP: A novel
signaling network as a critical rheostat for the biology and
maintenance of the normal stem cell and the cancer-initiating cell.
Curr Opin Genet Dev. 19:51–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hirsch E, Ciraolo E, Ghigo A and Costa C:
Taming the PI3K team to hold inflammation and cancer at bay.
Pharmacol Ther. 118:192–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dowling RJO, Topisirovic I, Fonseca BD and
Sonenberg N: Dissecting the role of mTOR: Lessons from mTOR
inhibitors. Biochim Biophys Acta. 1804:433–439. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kenerson HL, Aicher LD, True LD and Yeung
RS: Activated mammalian target of rapamycin pathway in the
pathogenesis of tuberous sclerosis complex renal tumors. Cancer
Res. 62:5645–5650. 2002.PubMed/NCBI
|
|
26
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lippert B: Cisplatin: Chemistry and
biochemistry of a leading anticancer drug. Wiley-VCH; Basel: pp.
31999
|
|
28
|
Bielawski K, Bielawska A, Popławska B and
Bołkun-Skórnicka U: Synthesis, DNA-binding affinity and
cytotoxicity of the dinuclear platinum(II) complexes with berenil
and amines ligands. Acta Pol Pharm. 65:363–370. 2008.PubMed/NCBI
|
|
29
|
Barcelo F, Ortiz-Lombardia M and Portugal
J: Heterogeneous DNA binding modes of berenil. Biochim Biophys
Acta. 1519:175–184. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nguyen B, Hamelberg D, Bailly C, Colson P,
Stanek J, Brun R, Neidle S and Wilson WD: Characterization of a
novel DNA minor-groove complex. Biophys J. 86:1028–1041. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Czarnomysy R, Bielawski K, Muszynska A,
Bielawska A and Gornowicz A: Biological evaluation of
dimethylpyridine-platinum complexes with potent antiproliferative
activity. J Enzyme Inhib Med Chem. 31:150–165. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bielawski K, Czarnomysy R, Muszyńska A,
Bielawska A and Popławska B: Cytotoxicity and induction of
apoptosis of human breast cancer cells by novel platinum(II)
complexes. Environ Toxicol Pharmacol. 35:254–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bielawska A, Popławska B, Surazyński A,
Czarnomysy R and Bielawski K: Cytotoxic efficacy of a novel
dinuclear platinum(II) complex in human breast cancer cells. Eur J
Pharmacol. 643:34–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bielawski K, Bielawska A, Popławska B,
Surazyński A and Czarnomysy R: The effect of a novel dinuclear
platinum complex with berenil and 2-picoline ligands on growth of
human breast cancer cells. Acta Pol Pharm. 67:609–614.
2010.PubMed/NCBI
|
|
35
|
Gornowicz A, Kałuża Z, Bielawska A,
Gabryel-Porowska H, Czarnomysy R and Bielawski K: Cytotoxic
efficacy of a novel dinuclear platinum(II) complex used with
anti-MUC1 in human breast cancer cells. Mol Cell Biochem.
392:161–174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pistritto G, Trisciuoglio D, Ceci C,
Garufi A and D'Orazi G: Apoptosis as anticancer mechanism: Function
and dysfunction of its modulators and targeted therapeutic
strategies. Aging (Albany NY). 8:603–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vousden KH and Lane DP: P53 in health and
disease. Nat Rev Mol Cell Biol. 8:275–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim HJ, Hawke N and Baldwin AS: NF-kappaB
and IKK as therapeutic targets in cancer. Cell Death Differ.
13:738–747. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dey N, Sun Y, Carlson JH, Wu H, Lin X,
Leyland-Jones B and De P: Anti-tumor efficacy of BEZ235 is
complemented by its anti-angiogenic effects via downregulation of
PI3K-mTOR-HIF1alpha signaling in HER2-defined breast cancers. Am J
Cancer Res. 6:714–746. 2016.PubMed/NCBI
|
|
40
|
Thomberry NA and Laxebnik Y: Caspases:
Enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wei X, Xu H and Kufe D: Human MUC1
oncoprotein regulates p53-responsive gene transcription in the
genotoxic stress response. Cancer Cell. 7:167–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lutz W and Nowakowska-Swirta E: Gene p53
mutations, protein p53, and anti-p53 antibodies as biomarkers of
cancer process. Int J Occup Med Environ Health. 15:209–218.
2002.PubMed/NCBI
|
|
43
|
Abeysinghe RD, Greene BT, Haynes R,
Willingham MC, Turner J, Planalp RP, Brechbiel MW, Torti FM and
Torti SV: p53-independent apoptosis mediated by tachpyridine, an
anti-cancer iron chelator. Carcinogenesis. 22:1607–1614. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kosugi M, Ahmad R, Alam M, Uchida Y and
Kufe D: MUC1-C oncoprotein regulates glycolysis and pyruvate kinase
M2 activity in cancer cells. PLoS One. 6:e282342011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Raina D, Kosugi M, Ahmad R, Panchamoorthy
G, Rajabi H, Alam M, Shimamura T, Shapiro GI, Supko J, Kharbanda S
and Kufe D: Dependence on the MUC1-C oncoprotein in non-small cell
lung cancer cells. Mol Cancer Ther. 10:806–816. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Woo JK, Choi Y, Oh SH, Jeong JH, Choi DH,
Seo HS and Kim CW: Mucin 1 enhances the tumor angiogenic response
by activation of the AKT signaling pathway. Oncogene. 31:2187–2198.
2012. View Article : Google Scholar : PubMed/NCBI
|