Introduction
Castleman's disease (CD) is a rare
lymphoproliferative disorder characterized by enlarged hyperplastic
lymph nodes. It has been six decades since the discovery of CD by
Dr Benjamin Castleman, who described a patient with a solitary
hyperplastic mediastinal lymph node in 1954 (1). With the development of clinical
examination and imaging technology, numerous previous studies have
investigated CD, including the pathophysiology, associated
inflammatory mediators and molecular therapies (2,3).
Generally, CD can be categorized into two subtypes based on the
anatomical distribution of disease: Unicentric CD (UCD) and
multicentric CD (MCD). UCD is the most common form, with localized
disease and usually being the hyaline vascular histological type
(4), whereas MCD is a much more
aggressive form with a plasma cell variant pattern histologically
(3). Pathological biopsy is required
for the diagnosis of CD when a patient presents with
lymphadenopathy, as UCD is often asymptomatic and MCD is frequently
accompanied by systemic manifestations, including fever, fatigue,
edema and weight loss (5). Laboratory
abnormalities have been observed in patients with CD, including
anemia, leukocytosis, thrombocytosis, hypergammaglobulinemia,
hypoalbuminemia and increased C-reactive protein expression levels
(5); however, these are usually
assigned to a differential diagnosis that includes rheumatic
diseases and lymphoma. Notably, the detection of dysregulated
plasma interleukin (IL)-6 level may be useful upon laboratory
examination, as previous studies have shed light on the critical
role of IL-6 in the disease process (2,6,7).
The aim of the present study was to investigate the
diagnosis, use of accessory examinations and treatment strategies
in 34 patients with CD treated in Drum Tower Hospital of Nanjing
University Medical School (Nanjing, China). The present study also
identified recent advances in elucidating the pathophysiology of
CD, and discussed available treatment options to improve the
understanding of this uncommon disease.
Patients and methods
Patients
A total of 34 patients with CD who were treated at
Drum Tower Hospital of Nanjing University Medical School (Nanjing,
China) between January 2006 and September 2014 were identified from
the pathological database. A total of 13 patients were men and 21
were women, with a median age of 47 years and a range of 24–71
years. Histological data were obtained from lymph node biopsies to
confirm the diagnosis of CD. All clinical data were acquired by
reviewing medical records and contacting patients by telephone.
Although the association between human immunodeficiency virus (HIV)
infection and CD has been observed in a previous study (8), the serological test results for HIV in
all the patients in the present study were negative. Written
informed consent was obtained from all study participants or their
legal guardian prior to enrollment in the present study. All
procedures performed in the present study involving human
participants were in accordance with the ethical standards of the
Human Subjects Institutional Committee of Drum Tower Hospital, and
with the 1964 Helsinki Declaration and its later amendments.
Category definitions
Based on the anatomical distribution of the disease,
patients were divided into two groups: The more common UCD and the
relatively less common MCD. As aforementioned, the UCD group
consisted of patients who displayed histological evidence of CD in
1 single group of lymph nodes without clinical or radiological
evidence of adenopathy elsewhere. Patients with MCD displayed
histological evidence of CD in ≥1 group of lymph nodes and
radiological or clinical evidence of additional adenopathy.
According to the histological criteria proposed by Keller et
al (4), CD was further classified
into two types: The hyaline vascular (HV) type and the plasma cell
(PC) type. Lymph nodes with characteristics intermediate between HV
and PC were categorized as a mixed type.
Data collection
Relevant clinical data, including gender, chief
complaint, clinical presentation, and lymph node distribution;
laboratory data including erythrocyte sedimentation rate (ESR),
C-reactive protein (CRP) level, leukocyte number, and
albumin/globulin ratio; radiological and pathological data were
collected to evaluate disease progression and treatment response.
Different treatment options, including surgery, chemotherapy and
radiation therapy, were recorded and assessed for clinical efficacy
in terms of post-treatment prognoses.
