Introduction
Laryngeal cancer is a malignant cancer commonly
occurring in the head and neck region, and was ranked as the sixth
most frequent cancer worldwide in 2004 (1,2). However
to date, the treatment strategy for laryngeal cancer is primarily
surgery with radiotherapy. Therefore, there is an urgent
requirement to investigate the causes and treatment strategies to
further improve the survival of patients.
A number of protein-coding and non-coding genetic
factors have been revealed to be abnormally expressed in laryngeal
cancer samples or cell lines (1,3–5). Correspondingly, two genes and microRNAs
(miRNA/miRs) are considered to be key biological factors in
laryngeal cancer, including SOX2 (5),
glucose transporter 1/3 (6), miR-196a
(7), miR-221 (8) and miR-34a/c (2). miRNAs are small noncoding RNA molecules,
which generally negatively regulate gene expression at the
post-transcriptional level. An increasing number of studies
indicate that ectopic expression of miRNAs is associated with
cancer pathogenesis. For example, aberrant miR-221 expression was
reported to accelerate the proliferation of M4e laryngeal carcinoma
cell line by suppressing apoptotic protease activating factor 1
(Apaf-1) (8). Inhibition of miR-221
in vitro increased the expression levels of Apaf-1 apoptotic
pathway proteins, including caspase-3 and −9 (8). Furthermore, miR-34a/c expression was
observed to be downregulated in human laryngeal squamous cell
carcinoma tissues, and upregulation of miR-34a/c in Hep-2 cells by
transfection significantly decreased cell proliferation and
migratory ability in vitro (2). It was previously reported that B cell
lymphoma 2 (BCL2), a central genetic apoptotic regulator (9), which is crucial for cell apoptosis,
migration and invasion (3), is
involved in the pathogenesis of laryngeal cancer (10–12). In
addition, research into other diseases has reported on the
association between miR-34c and BCL2 (13–15); there
is an inverse association between levels of miR-34c and expression
of BCL2. Knockdown of miR-34c upregulated anti-apoptotic protein
BCL2 to ameliorate apoptosis in the hippocampus or promote
embryonic stem cell (ESC) differentiation (13–15). Based
on these reports, the authors of the present study hypothesized
that there may be an association between miR-34c and BCL2, which is
involved in the pathogenesis of laryngeal cancer. However, to the
best our knowledge, to date, there have been no reports of direct
evidence for the association between miR-34c and BCL2 in laryngeal
cancer.
In order to investigate the association between
miR-34c and BCL2 in laryngeal cancer, miR-34c was overexpressed to
investigate the effect of miR-34c overexpression on viability,
apoptosis and BCL2 expression in M4e cells. In addition, a
luciferase reporter assay was employed to confirm whether BCL2 is a
direct target of miR-34c. The aim of the present study was to
provide further information on whether BCL2 may be regulated by
miR-34c and to investigate if there is any therapeutic target
potential of miR-34c in M4e cells.
Materials and methods
Cell lines and culture conditions
Culture of M4e cells was performed as reported
previously by Wang et al (16). Human laryngeal cancer cells M4e were
purchased from the American Type Culture Collection (Manassas, VA
USA). Briefly, the cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS,
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin at 37°C in a humidified atmosphere with 5%
CO2 and 95% air.
Lentiviral vector construction and
cell transfection
The transfection of M4e cells was performed via
Lipofectamine 2000® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Then, the cells were divided into three groups:
Control group (control), scrambled group (scramble), and
has-miR-34c transfected group (lenti-miR-34c). Total RNA in M4e
cells was extracted by using the TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). As previously reported by Yang et
al (17), the full length
pre-miR-34c oligonucleotide was synthesized by Shanghai GeneChem
Co., Ltd. (Shanghai, China) and was subsequently inserted into the
unique EcoRI site of the GV217 lentiviral vector (Shanghai GeneChem
Co., Ltd.), to construct a lentivirus encoding miR-34c, which is
named lenti-miR-34c. The nucleotide sequence of the construct was
then confirmed by sequencing. In accordance with Yang et al
(17), the complementary nucleotides
of miR-34c were cloned at one site between the AgeI and EcoRI sites
in GV280 lentiviral vector (Shanghai GeneChem Co., Ltd.) to
construct the specific inhibitor of miR-34c. M4e cells were seeded
in 6-well plates at a density of 5×104 cells/well and
cultured in DMEM media containing 10% FBS, 100 U/ml penicillin and
100 µg/ml streptomycin at 37°C with 5% CO2. When 30%
confluence was obtained, the cells were transfected with
lenti-miR-34c or scramble for 72 h. The efficiency of transfection
was evaluated by EGFP fluorescence intensity on a Nikon Eclipse 80i
microscope (Nikon USA, Melville, NY, USA).
