Introduction
The morbidity of colorectal cancer (CRC) ranks third
place in males and second place in females worldwide (1). In 2012, ~1.4 million new cases of CRC
were diagnosed, with ~694,000 CRC-associated mortalities (2). In previous years, the incidence of CRC
has continued to increase worldwide, particularly in developed
countries, accounting for >65% of newly diagnosed cases each
year (3). The risk factors of CRC
include lifestyle, inherited genetic disorders, family history of
colon cancer, exposure to radiation and other diseases, such as
inflammatory bowel disease, obesity and diabetes (4). The treatment for CRC includes surgery,
radiation therapy, chemotherapy and targeted therapy (4).
However, CRC remains one of the leading causes of
cancer-associated mortality worldwide. The main challenge is that
patients with CRC are not diagnosed at early stages, which results
in a poor prognosis, with a 5-year survival rate of 50–59%
(3). However, carcinoembryonic
antigen and cancer antigen 19-9 have been widely used as biomarkers
for CRC diagnosis and have also been shown to efficiently reduce
the mortality rate of patients with CRC (5,6).
Therefore, it is critical to identify the molecular markers, which
are able to monitor or predict the progression and prognosis of
patients with CRC and to investigate these potential biomarkers as
therapeutic targets to improve the survival quality of
patients.
Previous studies have highlighted the role of
chromosome structure on the regulation of genome transcriptional
status, which was frequently observed in diseases, particularly
cancer (7,8). Enhancer of zeste homolog 2 (EZH2), the
key component of polycomb repressive complex 2, has a crucial role
in the regulation of cell proliferation and cell cycle through gene
repression or histone H3 lysine 27 (H3K27) methylation (8). The methyltransferase activity of EZH2
lies in the catalytic domain of the C-terminal, which is also
termed the SET domain (9). Mutations
of the tyrosine 641 (Y641F, Y641N, Y641S, Y641C and Y641H) within
the SET domain results in reduced methylation of unmethylated H3K27
but enhanced methylation of the dimethylated version of H3K27
(H3K27me2), and thus represses the expression of polycomb targets
(10). An increasing body of evidence
has demonstrated that a specific cell or its behavior mainly relies
on the expression and repression of genes (11,12). There
is evidence that exogenous expression of EZH2 in mice may lead to
the development of myeloproliferative disorder (13). Another in vitro study that
aimed to investigate the role of EZH2 in human breast epithelial
cell lines demonstrated that expression of EZH2 causes neoplastic
transformation of epithelial cells, which highlighted that EZH2 may
perform an important role in cancer (14).
Additional studies have implicated the oncogenic
role of EZH2 in cancer. The overexpression of EZH2 was shown to be
associated with the poor prognosis of prostate cancer (15). In addition, overexpression of EZH2 in
a number of types of human cancer, including hepatocellular
carcinoma (16), breast cancer
(17), bladder cancer (18) and melanoma (19), has been observed. Furthermore, several
independent groups have revealed the mechanism of how EZH2 is
involved in the development and progression of a variety of types
of cancer (20). However, there is
limited knowledge on the expression of EZH2 in CRC. In the present
study, the expression status of EZH2 was analyzed in patients with
CRC, and its association with the prognosis of CRC was also
investigated.
Materials and methods
Cell lines
The noncancerous colon epithelial cell line (HCEC)
and the two CRC cell lines (HCT-116 and SW480) used in a
pre-experiment were purchased from the Institute of Biochemistry
and Cell Biology, Chinese Academy of Sciences (Shanghai, China).
The cells were cultured in complete Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) with 100 µg/ml streptomycin and 100 U/ml
penicillin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and
maintained in a humidified incubator (5% CO2, 37°C).
RNA interference
Knockdown of EZH2 in the CRC cell line was
accomplished with small interfering RNA (siRNA) duplex as
previously described (15). The EZH2
siRNA sequence (5′-AGUCUCAUGUACGCTGACUCUG-3′) was designed by
Genepharm, Inc. (Sunnyvale, CA, USA) to target the 85–106 region of
human EZH2. In vitro transient transfection was performed as
described previously (21).
Generation of EZH2 tyrosine 641
mutation cell line using the CRISPR/Cas9 complex
To investigate the effect of EZH2 Y641 mutations on
cell behavior, in vivo gene mutagenesis was performed on
HCEC cells using CRISPR/Cas9-based technology. The guide RNA (gRNA)
pairs and donor sequences were designed and synthesized by
GenScript (Nanjing, China). The gRNA was cloned into pSpCas9n-BB
(PX460) according manufacturer's recommendations and the sequences
were as follows: gRNA-1, 5′-GGATTTGCATGCTTAGTAAC-3′; and gRNA-2,
5′-TGCAGAAGTCCAGGCTGAAA-3′. The donor sequences were presented in
Table I and cloned into the EcoRV
site of pUC57 (GenScript).
 | Table I.Donor sequences used in this
study. |
Table I.