Results
Location and histological
features
There was a total of 27 patients with UCD and 7 with
MCD. The median ages of the patients with UCD and MCD were 48 and
46 years, respectively. By definition, UCD was localized to one
site. Of the 27 unicentric patients, 9 presented with UCD of the
retroperitoneum (33.3%), 6 with UCD of the neck (22.2%), 4 with UCD
of the mediastinum (14.8%), 3 with UCD of the groin (11.1%), 2 with
UCD of the pelvic cavity (7.4%), 2 with UCD of the armpit (7.4%)
and 1 with UCD of the mesentery (3.7%). Among the patients with
MCD, 6 patients presented with MCD of the armpit. HVCD was
identified in 22 patients (88%) with UCD and in 3 patients with MCD
(12%). Conversely, PCCD was observed in 5 patients (55.5%) with UCD
and in 4 patients with MCD (44.5%). No mixed variants were
identified in these patients.
Clinical manifestations and signs
The relevant major symptoms in the 34 patients with
CD are presented in Table I. In
general, 14 patients with UCD were detected on routine examination
without displaying symptoms and the anatomical regions involved
included the retroperitoneum, mediastinum, mesentery and pelvic
cavity. A total of 11 patients with UCD had swollen lymph nodes and
were admitted to hospital for lymph node biopsy, and the other 2
patients with UCD were identified by lymph node biopsy during
radical surgery for liver cancer and pancreatic cancer, which were
initially recognized as lymph node metastasis. Of the 7 patients
with MCD, 5 patients displayed systemic manifestations, including
fever and fatigue, and 1 patient had breathing difficulties induced
by enlarged lymph nodes compressing the trachea. The remaining 2
patients did not present with any clinical symptoms, and were
initially identified by routine examination of computed tomography
(CT) scans.
 | Table I.Clinical manifestations and
indicators. |
Table I.
Clinical manifestations and
indicators.
| Category | UCD | MCD |
|---|
| Histological feature,
n |
|
|
| HV
type | 22 | 3 |
| PC
type | 5 | 4 |
|
Mixed-type | 0 | 0 |
| Gender |
|
|
|
Male/female, n | 9/18 | 4/3 |
| Median
age, years | 48 | 46 |
| Chief complaint,
n |
|
|
| Routine
examination | 14 | 2 |
|
Accidental touch | 11 | 1 |
|
Compression symptoms | 0 | 1 |
| Other
signs | 2 | 0 |
| Clinical
presentation, n |
|
|
|
Fever | 0 | 5 |
|
Fatigue | 0 | 6 |
| Weight
loss | 0 | 3 |
|
Edema | 0 | 1 |
|
Anemia | 3 | 5 |
| ESR
elevated | 3 | 5 |
| CRP
elevated | 5 | 5 |
|
Albumin/globulin
decreased | 6 | 5 |
| Decreased
leukocytes | 2 | 4 |
| Pleural
effusion | 0 | 3 |
|
Ascites | 0 | 1 |
|
Pericardial effusion | 0 | 2 |
| Skin
lesion | 0 | 2 |
| Numbness
of extremities | 0 | 1 |
|
Hepatosplenomegaly | 0 | 3 |
| Lymph node
distribution, n |
|
|
| Neck | 6 | 3 |
|
Mediastinum | 4 | 3 |
|
Retroperitoneum | 9 | 4 |
|
Armpit | 2 | 6 |
| Pelvic
cavity | 2 | 1 |
|
Groin | 3 | 5 |
|
Mesentery | 1 | 0 |
Laboratory and radiological
examinations
Numerous frequently recorded abnormal laboratory
values were analyzed in patients with UCD and MCD. In patients with
UCD, an elevated immunoglobulin G level was observed in 6 patients
(22.2%), reduced hemoglobin levels in 3 patients (11.1%), a reduced
leukocyte number in 2 patients (7.4%), and an elevated C-reactive
protein expression level and erythrocyte sedimentation rate in 5
(18.5%) and 6 patients (22.2%), respectively. An elevated
immunoglobulin G expression level was revealed in 5 patients
(71.4%) with MCD. An elevated erythrocyte sedimentation rate and
C-reactive protein expression level were demonstrated in 5
patients. Compared with patients with UCD, skin lesions and
numbness of the extremities were also observed in 2 and 1 patient,
respectively, in the MCD group. These 2 patients with skin lesions
were also diagnosed with polyneuropathy, organomegaly,
endocrinopathy, monoclonal gammopathy and skin changes
syndrome.