Cell viability assay
MTT assay (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was used to determine the relative cell viability of
infected M4e cells at 24, 48, 72 and 96 h post-transfection
(2). Control and infected M4e cells
by lenti-miR-34c were seeded in 96-well plates at a density of
1×103 cells/well. M4e cells were incubated in 100 µl
DMEM with 10 µl MTT solution at 37°C for 2 h. The optical density
was determined at an absorbance wavelength of 570 nm. Each
experiment was performed in triplicate.
Apoptosis assay
Cell apoptosis was determined using the Annexin V
apoptosis detection kit (KeyGen Biotech, Nanjing, China), as
previously described (4). In brief,
the cells were washed twice with phosphate-buffered saline (PBS),
resuspended in 1× binding buffer (BD Biosciences, San Jose, CA,
USA) and dual staining was performed with Annexin V/allophycocyanin
(APC) and propidium iodide (PI) for 10 min at the room temperature
in darkness, according to the manufacturer's protocol. M4e cells
that were untreated were used as the internal control. Each
experiment was repeated three times. The percentage of M4e cells
undergoing apoptosis was determined by flow cytometric analysis
using a flow cytometer (FACSCalibur; BD Biosciences, San Jose, CA,
USA) and FlowJo Software (version 10.4; FlowJo LLC, Ashland, OR,
USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
For miR-21 and BCL2 mRNA analyses, total RNA was
extracted from M4e cells using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Then, 4 µg of total RNA was converted to cDNA using PrimeScript II
1st Strand cDNA Synthesis kit (Takara Bio Inc., Otsu, Japan)
according to the manufacturer's protocol. For the detection of
miR-21 expression, stem-loop RT-qPCR was performed using SYBR
Premix Ex Taq™ kit (Takara Bio Inc., Otsu, Japan) and a LightCycler
480 instrument (Roche Diagnostics, Basel, Switzerland) equipped
with Light-Cycler Software Version 1.5 (Roche Diagnostics)
following the manufacturer's instructions. The PCR cycling
consisted of 95°C for 5 min, followed by 40 cycles of 95°C for 30
sec, 57°C for 30 sec and 72°C for 30 sec. U6 small RNA was used as
the reference RNA to normalize relative expression of miR-21. BCL2
mRNA expression was detected via RT-qPCR using QuantiTect SYBR
Green RT-PCR kit (Qiagen GmbH, Hilden, Germany). β-actin was used
to normalize the relative expression level of BCL2 mRNA. . Fold
changes were calculated using the 2−ΔΔCt method
(5). The sequences of RT-qPCR primers
were as follow: miR-21, Stem-loop RT-qPCR primer:
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAACA-3′, Forward:
5′-GCCCGCTAGCTTATCAGACTGATG-3′, Reverse: 5′-GTGCAGGGTCCGAGGT-3′;
U6, RT primer:
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAAATATG-3′,
Forward: 5′-GCGCGTCGTGAAGCGTTC-3′, Reverse: 5′-GTGCAGGGTCCGAGGT-3′;
BCL2 mRNA, Forward: 5′-CTGTGCTGCTATCCTGC-3′, Reverse:
5′-TGCAGCCACAATACTGT-3′; β-actin, Forward:
5′-TCACCCACACTGTGCCCCATCTACGA-3′, Reverse:
5′-CAGCGGAACCGCTCATTGCCAATGG-3′.
Western blot analysis
Western blot analysis was performed as previously
described by Li et al (3). M4e
cells were harvested after being digested with 0.25% trypsin
(Thermo Fisher Scientific, Inc.) for 2 min at 37°C, washed in PBS
and resuspended in radioimmunoprecipitation assay cell lysis
solution (Beyotime Institute of Biotechnology, Shanghai, China) for
30 min at 4°C. Protein concentration was determined using the
bicinchoninic acid assay (Pierce; Thermo Fisher Scientific, Inc.).