Donor sequences used in this
study.
| Mutation type | Sequence |
|---|
| EZH2 Y641F |
ctgaacgatggtcattgcagaggaccaacaccaccaaaaggttttctgtaagacagagattcttatctgctgtataaggaaaacataatgttcatagccattctcagcagctttcacgttgactgaagctgtgtgcccaattactgccttagaacaaacaggtctgaggatttacagtgatagcttttgttttcattctgtagtctactttgtccccagtccattttcaccctccttttttgatgatgtgattgtgttttattctctagcatctattgctggcaccatctgacgtggcaggctgggggatttttatcaaagatcctgtgcagaaaaatgaattcatctcagaaTTGtgtggagaggtaaggcactgataacctgtattcaggtggcattgtatatactaactttactttattttagattgattttattaggtaagtctgtgggtttgattggaaatgaattgccataaactgccttttcagcctggacttctgcatgtttgtggatttgcatgcttagtaactggattgtgctgggcgcggtggccgactcctgcaatcccagcactttgggaggccgaggcaggtggattgcttgagctcaggagttggagaccagcatgggcaacatggcaagaccccattgctacaaaaaatgcaaaaattagccgggcgtggtggtgcatacttgtagtcccagctacttgggagc |
| EZH2 Y641N |
ctgaacgatggtcattgcagaggaccaacaccaccaaaaggttttctgtaagacagagattcttatctgctgtataaggaaaacataatgttcatagccattctcagcagctttcacgttgactgaagctgtgtgcccaattactgccttagaacaaacaggtctgaggatttacagtgatagcttttgttttcattctgtagtctactttgtccccagtccattttcaccctccttttttgatgatgtgattgtgttttattctctagcatctattgctggcaccatctgacgtggcaggctgggggatttttatcaaagatcctgtgcagaaaaatgaattcatctcagaaAAGtgtggagaggtaaggcactgataacctgtattcaggtggcattgtatatactaactttactttattttagattgattttattaggtaagtctgtgggtttgattggaaatgaattgccataaactgccttttcagcctggacttctgcatgtttgtggatttgcatgcttagtaactggattgtgctgggcgcggtggccgactcctgcaatcccagcactttgggaggccgaggcaggtggattgcttgagctcaggagttggagaccagcatgggcaacatggcaagaccccattgctacaaaaaatgcaaaaattagccgggcgtggtggtgcatacttgtagtcccagctacttgggagc |
| EZH2 Y641S |
ctgaacgatggtcattgcagaggaccaacaccaccaaaaggttttctgtaagacagagattcttatctgctgtataaggaaaacataatgttcatagccattctcagcagctttcacgttgactgaagctgtgtgcccaattactgccttagaacaaacaggtctgaggatttacagtgatagcttttgttttcattctgtagtctactttgtccccagtccattttcaccctccttttttgatgatgtgattgtgttttattctctagcatctattgctggcaccatctgacgtggcaggctgggggatttttatcaaagatcctgtgcagaaaaatgaattcatctcagaaAGUtgtggagaggtaaggcactgataacctgtattcaggtggcattgtatatactaactttactttattttagattgattttattaggtaagtctgtgggtttgattggaaatgaattgccataaactgccttttcagcctggacttctgcatgtttgtggatttgcatgcttagtaactggattgtgctgggcgcggtggccgactcctgcaatcccagcactttgggaggccgaggcaggtggattgcttgagctcaggagttggagaccagcatgggcaacatggcaagaccccattgctacaaaaaatgcaaaaattagccgggcgtggtggtgcatacttgtagtcccagctacttgggagc |
| EZH2Y641C |
ctgaacgatggtcattgcagaggaccaacaccaccaaaaggttttctgtaagacagagattcttatctgctgtataaggaaaacataatgttcatagccattctcagcagctttcacgttgactgaagctgtgtgcccaattactgccttagaacaaacaggtctgaggatttacagtgatagcttttgttttcattctgtagtctactttgtccccagtccattttcaccctccttttttgatgatgtgattgtgttttattctctagcatctattgctggcaccatctgacgtggcaggctgggggatttttatcaaagatcctgtgcagaaaaatgaattcatctcagaaTGCtgtggagaggtaaggcactgataacctgtattcaggtggcattgtatatactaactttactttattttagattgattttattaggtaagtctgtgggtttgattggaaatgaattgccataaactgccttttcagcctggacttctgcatgtttgtggatttgcatgcttagtaactggattgtgctgggcgcggtggccgactcctgcaatcccagcactttgggaggccgaggcaggtggattgcttgagctcaggagttggagaccagcatgggcaacatggcaagaccccattgctacaaaaaatgcaaaaattagccgggcgtggtggtgcatacttgtagtcccagctacttgggagc |
| EZH2 Y641H |
ctgaacgatggtcattgcagaggaccaacaccaccaaaaggttttctgtaagacagagattcttatctgctgtataaggaaaacataatgttcatagccattctcagcagctttcacgttgactgaagctgtgtgcccaattactgccttagaacaaacaggtctgaggatttacagtgatagcttttgttttcattctgtagtctactttgtccccagtccattttcaccctccttttttgatgatgtgattgtgttttattctctagcatctattgctggcaccatctgacgtggcaggctgggggatttttatcaaagatcctgtgcagaaaaatgaattcatctcagaaCACtgtggagaggtaaggcactgataacctgtattcaggtggcattgtatatactaactttactttattttagattgattttattaggtaagtctgtgggtttgattggaaatgaattgccataaactgccttttcagcctggacttctgcatgtttgtggatttgcatgcttagtaactggattgtgctgggcgcggtggccgactcctgcaatcccagcactttgggaggccgaggcaggtggattgcttgagctcaggagttggagaccagcatgggcaacatggcaagaccccattgctacaaaaaatgcaaaaattagccgggcgtggtggtgcatacttgtagtcccagctacttgggagc |
To generate EZH2 tyrosine 641 mutant cell lines, two
gRNA constructs along with the donor sequence construct were
co-transfected into the HCEC cell line using Lipofectamine
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol (22). The
transfected cells were cultured in the aforementioned conditions
with the addition of ampicillin (Sigma-Aldrich; Merck KGaA) as a
selective marker. Following continuous cultivation under the
antibiotic selective pressure, several single colonies were
successfully obtained in every independent experiment aimed to
obtain tyrosine mutant cell lines. The successful introduction of
tyrosine mutation in EZH2 gene was verified by Sanger Sequencing
(GenScript).