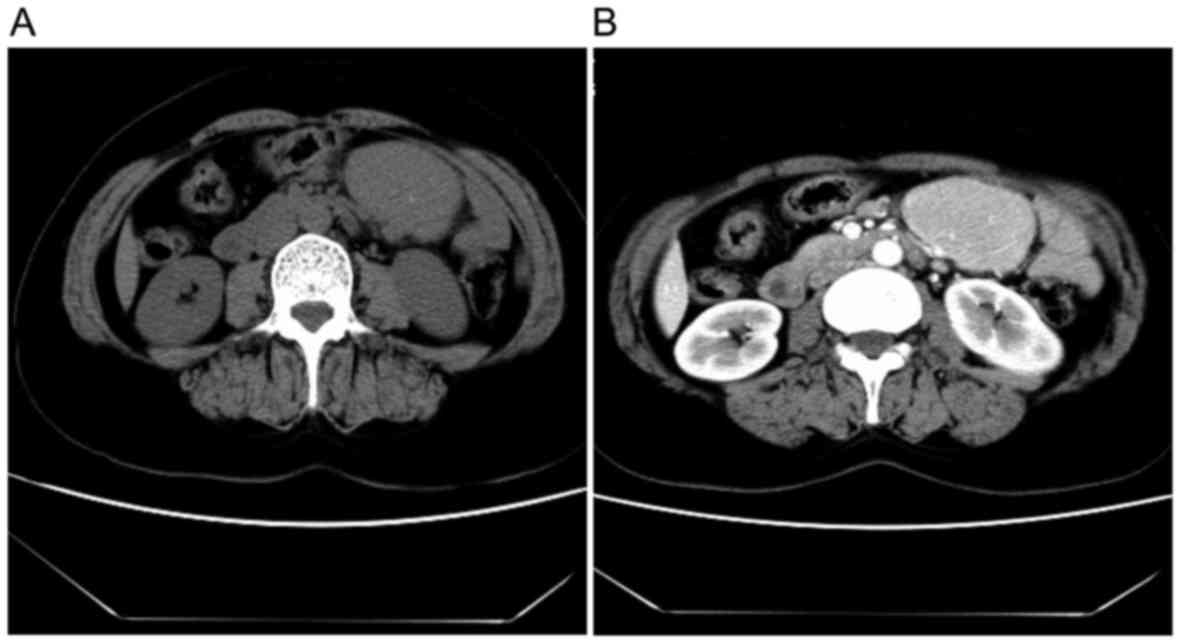
All patients with UCD underwent CT scans (Fig. 1), which displayed an atypical
homogeneous soft tissue mass with moderate enhancement following
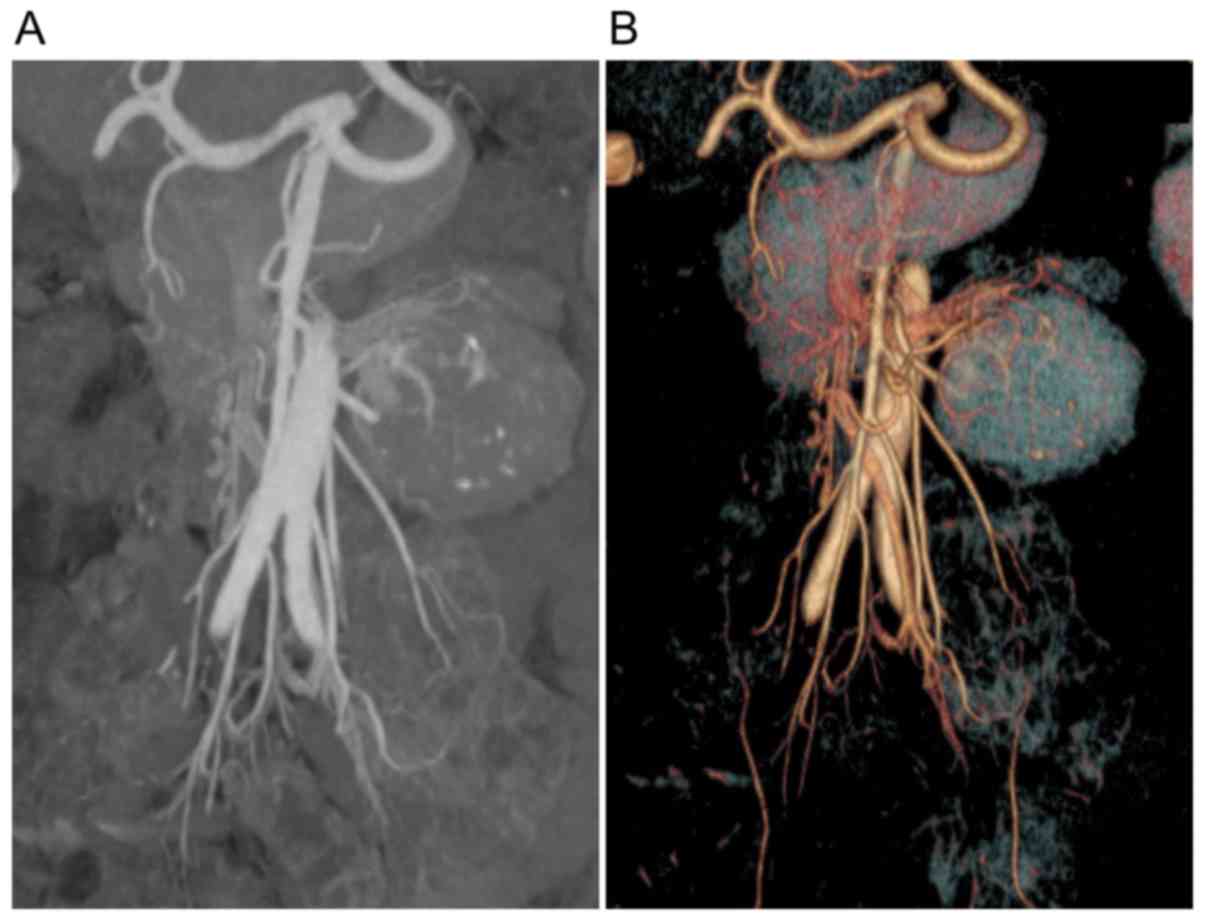
injection of contrast material. A total of 12 patients with UCD
also underwent multidetector computed tomography (MDCT) angiography
(9) to clarify the association
between enlarged tissue, blood vessels and peripheral vital organs
(Fig. 2). MCD was characterized by
predominant lymphadenopathy, which involved peripheral lymph nodes.
Furthermore, patients in the MCD group presented with effusion on
CT scans, involving the chest (3 patients), the peritoneum (1
patient) and the pericardium (2 patients). A single patient
underwent position emission tomography (PET)-CT examination, and
multiple intrahepatic low densities were revealed with markedly
increased metabolism. The largest standardized uptake value (SUV)
was 11.3 and the median SUV was 8.5. The present study also
observed enlarged lymph nodes in the mediastinal, bilateral
axillary, hilar and groin regions, with a median SUV of 3.1.
Treatment and prognosis
A total of 22 patients with UCD underwent laparotomy
for complete resection of the tissue mass, and 5 patients underwent
surgery using the laparoscopic approach (the retroperitoneum in 4
and the mesentery in 1). None of the patients with UCD received
further chemotherapy or radiotherapy, as there was no evidence of
CD during the follow-up period. All patients survived with the
exception of 1 patient who succumbed to pancreatic cancer. The mean
follow-up period was 49.2±31.5 months (mean ± standard error of the
mean).