Cell lysates with equal amounts of protein (20 µg/well) were
separated on 10% SDS-PAGE gels by electrophoresis, and transferred
to polyvinylidene difluoride (PVDF) membranes (Invitrogen; Thermo
Fisher Scientific, Inc.). PVDF membranes were blocked with 5%
skimmed milk in TBST at room temperature with agitation for 1 h.
The PVDF membranes were incubated with the primary antibody against
BCL2 (ab59348; dilution, 1:1,000; Epitomics; Abcam, Cambridge, UK)
at 4°C for 12 h. The membranes were then washed three times with
TBST each for 10 min and incubated with goat anti-rabbit
immunoglobulin G antibodies (ab7090; dilution, 1:1,000; Epitomics;
Abcam) at room temperature for 1 h. The blots were washed with
TBST, and then polypeptide bands were detected using ECL Plus
Western Blotting Detection Reagents (Merck KGaA, Darmstadt,
Germany) and were exposed to X-ray film (Kodak, Rochester, NY,
USA). The intensity of the bands was quantified using Image Lab™
Software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). β-actin
(ab8227; dilution, 1:1,000; Epitomics; Abcam) was used as an
internal reference control.
Luciferase reporter assay
A luciferase reporter assay was performed, according
to the methods provided by Li et al (3). In brief, M4e cells were seeded and
cultured in 6-well plates at a density of 5×104
cells/well. Upon reaching 80% confluence, the cells were
transfected with either pGL3-BCL2-WT (wildtype) or pGL3-BCL2-MUT
(mutant) vector containing firefly luciferase together with miR-34c
inhibitor or negative control. M4e cells were transfected with WT
or mutant reporter plasmid using Lipofectamine 2000® for
6 h, and were subsequently co-transfected with miR-34c inhibitor or
negative control for 36 h. The dual luciferase assay system
(Promega Corporation, Madison, WI, USA) was used to measure
luciferase activity, according to the manufacturer's protocol.
Statistical analysis
All data are expressed as the mean ± standard
deviation of three independent experiments. The differences between
two groups were assessed using an unpaired, two-tailed Student's
t-test, and multi-group comparisons were analyzed by one-way
analysis of variance with Bonferroni analyses performed as post-hoc
tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-34c inhibits the viability of M4e
cells in vitro
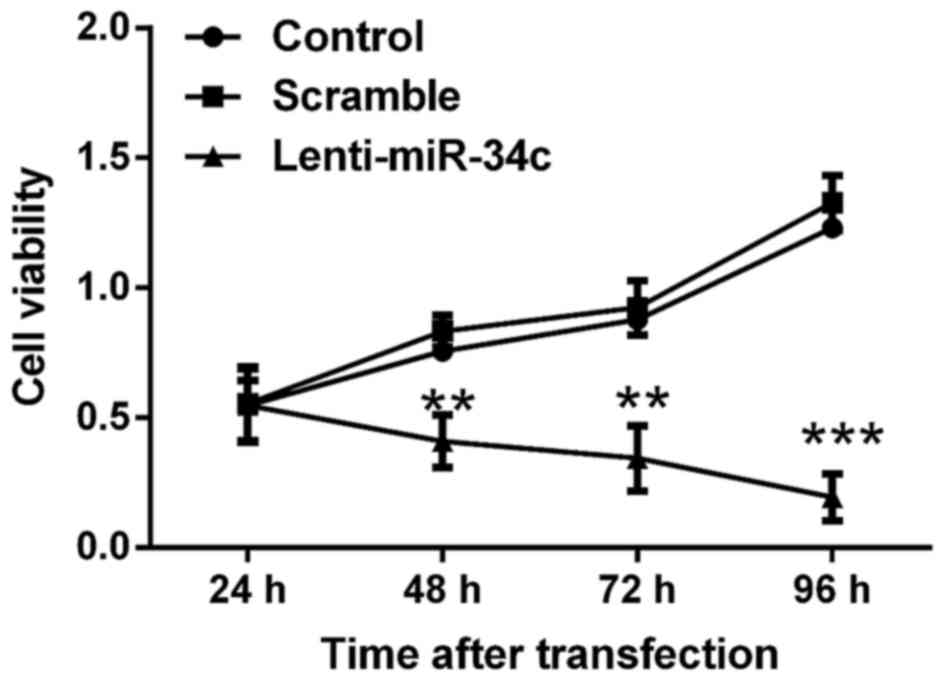
The viability of M4e cells was assessed by MTT
assay. As indicated in Fig. 1, the
viability of M4e cells was inhibited following transfection with
lenti-miR-34c for 72 h. Cell viability in the control and scramble
groups gradually increased and reached the highest level at 96 h
post-transfection. By contrast, the cell viability in lenti-miR-34c
transfected-group gradually decreased to the lowest level at 96 h
post-transfection. Significant differences in cell viability were
determined between the control or scramble group and lenti-miR-34c
group at 48, 72 and 96 h post-transfection (P<0.01 or
P<0.001).