Cell proliferation assay
The cell proliferation of wild-type cell lines
(HCEC, HCT116 and SW480) and the mutant cell lines (HCEC/Y641F,
HCEC/Y641N, HCEC/Y641S, HCEC/Y641C and HCEC/Y641H) were assessed by
the widely used MTT assay. In brief, the cells in the logarithmic
growth phase were harvested and the cell density was adjusted to
~5×104/ml. A total of 100 µl cell suspension for each
cell line was seeded onto 96-well plates with a final cell density
of ~5,000 cells/well and cultured in the described medium and
atmosphere. MTT (20 µl; Sigma-Aldrich; Merck KGaA) was added after
24, 48 and 72 h of incubation, followed by 4 h incubation in the
same conditions, and the supernatant was then removed by
centrifugation (1,000 × g for 10 min at room temperature). A total
of 150 µl DMSO was added to each well, and the plates were
oscillated at a lower speed (100 rpm) until the crystals fully
dissolved. The absorbance of each well was measured at a wavelength
of 570 nm using Thermo Multiskan Spectrum (Thermo Fisher
Scientific, Inc.). Each experiment was repeated three times under
the same conditions.
Clinical patients
A total of 95 patients with CRC who underwent
surgical treatment between March 2009 and June 2011 at Guangzhou
First People's Hospital (Guangzhou, China) were enrolled in the
present study. The cohort included 54 males and 41 females; age
range, 39 to 76 years; average age of 57.3 years. In addition, none
of the patients had received any anticancer treatments, including
radiation, chemotherapy and surgery. The colorectal tissues were
surgically removed from patients with CRC, and the matched
non-cancerous tissues were obtained from the distal edge of the
resection ≥5 cm from the tumor. The tissues were immediately frozen
with liquid nitrogen and preserved at −80°C for further
experiments. According to the tumor-node-metastasis (TNM) staging
system (23), the patients enrolled
in the present study were classified into stages I–IV. In addition,
the histological grade was confirmed by microscopic examination and
conducted by an independent pathologist. Other clinicopathological
data, including tumor size, age, sex and distant metastasis were
also collected (Table II), and the
prognostic factors and disease progression were retrospectively
collected. The present study was approved and monitored by the
Ethics Committee of Guangzhou First People's Hospital, and written
consent was obtained from all the recruited patients.
 | Table II.Association between EZH2 expression
and clinicopathological features of colorectal cancer. |
Table II.
Association between EZH2 expression
and clinicopathological features of colorectal cancer.
|
|
| EZH2
expression |
|
|---|
|
|
|
|
|
|---|
| Variable | No. of cases | High | Low | P-value |
|---|
| Sex |
|
|
|
|
|
Male | 54 | 40 | 14 | NS |
|
Female | 41 | 26 | 15 |
|
| Age, years |
|
|
|
|
|
≥50 | 49 | 34 | 15 | NS |
|
<50 | 46 | 32 | 12 |
|
| Tumor size, cm |
|
|
|
|
| ≥5 | 63 | 48 | 15 | 0.046 |
|
<5 | 32 | 18 | 14 |
|
| Lymph node
metastasis |
|
|
|
|
|
Absent | 53 | 42 | 11 | 0.041 |
|
Present | 42 | 24 | 18 |
|
| Histological
differentiation |
|
|
|
|
|
Well/moderate | 44 | 26 | 18 | 0.041 |
|
Poor | 51 | 40 | 11 |
|
| Tumor stage |
|
|
|
|
|
I–II | 59 | 46 | 13 | 0.021 |
|
III–IV | 36 | 20 | 16 |
|
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from cell lines and all 95 frozen tumor
tissues was isolated using the miRNeasy Mini kit (Qiagen,
Nordrhein-Westfalen, Germany) according to the detailed protocols
provided by the manufacturer. The isolated mRNA was then treated
with DNase (Invitrogen; Thermo Fisher Scientific, Inc.), and cDNA
was synthesized using the Universal cDNA synthesis kit II (Exiqon
A/S, Vedbaek, Denmark) according to the manufacturer's
protocol.
RT-qPCR was performed on the ABI PRISM 7300 sequence
detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) using SYBR-Green PCR Master mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
To estimate the level of EZH2 mRNA expression, GAPDH expression
level in the corresponding tissue was used an internal control. The
primers were synthesized by Invitrogen (Thermo Fisher Scientific,
Inc.), and the sequences were as follows: EZH2 sense,
5′-TTGTTGGCGGAAGCGTGTAAAATC-3′; and antisense,
5′-TCCCTAGTCCCGCGCAATGAGC-3′; GAPDH sense,
5′-TGAACGGGAAGCTCACTGG-3′; and antisense,
5′-TCCACCACCCTGTTGCTGTA-3′. The detailed PCR cycling conditions
were as follows: Initial denaturation at 95°C for 10 min, followed
by 40 cycles of denaturation at 95°C for 1 min, and
annealing/extension at 56°C for 1 min. All samples were performed
in triplicate and normalized to internal controls. The fold-changes
or relative EZH2 expression levels were calculated based on the
2−ΔΔCq method (24). The
75th percentile of EZH2 expression level was used as cut-off value
to classified the patients into either the overexpressed or
underexpressed group.