In the 7 patients with MCD, 2 underwent cervical
lymph node dissection and retroperitoneal lymph node dissection,
respectively, and received glucocorticoid treatment. Patient 1
underwent cervical lymph node dissection and survived with a
follow-up period of 12 months. Patient 2 received retroperitoneal
lymph node dissection and succumbed 2 months after surgery. A total
of 5 patients underwent lymph node biopsy and 2 declined treatment
following the definite diagnosis of CD. No follow-up data was
available for these 2 patients. The remaining 3 patients received
various treatments: 1 patient received intravenous siltuximab at a
dose of 12 mg/kg every 3 weeks for 42 weeks and survived, and 1
patient received weekly intravenous rituximab (375
mg/m2/dose) combined with cyclophosphamide, doxorubicin,
vincristine and prednisolone chemotherapy (CHOP for 8 cycles). The
remaining patient was administered methylprednisolone (1 g/day × 5
days) combined with Imuran (2 mg/kg every day for 12 weeks) and
hydroxyurea (40 mg/kg 2 times every week for 12 weeks) orally, and
received autologous hematopoietic stem cell transplantation 3
months after failure to control the disease. These 3 patients
survived with a median follow-up period of 69 months.
Discussion
CD is an uncommon disease that comprises a
heterogeneous group of lymphoproliferative disorders (10). Currently, no guideline is available
for this disease due to its low incidence and complicated
pathophysiology. The diagnosis of CD is established by histological
definition and further classified by centricity, with no specific
relevant clinical features, and accessory examinations (11). The treatment of CD, particularly MCD,
remains under investigation (2). The
present study was a retrospective analysis of unicentric and
multicentric CD involving 34 patients treated in Drum Tower
Hospital of Nanjing University Medical School. Although numerous
similar studies have also been performed with different numbers of
patients, the present study attempted to develop a strategy using
these previous experiences and aimed to provide useful data.
CD can occur at any age (12), and there are no ethnicity or gender
differences with regard to the incidence of CD (12). Studies performed to examine age
disparity demonstrated that the majority of patients with UCD were
30–50 years old, whereas patients with MCD were 50–70 years old
(13). In the present study, the
median age in the UCD group was 48 years, and 46 years in the MCD
group. According to a previous study, all lymph nodes are
susceptible to CD (13). The majority
of lesions in UCD patients occur in the mediastinum, followed by
other sites, including the neck, abdomen, retroperitoneum and
axilla (14,15). A similar pattern of distribution was
observed in the present study, with the retroperitoneum being the
most common site (33.3%), followed by the neck (22.2%) and
mediastinum (14.8%). A total of 6 cases of MCD involved the axilla
and only 1 involved the cervical pelvis.
According to a previous study, the majority patients
with UCD are asymptomatic and MCD is most commonly reported with
systemic symptoms, which may be attributed to vIL-6 (a viral
homologue of IL-6 encoded by Kaposi's sarcoma herpesvirus)
(3), including fever, fatigue and
weight loss. In the present study, half of the patients with UCD
were identified by routine examination, and the other half were
identified to have enlarged, painless lymph nodes. These patients
did not demonstrate any symptoms, which was consistent with a
previous report (3). A total of 2
patients who were admitted to hospital with other symptoms were
diagnosed by lymph node biopsy during surgery for cancer. It was
also revealed that nearly all patients with MCD had associated
systemic symptoms, with fatigue being the most common (85.7%),
followed by fever (71.4%). Therefore, when patients are admitted to
hospital with a chronic inflammatory status, the diagnosis of CD
should also be taken into consideration in addition to
hematological tumors.
Abnormalities in the diagnostic examinations,
including elevated C-reactive protein expression level and
erythrocyte sedimentation rate, were mostly observed in the
patients with MCD in the present study, which is consistent with
the results of a previous study (3).