miR-34c induces the apoptosis of M4e
cells in vitro
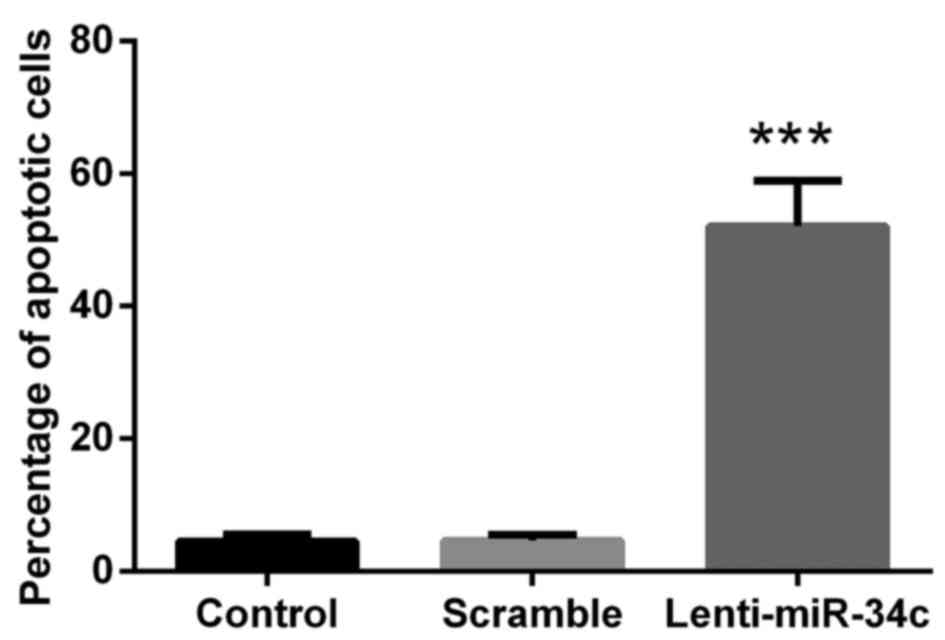
M4e cells were stained with Annexin V/APC and PI to
evaluate the percentage of cells undergoing apoptosis in the three
treatment groups. The results indicated that lenti-miR-34c
transfection was able to promote the apoptosis of M4e cells, where
the percentage of apoptotic cells in the lenti-miR-34c group was
~65% and the percentage in the control groups was 6% (P<0.001;
Fig. 2).