Western blot analysis
All 95 frozen tumor tissues and cellular protein
samples were isolated using the method described in a previously
published study (14) with slight
modifications. Initially, the tissue or cell samples were
homogenized with homogenization buffer [1 M Tris HCl pH 7.5, 1%
Triton X-100, 1% Nonidet P-40 (NP-40), 10% SDS, 0.5% sodium
deocycholate, 0.5 M EDTA, 10 µg/ml leupeptin, 10 µg/ml aprotinin
and 1 M PMSF] and centrifuged at 10,000 × g for 30 min at 4°C. The
protein concentration was determined using the standard Bradford
method.
Total protein (50 µg) was loaded to each lane of a
10% SDS-PAGE gel and transferred to a nitrocellulose membrane. The
membranes were then blocked with 5% fat-free milk in PBS for ~2 h
at room temperature and incubated with antibodies against EZH2
(cat. no. 4905; dilution, 1:1,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA) and GAPDH (cat. no. 2118; dilution, 1:1,000; Cell
Signaling Technology, Inc.) in PBS containing 5% milk for ~1 h at
room temperature. The membranes were washed three times with PBS
buffer and then incubated with horseradish peroxidase-conjugated
secondary antibodies (cat. no. 7074; dilution, 1:500; Cell
Signaling Technology, Inc.) for 1 h at room temperature. The
specific band was developed using an enhanced chemiluminescence
reagent (ECL reagent; NEN Life Science Products; PerkinElmer, Inc.,
Waltham, MA, USA) in a gel imaging system (ImageQuant 300/RT ECL;
GE Healthcare, Chicago, IL, USA). The band was analyzed using an
Image J 1.37 software (National Institutes of Health, Bethesda, MA,
USA). A total of three independent experiments were performed.
Immunohistochemical analysis
To visualize the localization of EZH2 in tumor
tissues obtained from patients with CRC, immunohistochemical
staining was performed. The tissues were fixed in 4% (v/v) formalin
at 4°C for 48 h and embedded in paraffin, and dewaxed in xylene and
rehydrated in graded ethanol solutions (100, 95, 90, 80, 70 and
50%). Antigen retrieval was performed by immersing sections (5 µm)
in 10 mM citrate buffer (pH 6.0) for between 15 and 20 min at 95°C,
prior to incubation with 0.3% hydrogen peroxide for 15 min at room
temperature to block endogenous peroxidase activity. The sections
were incubated with 5% goat serum (CWBiotech, Beijing, China) to
block the non-specific binding sites at 37°C for 30 min. The
sections were then incubated overnight with the anti-EZH2 antibody
(cat. no. 4905; dilution, 1:100; Cell Signaling Technology, Inc.)
at 4°C. All sections were processed using the
peroxidase-anti-peroxidase method. Subsequent to washing, the
sections were counterstained with hematoxylin for 10 min at room
temperature, dehydrated and mounted with a coverslip. The Olympus
BX61-32S04 microscope was used to observe the stained tissues. The
immunostaining scores for each section were evaluated in a blinded
manner by two independent pathologists. The staining intensity (SI)
was scored on a scale of 0 to 3: Negative (0), weak positive
(1), moderately positive (2), and strongly positive (3). The percentage of positive cells (PP) was
regarded as: None (0), <10% (1),
11–50% (2), 51–80% (3) and ≥80% (4). The product of SI and PP is the
Immunoreactive score (0–12). A score of 0–2 was regarded as low,
3–12 as positive.
Statistical analysis
Statistical analysis was performed with SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). The values are presented
as the mean ± standard deviation, and P<0.05 was considered to
indicate a statistically significant difference. One-way analysis
of variance (ANOVA) followed by Tukey's multiple comparisons test
was used for the comparison of means. The association between EZH2
expression and the clinicopathological characteristics was analyzed
using χ2 test. Survival analysis was performed using the
Kaplan-Meier log-rank test. Univariate and multivariate analyses
were performed using Cox proportional hazard models.