The radiological characteristics of CD can also be observed in
benign or malignant lymphomatous tumors and other mediastinal
masses. Various histological subtypes demonstrated moderate
differences on contrast-enhanced CT scans. HVCD (hyaline-vascular
CD) and mixed-CD (mixed type of hyaline-vascular and plasma cell
CD) revealed marked enhancement in the arterial phase, whereas PCCD
(plasma cell CD) demonstrated less enhancement in the arterial
phase and delayed enhancement. These differences may be the result
of increased angiogenesis in the HVCD and mixed-CD lesions. The use
of PET-CT has improved the diagnosis of CD, as it is able to
identify all enlarged lymph nodes with low FDG uptake (16). A patient in the present study
underwent PET-CT examination and multiple enlarged lymph nodes were
observed. As this patient also presented with intrahepatic low
density, which indicated hepatocellular carcinoma with lymph node
metastasis, an inguinal lymph node biopsy was performed and the
histological results revealed CD. Therefore, PET-CT may be useful
for the evaluation of enlarged lymph nodes, but demonstrates less
efficiency in the diagnosis of CD. We also recommend MDCT as a
regular accessory examination for patients with an enlarged tissue
mass, as it has advantages in the perioperative evaluation of the
association between blood vessels, tissue masses and the
surrounding organs.
CD has three histological variants: HVCD, PCCD and a
mixed type. HVCD has been reported in 90% of patients with UCD, but
rarely in patients with MCD, whereas PCCD has been reported in only
10% of patients with UCD and in 80–90% of patients with MCD
(9). In the present study, the HV
type was identified in 81.5% of patients with UCD, and the PC type
was observed in 57.1% of patients with MCD which was slightly lower
compared with one recent study from China (17). The histological characteristics of
HVCD consisted of distinctive dysplasia follicles with regressed
germinal centers and a broad mantle zone of lymphocytes, which
formed a concentric ring (known as the ‘onion-skin’ arrangement)
(18). Another important feature of
HVCD is increased interfollicular vascularity with hyalinized
vessels, which may penetrate the germinal center and result in
inflammatory cell infiltration (19).
The differential diagnosis of HVCD in histological samples includes
thymoma and angioimmunoblastic differentiated lymphadenopathy
(20). The histological features of
PCCD are usually not so distinctive and differ from those of HVCD,
with less interfollicular vascularity of hyalinized vessels and
onion-skin arrangement. The majority of PCCD presents with
proliferation of follicles and plasma cell infiltration accompanied
by Russell bodies (21). The
differential diagnosis of PCCD in histological samples includes
autoimmune diseases, malignancies and reactive lymph node
hyperplasia (3,22). It should be noted that the
histological type is of secondary importance compared with the
unicentric or multicentric nature of the disease, as no outcome
differences have been revealed between PC and HV type in patients
with unicentric or multicentric disease (15).
The diagnosis of CD depends on pathological results.
The clinical presentation of CD is characterized by asymptomatic or
typical B-symptoms in conjunction with one or multiple tender or
tender to touch lymph nodes. The aforementioned abnormal laboratory
values may also be observed. These presentations may lead to the
initial diagnosis of lymphoma (10,23).
Although elevated serum expression levels of IL-6, as well as
circulating human herpes virus-8 particles, may be useful for
differential diagnosis, these two indicators are not widely nor
commonly used in clinical practice (17). The size of the swollen lymph node in
UCD, with a mean value of 5.7 cm, may be another useful indicator,
as the typical size of lymphoma lymph nodes is smaller (15).
Numerous pathophysiology studies on CD have
demonstrated promising results that may guide treatment; however,
current therapy remains largely based on published case reports
only (10,23,24). Talat
et al (15) reviewed 404
published cases and concluded that unicentric and multicentric
diseases were separate entities, with a different response to
treatment and long-term outcome. The biological behavior of UCD
tends to be similar to benign disease, and the standard therapy for
UCD is surgical excision, which has been proven to be curative if
complete and en-bloc resection are performed (15). In our clinical practice, stromal
tumors or lymphoma may be the initial working diagnosis, which may
prompt a wedge resection of the tissue to achieve a classification.