Effect of miR-34c on mRNA and protein
levels of BCL2
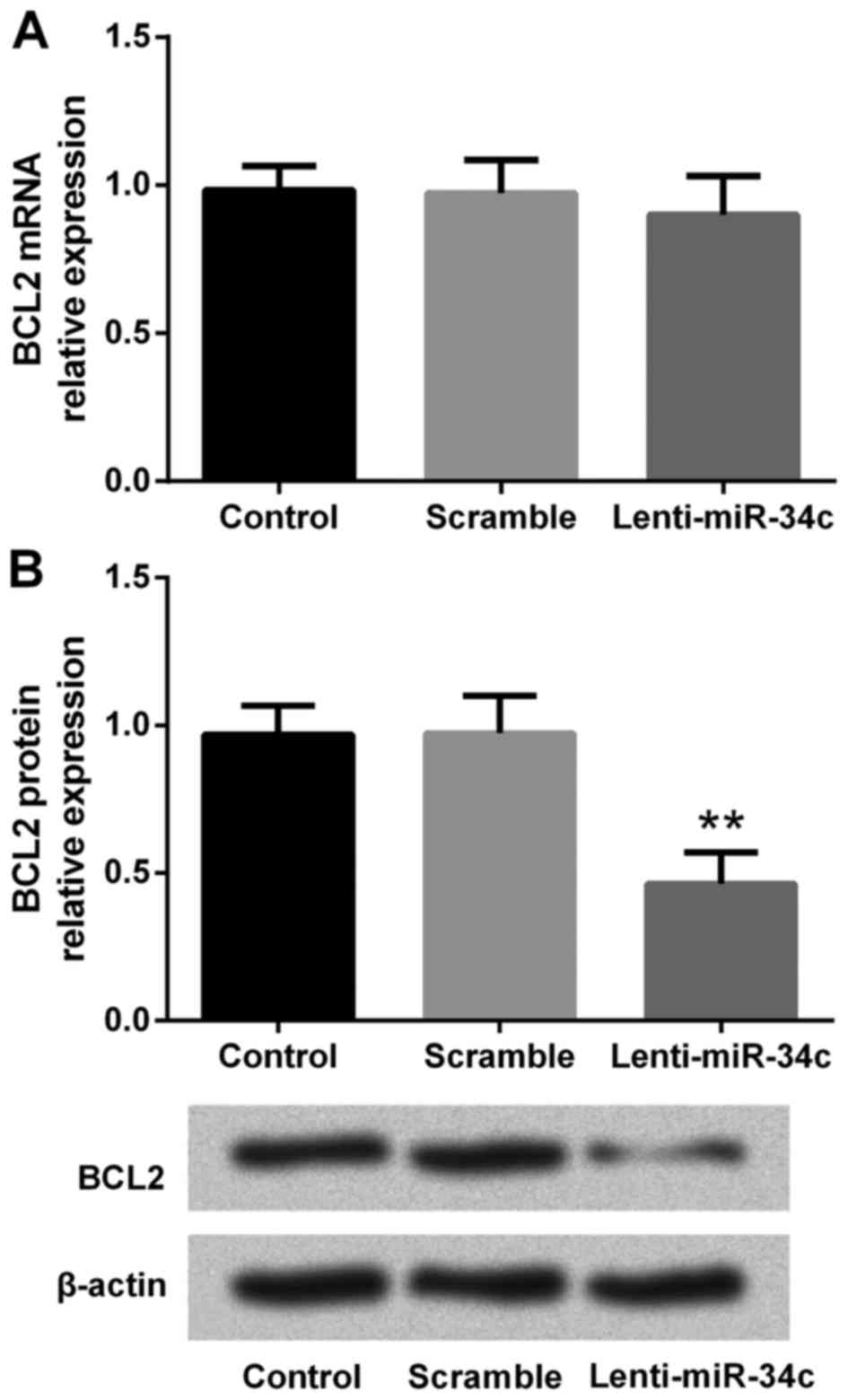
To investigate the effect of lenti-miR-34c
transfection on BCL2 expression in M4e cells, RT-qPCR and western
blot analyses were performed to analyze the levels of mRNA and
protein, respectively. RT-qPCR results indicated that BCL2 mRNA
expression did not change following lenti-miR-34c transfection
(Fig. 3A). However, western blot
analysis revealed that the level of BCL2 protein was significantly
downregulated by lenti-miR-34c transfection compared with the
control and scramble groups (P<0.01; Fig. 3B, C). This indicated that BCL2 may be
a putative target of miR-34c.