Results
EZH2 is overexpressed in CRC cell
lines
To date, little is known about the role of EZH2 in
the development and progression of CRC, therefore, the levels of
EZH2 mRNA and protein expression were analyzed in CRC cell lines. A
total of two CRC cell lines (HCT-116 and SW480) and one normal
colon epithelial cell line (HCEC) were selected in the present
study. RT-qPCR was performed to estimate the level of EZH2
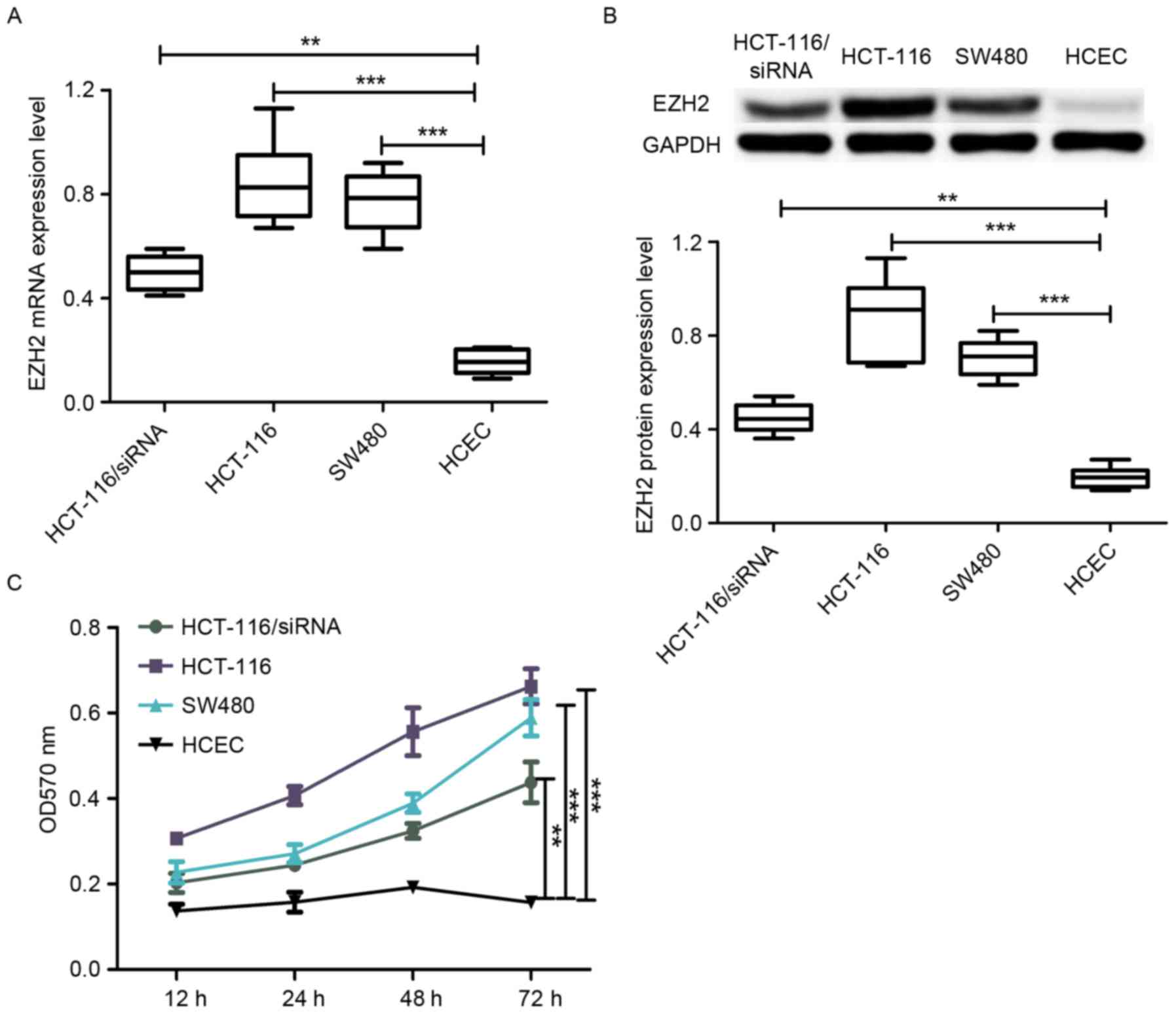
mRNA expression in these cell lines. As shown in Fig. 1A, the level of EZH2 expression
in the HCT-116 and SW480 cell lines was significantly higher
compared with the HCEC cell line. In addition, the level of protein
expression in the aforementioned cell lines harvested in the same
conditions was analyzed. As with the results obtained from RT-qPCR,
the protein expression level of EZH2 in HCT-116 and SW480 cells was
higher compared with HCEC cells (Fig.
1B). The observation that EZH2 expression in CRC cell lines was
much higher compared with the normal colon epithelial cell line
(HCEC) indicates that EZH2 may perform an important role in the
development of CRC.
EZH2 overexpression promotes cell
proliferation
To further investigate the role of EZH2
overexpression on the proliferation of CRC cell lines and normal
colon epithelial cell line, the cell proliferation rate of HCEC,
HCT-116 and SW480 was analyzed using the well-known MTT assay. The
results are depicted in Fig. 1C,
where the cell proliferation rate in the CRC HCT-116 and SW480 cell
lines was markedly higher compared with the HCEC cell line, which
showed that EZH2 overexpression promoted cell proliferation. In
addition, the cell proliferation rate of HCT-116 was higher
compared with SW480 (Fig. 1C), which
is consistent with comparisons of EZH2 expression level between
HCT-116 and SW480 cells.
In addition, the level of EZH2 mRNA and protein
expression was analyzed in the EZH2-specific siRNA-transfected
HCT-116 cell line. The results revealed that EZH2 expression was
downregulated in the siRNA-transfected HCT-116 cell line compared
with the normal HCT-116 cell line (Fig.
1A and B). Importantly, the cell proliferation rate of the
siRNA-transfected HCT-116 cell line was lower compared with the
wild-type HCT-116 cell line. Taken together (Fig. 1C), EZH2 overexpression was able to
promote cell proliferation and the degree of promotion was observed
to be associated with the level of EZH2 expression.