Under these circumstances, once a rapid pathological diagnosis of
UCD has been established, complete resection of the lymph node
and/or surrounding lymph nodes is performed to achieve a surgical
cure. With the exception of lymphoma, accurate staging of the
disease to eliminate the possibility of MCD should also be
determined prior to surgery. By performing MDCT, it is possible to
achieve an improved perioperative evaluation, which may aid
selection of a laparotomy or a laparoscopic approach. In the
present study, all the patients with UCD underwent complete
surgical resection with free resection margins and survived with an
excellent prognosis. A total of 5 patients who underwent
laparoscopic surgery achieved faster recovery, which was due to the
advantage of laparoscopy in gaining access to difficult areas of
the abdomen, as well as improved visualization with bloodless
dissection. No patients underwent complete excision received
further therapies as no evidence of disease recurrence was
discovered in these patients, which was consistent with other
reports on the critical role of surgical excision in UCD treatment
(15,17).
The biological behavior of MCD has an invasive
characteristic, with difficulties in controlling disease
progression and a high rate of recurrence. Furthermore, MCD may
result in secondary lesions, including plasma lymphoma or
follicular dendritic sarcoma (5,25).
Therefore, MCD is a systemic disorder, with no definitive standard
treatment regimens available. Although surgical removal may be
necessary for patients with MCD in whom a pre-operative diagnosis
is not possible, surgical intervention in the majority of cases
provides no observable long-term benefit (15). Combined therapy is the most frequently
used treatment, and certain regimens demonstrated promising
results, whereas others did not (2).
As the critical role of IL-6 in disease pathogenesis is being
clarified, a molecular target of IL-6 may be a promising option. In
a previous multinational, randomized, placebo-controlled study,
intravenous administration of siltuximab at a dose of 11 mg/kg
revealed promising results in patients with stable MCD (26). In the present study, 2 patients
received molecular target therapy based on immunohistochemical
results of positive cluster of differentiation (CD) 20 and IL-6.
These 2 HIV-negative patients survived, which was consistent with
the previous reports (2,26). In patients with disease progression,
more research is required and autologous hematopoietic stem cell
transplantation may be a treatment option.
In conclusion, the present study investigated a
number of patients with CD in a single institution. The
characteristics of CD were investigated, in terms of symptoms,
signs, laboratory examinations and pathological features, and we
shared our experience in the treatment of CD. It was revealed that
MDCT is beneficial for perioperative evaluation and that the
laparoscopic approach is suitable for certain UCD patients with an
abdominal tissue mass. In patients with MCD, molecular therapy
targeting IL-6 and CD20, as well as autologous hematopoietic stem
cell transplantation, may be useful.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant nos. 81500432, 81372364 and
81571563), the Fundamental Research Fund for the Central
Universities (grant no. 021414380106), the China Postdoctoral
Science Foundation (grant no. 2014M552695) and the People's
Liberation Army Youth Culturing Project of Medical Sciences (grant
no. 14QNP009).
References
|
1
|
Castleman B and Towne VW: Case records of
the Massachusetts general hospital; weekly clinicopathological
exercises; founded by Richard C. Cabot. N Engl J Med. 251:396–400.
1954.PubMed/NCBI
|
|
2
|
El-Osta HE and Kurzrock R: Castleman's
disease: From basic mechanisms to molecular therapeutics.
Oncologist. 16:497–511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Waterston A and Bower M: Fifty years of
multicentric Castleman's disease. Acta Oncol. 43:698–704. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keller AR, Hochholzer L and Castleman B:
Hyaline-vascular and plasma-cell types of giant lymph node
hyperplasia of the mediastinum and other locations. Cancer.
29:670–683. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Casper C: The aetiology and management of
Castleman disease at 50 years: Translating pathophysiology to
patient care. Br J Haematol. 129:3–17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshizaki K, Matsuda T, Nishimoto N,
Kuritani T, Taeho L, Aozasa K, Nakahata T, Kawai H, Tagoh H, Komori
T, et al: Pathogenic significance of interleukin-6 (IL-6/BSF-2) in
Castleman's disease. Blood. 74:1360–1367. 1989.PubMed/NCBI
|
|
7
|
Vinzio S, Ciarloni L, Schlienger JL, Rohr
S, Méchine A and Goichot B: Isolated microcytic anemia disclosing a
unicentric Castleman disease: The interleukin-6/hepcidin pathway?