miR-34c modulates BCL2 expression by
targeting BCL2
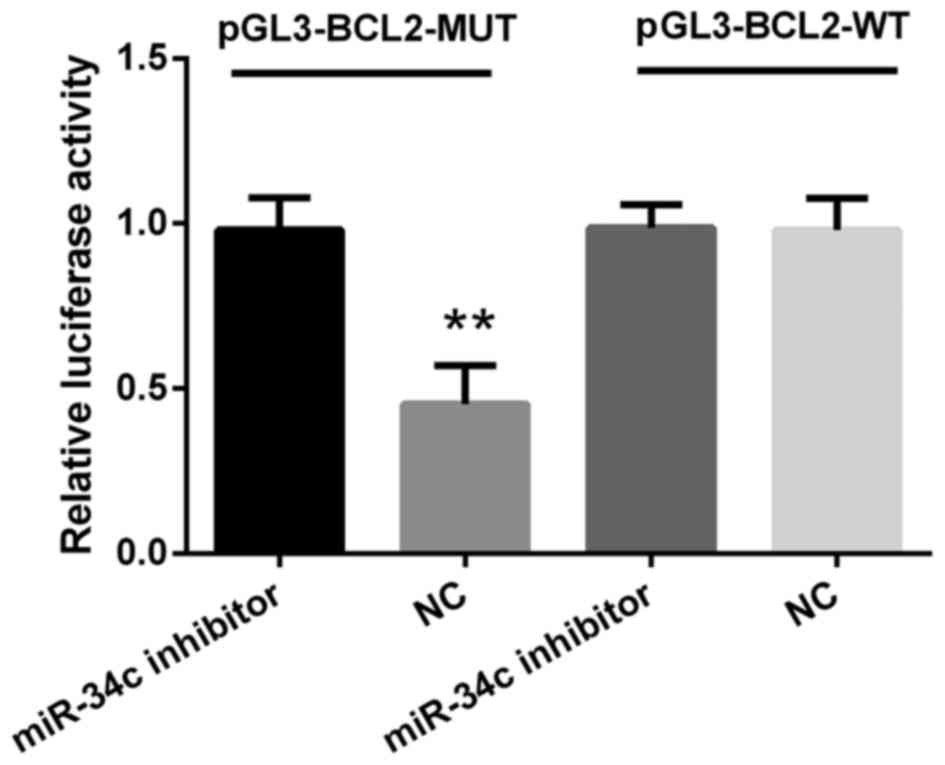
To verify whether BCL2 was a putative target of
miR-34c, M4e cells were co-transfected with either pGL3-BCL2-WT or
pGL3-BCL2-MUT vector containing firefly luciferase together with
miR-34c inhibitor or negative control. As indicated in Fig. 4, luciferase activity in M4e cells
co-transfected with miR-34c negative control and pGL3-BCL2-MUT was
decreased compared with the miR-34c inhibitor (P<0.01). In
addition, no significant differences were observed between cells
transfected with WT plasmids when co-transfected with miR-34c
inhibitor. The data presented in this present study indicated that
BCL2 may be directly regulated by miR-34c.
Discussion
Recent studies in cancer research have revealed that
hsa-miR-34c is downregulated in human laryngeal squamous cell
carcinoma tissues, and ectopic expression of miR-34a/c
significantly induces the proliferative and migratory capacities of
Hep-2 cells, in vitro (2). The
imbalance between cell proliferation and apoptosis is a phenotypic
characteristic of cancer, which is largely accountable for the
pathogenesis of this disease. In the present study, the results
indicated that infection with lenti-miR-34c was able to significant
induce apoptosis and inhibit proliferation of M4e cells.
Furthermore, it was revealed that BCL2 may a putative target of
miR-34c.
Over the past decades, numerous miRNAs and genes
have been verified to associate with and play crucial roles in
regulating biological processes, including pathopoiesis and
tumorigenesis (2,18,19). The
target genes of these miRNAs, namely miR-34c (2), miR-196a (7), miR-221 (8), miR-203 (20), miR-27a (21) and miR-106b (22) have been reported to be associated with
laryngeal carcinoma. Furthermore, multiple miRNAs have been
demonstrated to exhibit tumor suppressive effects in several types
of cancer (2). For example, miR-34a/c
was reported to be downregulated in human laryngeal squamous cell
carcinoma, and overexpression of miR-34a/c in Hep-2 cells by
transfection, decreased the proliferative and migratory abilities
of the cells (2). In Li et al
(2), RT-qPCR analysis indicated that
UDP-N-acetyl-α-D-galactosamine: polypeptide-N-acetylgalactosaminyl
transferase 7 (GALNT7) expression was negatively regulated by
miR-34a/c in Hep-2 cells. Further data from the luciferase reporter
assay determined that GALNT7 was a target of miR-34a/c. These
results highlighted that miR-34a/c, and its novel target GALNT7 may
serve as novel potential biomarkers for human laryngeal squamous
cell carcinoma therapy.