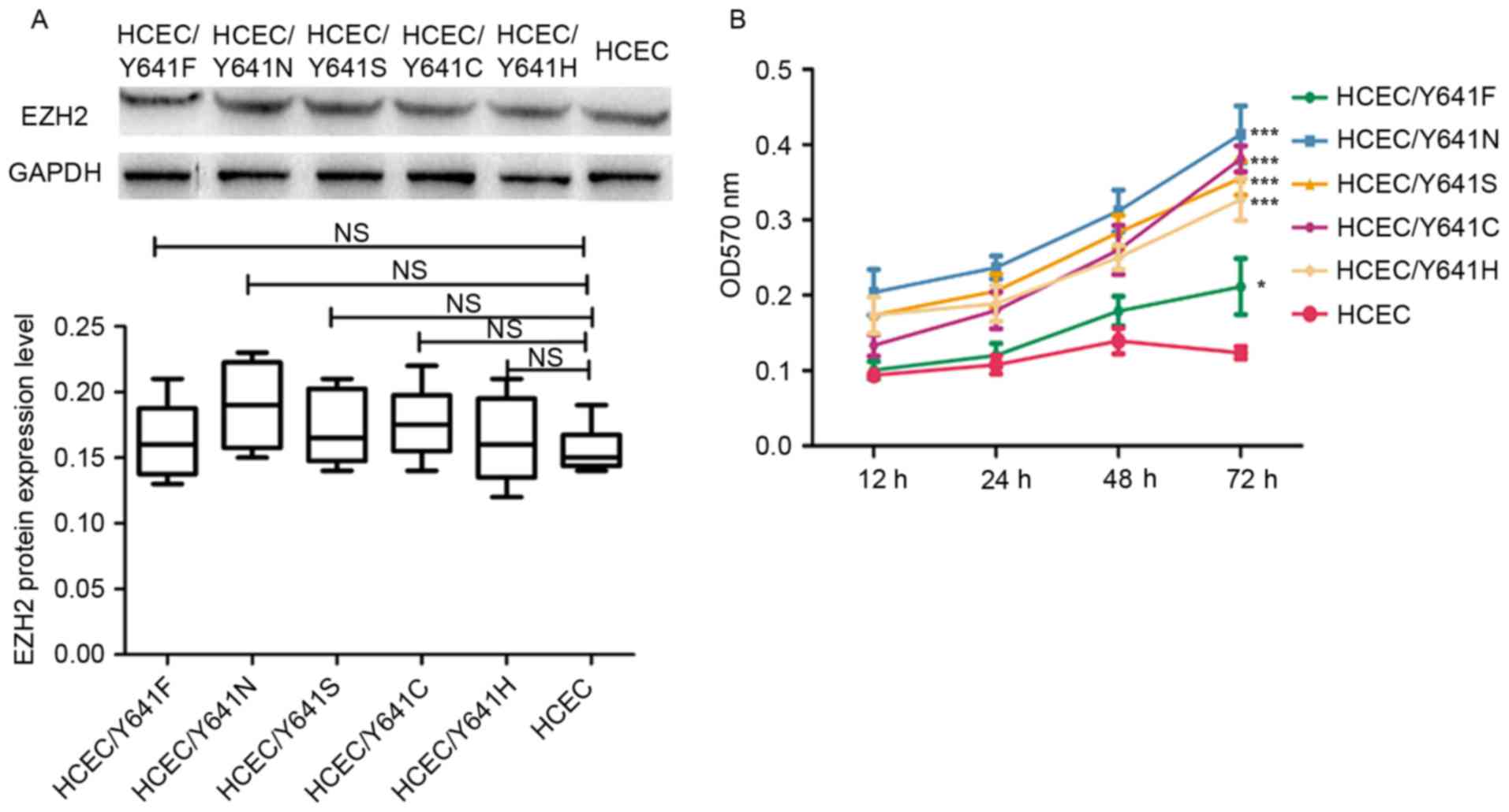
Effects of EZH2 tyrosine 641 mutations
on cell proliferation
Multiple studies have demonstrated that EZH2 is easy
to mutate, particularly in tyrosine 641 (8,10).
Notably, mutations in this specific site will affect the activity
of EZH2, either gain of function or loss of function, and thus
bring a discrepancy effect on the downstream target genes. In the
present study, mutagenesis was performed on the tyrosine 641 of
EZH2 using the CRISPR/Cas9-based gene editing technology to
introduce Y641F, Y641N, Y641S, Y641C and Y641H mutations to EZH2.
The gRNA pairs and donor sequences were designed according to the
instructions in previous published studies. The HCEC cell line was
co-transfected with The gRNA and donor constructs using
Lipofectamine and the successful introduction of mutation was
verified by genome sequencing (data not shown). The cell lines
containing the desired mutations were termed HCEC/Y641F,
HCEC/Y641N, HCEC/Y641S, HCEC/Y641C and HCEC/Y641H.
The protein expression of the mutant EZH2 in the
HCEC cell line was then analyzed by western blot analysis. The
results presented in Fig. 2A
demonstrated that the expression level of EZH2 variants was almost
the same as wild-type EZH2, which indicates that the expression of
EZH2 was not affected by the point mutation. The cell proliferation
rate of HCEC cells with the wild-type EZH2 or mutated EZH2 was then
analyzed. According to the results shown in Fig. 2B, the cell lines with EZH2 mutations
had a significantly higher cell proliferation rate compared with
the wild-type HCEC cell line (P<0.05), which is consistent with
previous findings (9). The present
study therefore provided more support that EZH2 may have an
important role in the malignancy of colorectal cell and is a
potential treatment target for CRC.