Eur J Intern Med. 19:367–369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reddy D and Mitsuyasu R: HIV-associated
multicentric Castleman disease. Curr Opin Oncol. 23:475–481. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen XF, Guan WX, Cao K, Wang H and Du JF:
Small bowel volvulus with jejunal diverticulum: Primary or
secondary? World J Gastroenterol. 21:10480–10484. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dispenzieri A: Castleman disease. Cancer
Treat Res. 142:293–330. 2008.PubMed/NCBI
|
|
11
|
Műzes G, Sipos F, Csomor J and Sréter L:
Multicentric Castleman's disease: A challenging diagnosis. Pathol
Oncol Res. 19:345–351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herrada J, Cabanillas F, Rice L, Manning J
and Pugh W: The clinical behavior of localized and multicentric
Castleman disease. Ann Intern Med. 128:657–662. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bonekamp D, Horton KM, Hruban RH and
Fishman EK: Castleman disease: The great mimic. Radiographics.
31:1793–1807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Frizzera G: Castleman's disease and
related disorders. Semin Diagn Pathol. 5:346–364. 1988.PubMed/NCBI
|
|
15
|
Talat N, Belgaumkar AP and Schulte KM:
Surgery in Castleman's disease: A systematic review of 404
published cases. Ann Surg. 255:677–684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barker R, Kazmi F, Stebbing J, Ngan S,
Chinn R, Nelson M, O'Doherty M and Bower M: FDG-PET/CT imaging in
the management of HIV-associated multicentric Castleman's disease.
Eur J Nucl Med Mol Imaging. 36:648–652. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye B, Gao SG, Li W, Yang LH, Zhao SH, Ma
K, Zhu XL, Liu XY and Sun KL: A retrospective study of unicentric
and multicentric Castleman's disease: A report of 52 patients. Med
Oncol. 27:1171–1178. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meador TL and McLarney JK: CT features of
Castleman disease of the abdomen and pelvis. AJR Am J Roentgenol.
175:115–118. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mangini M, Aiani L, Bertolotti E,
Imperatori A, Rotolo N, Paddeu A, Uccella S, Carrafiello G and
Fugazzola C: Parapancreatic Castleman disease: Contrast-enhanced
sonography and CT features. J Clin Ultrasound. 35:207–211. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Noh OK, Lee SW, Lee JW, Kim SY, Kim CS,
Choi EK, Kim JH and Ahn SD: Cases report of unicentric Castleman's
disease: Revisit of radiotherapy role. Radiat Oncol J. 31:48–54.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frizzera G, Banks PM, Massarelli G and
Rosai J: A systemic lymphoproliferative disorder with morphologic
features of Castleman's disease. Pathological findings in 15
patients. Am J Surg Pathol. 7:211–231. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee HJ, Jeon HJ, Park SG and Park CY:
Castleman's disease of the spleen. World J Gastroenterol.
21:1675–1679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bowne WB, Lewis JJ, Filippa DA, Niesvizky
R, Brooks AD, Burt ME and Brennan MF: The management of unicentric
and multicentric Castleman's disease: A report of 16 cases and a
review of the literature. Cancer. 85:706–717. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soumerai JD, Sohani AR and Abramson JS:
Diagnosis and management of Castleman disease. Cancer Control.
21:266–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weisenburger DD, Nathwani BN, Winberg CD
and Rappaport H: Multicentric angiofollicular lymph node
hyperplasia: A clinicopathologic study of 16 cases. Hum Pathol.
16:162–172. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Rhee F, Casper C, Voorhees PM, Fayad
LE, van de Velde H, Vermeulen J, Qin X, Qi M, Tromp B and Kurzrock
R: A phase 2, open-label, multicenter study of the long-term safety
of siltuximab (an anti-interleukin-6 monoclonal antibody) in
patients with multicentric Castleman disease. Oncotarget.
6:30408–30419. 2015. View Article : Google Scholar : PubMed/NCBI
|