Another miRNA of interest, miR-27a, has been
reported to be an important regulator in laryngeal carcinoma
(21). As previously reported by Tian
et al (21), miR-27a and
polo-like kinase 2 (PLK2), are critically involved in controlling
cell cycle progression. In addition, the expression level of
miR-27a was significantly upregulated in laryngeal carcinoma
tissues compared with adjacent non-tumor tissues, respectively.
Transfection of miR-27a mimics increased proliferation and colony
formation and decreased the percentage of cells undergoing
apoptosis and the level of PLK2 protein expression in Hep-2 cells.
These data suggested that miR-27a acts as an oncogene in laryngeal
squamous cell carcinoma via regulating PLK2 protein and implicated
miR-27a to be a potential biomarker in the diagnosis and therapy
for laryngeal carcinoma.
BCL2 contributes a crucial role in cell death,
apoptosis, migration and invasion (3,9,23,24).
Previous studies have reported that BCL2 is involved in the
pathogenesis of laryngeal cancer (10–12). In
the present study, it was observed that transfection of M4e cells
with lenti-miR-34c significantly inhibited cell viability and BCL-2
protein expression, and increased the percentage of cells
undergoing apoptosis. Furthermore, luciferase reporter assay
revealed that transfection of miR-34c negative control decreased
luciferase activity in M4e cells co-transfected with pGL3-BCL2-MUT
plasmid, compared with the miR-34c inhibitor.
In conclusion, the results from the present study
demonstrated that miR-34c was a growth inhibitor of M4e cells, and
there was a negative association between miR-34c and its target
BCL2. Downregulated miR-34c may contribute to the malignancy of
human laryngeal carcinoma via BCL2-associated pathways.
Furthermore, miR-34c and BCL2 may serve as biomarkers for the
diagnosis and treatment of laryngeal carcinoma.
Glossary
Abbreviation
Abbreviations:
References
|
1
|
Zhang F, Xu Z, Wang K, Sun L, Liu G and
Han B: microRNA and gene networks in human laryngeal cancer. Exp
Ther Med. 10:2245–2252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li W, Ma H and Sun J: microRNA-34a/c
function as tumor suppressors in Hep-2 laryngeal carcinoma cells
and may reduce GALNT7 expression. Mol Med Rep. 9:1293–1298. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, Yan L, Zhang W, Wang H, Chen W, Hu N
and Ou H: miR-21 inhibitor suppresses proliferation and migration
of nasopharyngeal carcinoma cells through down-regulation of BCL2
expression. Int J Clin Exp Pathol. 7:3478–3487. 2014.PubMed/NCBI
|
|
4
|
Ren J, Zhu D, Liu M, Sun Y and Tian L:
Downregulation of miR-21 modulates Ras expression to promote
apoptosis and suppress invasion of Laryngeal squamous cell
carcinoma. Eur J Cancer. 46:3409–3416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang N, Hui L, Wang Y, Yang H and Jiang X:
SOX2 promotes the migration and invasion of laryngeal cancer cells
by induction of MMP-2 via the PI3K/Akt/mTOR pathway. Oncol Rep.
31:2651–2659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Starska K, Forma E, Jóźwiak P, Bryś M,
Lewy-Trenda I, Brzezińska-Błaszczyk E and Krześlak A: Gene and
protein expression of glucose transporter 1 and glucose transporter
3 in human laryngeal cancer-the relationship with regulatory
hypoxia-inducible factor-1α expression, tumor invasiveness, and
patient prognosis. Tumor Biol. 36:2309–2321. 2015. View Article : Google Scholar
|
|
7
|
Saito K, Inagaki K, Kamimoto T, Ito Y,
Sugita T, Nakajo S, Hirasawa A, Iwamaru A, Ishikura T, Hanaoka H,
et al: MicroRNA-196a is a putative diagnostic biomarker and
therapeutic target for laryngeal cancer. PLoS One. 8:e714802013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun X, Liu B, Zhao XD, Wang LY and Ji WY:
MicroRNA-221 accelerates the proliferation of laryngeal cancer cell
line Hep-2 by suppressing Apaf-1. Oncol Rep. 33:1221–1226. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cimmino A, Calin GA, Fabbri M, Iorio MV,
Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, et
al: miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl
Acad Sci USA. 102:pp. 13944–13949. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin YT, Kayser S, Kemp BL, Ordonez NG,
Tucker SL, Clayman GL, Goepfert H, Luna MA, Batsakis JG and
El-Naggar AK: The prognostic significance of the biomarkers
p21WAF1/CIP1, p53, and bcl-2 in laryngeal squamous cell carcinoma.