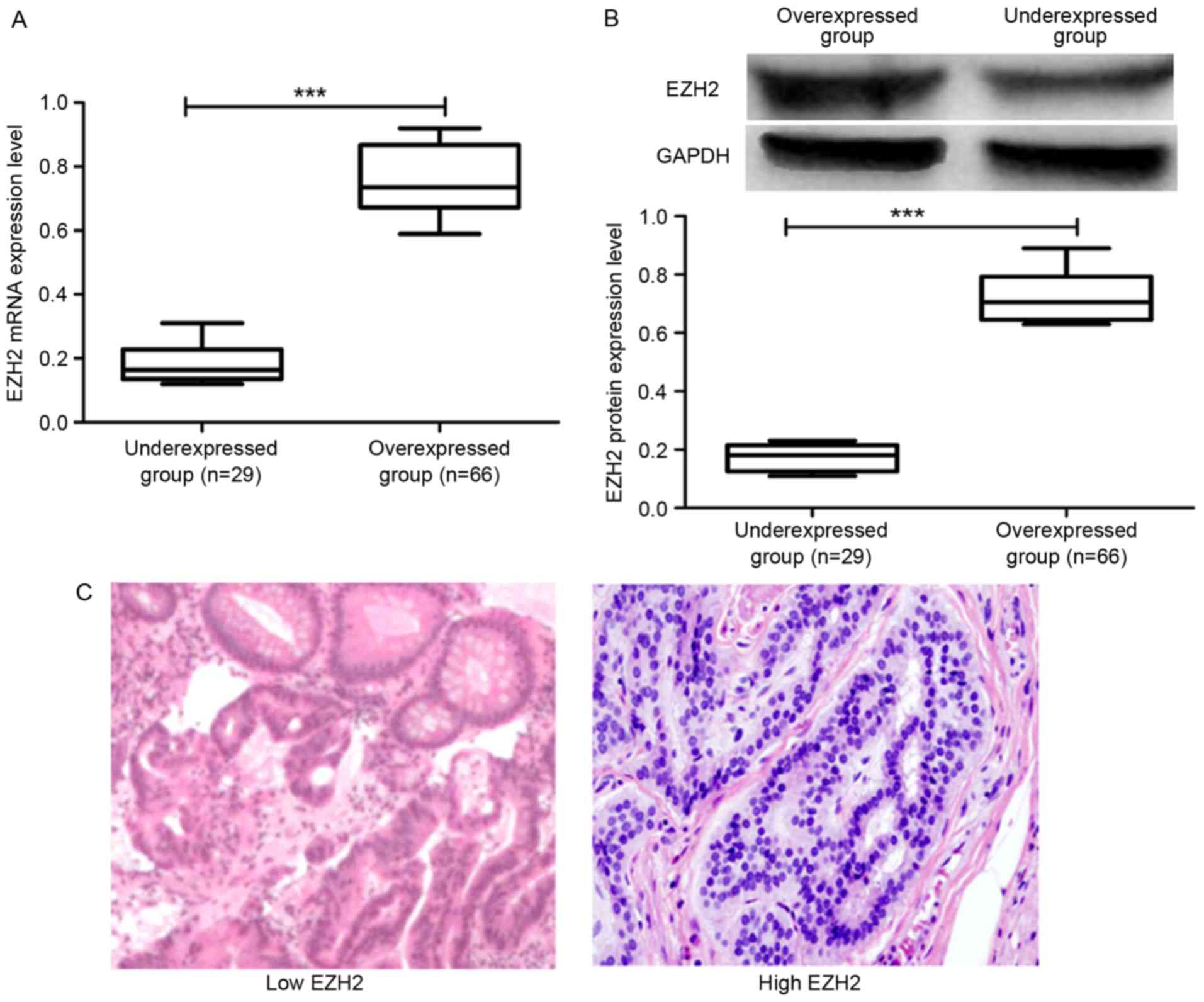
Overexpression of EZH2 in patients
with CRC
In addition to analyzing the EZH2 expression in CRC
cell lines, the expression of EZH2 was examined in patients with
CRC. In total, 95 pairs of tumor tissues and adjacent non-cancerous
tissues were obtained from the patients with CRC involved in the
present study and the expression pattern of EZH2 in all the
collected tissues was analyzed. Initially, RT-qPCR was performed to
analyze the level of EZH2 expression in CRC tissues. It was
observed that 66 of 95 (69.47%) of the cancer tissue samples
exhibited high EZH2 expression and 29 of 95 (30.53%) demonstrated
low expression (Fig. 3A). Patients
with CRC were then classified into overexpressed and
under-expressed groups according to the results obtained from
RT-qPCR. Furthermore, the protein expression level of EZH2 in the
archived tumor tissues was analyzed by western blot analysis. The
results presented in Fig. 3B
demonstrated that the protein expression level of EZH2 in the
overexpressed group was higher compared with the under-expressed
group, which was consistent with the mRNA expression results
obtained from RT-qPCR.
Immunohistochemical staining was performed to
analyze the expression of EZH2 in the CRC tissues in addition to
RT-qPCR and western blot analysis. The results shown in Fig. 3C demonstrated that the level of EZH2
expression as detected by immunohistochemical staining in the
samples from the overexpressed group was higher compared with the
under-expressed group. These results demonstrated that EZH2 was
highly expressed in patients with CRC.