Cancer. 82:2159–2165. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Du J, Chen G, Vlantis A, Chan P, Tsang R
and van Hasselt C: Resistance to apoptosis of HPV 16-infected
laryngeal cancer cells is associated with decreased Bak and
increased Bcl-2 expression. Cancer Lett. 205:81–88. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gurlek U, Abakay CD, Ozkan L, Saraydaroglu
O, Kurt M and Cetintas SK: The evaluation of bcl-2 expression as a
prognostic marker in early stage laryngeal cancer. Tumori.
99:682–688. 2013.PubMed/NCBI
|
|
13
|
Zirnheld AL, Regalado EL, Shetty V,
Chertkow H, Schipper HM and Wang E: Target genes of circulating
miR-34c as plasma protein bio markers of Alzheimer's disease and
mild cognitive impairment. J Aging Sci. 5:e1252017.
|
|
14
|
Cao SE, Tian J, Chen S, Zhang X and Zhang
Y: Role of miR-34c in ketamine-induced neurotoxicity in neonatal
mice hippocampus. Cell Biol Int. 39:164–168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang S, Yu M, Liu C, Wang L, Hu Y, Bai Y
and Hua J: MIR-34c regulates mouse embryonic stem cells
differentiation into male germ-like cells through RARg. Cell
Biochem Funct. 30:623–632. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang HX and Tang C: Galangin suppresses
human laryngeal carcinoma via modulation of caspase-3 and AKT
signaling pathways. Oncol Rep. 38:703–714. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang S, Li WS, Dong F, Sun HM, Wu B, Tan
J, Zou WJ and Zhou DS: KITLG is a novel target of miR-34c that is
associated with the inhibition of growth and invasion in colorectal
cancer cells. J Cell Mol Med. 18:2092–2102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Valastyan S, Reinhardt F, Benaich N,
Calogrias D, Szász AM, Wang ZC, Brock JE and Richardson AL:
Retraction notice to: A pleiotropically acting MicroRNA, miR-31,
inhibits breast cancer metastasis. Cell. 161:4172015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bian K, Fan J, Zhang X, Yang XW, Zhu HY,
Wang L, Sun JY, Meng YL, Cui PC, Cheng SY, et al: MicroRNA-203
leads to G1 phase cell cycle arrest in laryngeal carcinoma cells by
directly targeting survivin. FEBS Lett. 586:804–809. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian Y, Fu S, Qiu GB, Xu ZM, Liu N, Zhang
XW, Chen S, Wang Y, Sun KL and Fu WN: MicroRNA-27a promotes
proliferation and suppresses apoptosis by targeting PLK2 in
laryngeal carcinoma. BMC Cancer. 14:6782014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ying X, Kai W, Wei G, Zhang C, Huang F,
Wen S and Wang B: MicroRNA-106b regulates the tumor suppressor
RUNX3 in laryngeal carcinoma cells. FEBS Lett. 587:3166–3174. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kusunoki S, Kato K, Tabu K, Inagaki T,
Okabe H, Kaneda H, Suga S, Terao Y, Taga T and Takeda S: The
inhibitory effect of salinomycin on the proliferation, migration
and invasion of human endometrial cancer stem-like cells. Gynecol
Oncol. 129:598–605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang TQ, Lu XJ, Wu TF, Ding DD, Zhao ZH,
Chen GL, Xie XS, Li B, Wei YX, Guo LC, et al: MicroRNA-16 inhibits
glioma cell growth and invasion through suppression of BCL2 and the
nuclear factor-κB1/MMP9 signaling pathway. Cancer Sci. 105:265–271.
2014. View Article : Google Scholar : PubMed/NCBI
|