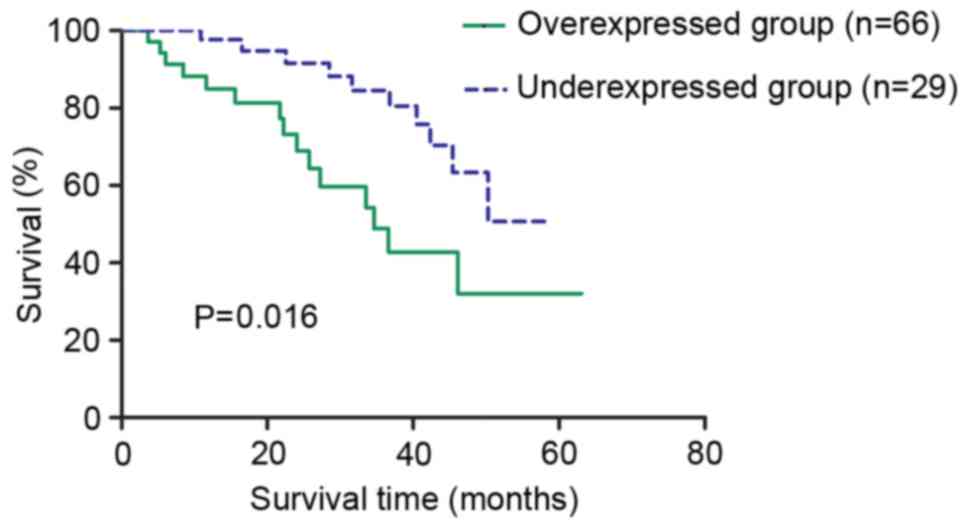
Clinical significance of EZH2
expression in CRC
The association between EZH2 expression in patients
with CRC and the different clinicopathological characteristics was
analyzed (Table II). High EZH2
expression was significantly associated with tumor stage (P=0.021),
tumor size (P=0.046), histological differentiation (P=0.041) and
lymph node metastasis (P=0.041), respectively. The overall survival
(OS) rate of the patients from overexpressed and under-expressed
groups was examined using the log-rank test. As a result, a
statistically significant difference was observed between these two
groups (P=0.016; Fig. 4). The 5-year
OS rates for the overexpressed group and under-expressed group were
32.08 and 50.68%, respectively, indicating that the patients with
low EZH2 expression had a longer survival expectation compared with
the patients with high expression.
EZH2 overexpression is associated with
poor prognosis in CRC
Univariate analysis and multivariate analysis were
performed to examine the association of clinicopathological
characteristics, including EZH2 expression, age, sex, tumor size,
lymph node metastases, histological differentiation, TNM stage with
the survival of patients with CRC. It was concluded that tumor
size, tumor stage, lymph node metastases, histological
differentiation and EZH2 expression level could be reconsidered as
independent prognosis factors for patients with CRC (Table III). The present study demonstrates
the importance of EZH2 expression in the development and
progression of CRC.
 | Table III.Univariate analysis and multivariate
analyses of overall survival in patients with colorectal
cancer. |
Table III.
Univariate analysis and multivariate
analyses of overall survival in patients with colorectal
cancer.
| A, Univariate
analysis |
|---|
|
|---|
| Variables | HR | 95% CI | P-value |
|---|
| EZH2
expression | 2.872 | 1.204–6.349 | 0.009 |
| Age | 2.142 | 0.936–4.898 | 0.071 |
| Sex | 1.983 | 0.851–4.619 | 0.113 |
| Tumor size | 2.290 | 1.019–5.148 | 0.045 |
| Lymph node
metastases | 2.297 | 1.022–5.164 | 0.044 |
| Histological
differentiation | 2.448 | 1.107–5.414 | 0.027 |
| Tumor stage | 2.717 | 1.209–6.108 | 0.016 |
|
| B, Multivariate
analysis |
|
|
Variables | HR | 95% CI | P-value |
|
| EZH2
expression | 2.625 | 1.204–5.722 | 0.015 |
| Age | – | – | – |
| Sex | – | – | – |
| Tumor size | 2.499 | 1.128–5.537 | 0.024 |
| Lymph node
metastases | 2.485 | 1.122–5.505 | 0.025 |
| Histological
differentiation | 2.551 | 1.168–5.573 | 0.019 |
| Tumor stage | 2.737 | 1.270–5.900 | 0.010 |
Discussion
An increasing number of studies have indicated that
the chromosome structure is a key factor to regulate the
development and progression of various types of human cancer
(10). EZH2 has long been recognized
as the most important participant to induce histone H3 lysine 27
(H3K27) methylation (7). Clinical
study data has shown the oncogenic role of EZH2 in several types of
human cancer including breast cancer and ovarian cancer (14,25).
Notably, an in vitro experiment involving the EZH2 inhibitor
in prostate cancel models demonstrated that EZH2 inhibitor was able
to significantly increase death of mouse and human prostate cancer
cells (26). Furthermore, in a
preclinical study aimed to investigate the treatment function of
EZH2 inhibitor on non-small cell lung cancer, researchers found the
EZH2 inhibitor had differing effects depending on the mutations
present (27). Although the
development of EZH2 inhibitor is at an early stage, it provides a
new potential strategy for cancer treatment (28,29).
However, little is known about the role of EZH2
expression in CRC and, to the best of our knowledge, this is the
first study reported to investigate the expression status of EZH2
in CRC and also its role in the development and progression of CRC.
The expression status of EZH2 in CRC and normal colon epithelial
cell lines was examined with siRNA transfection. It was observed
that the expression level of EZH2 was significantly higher in CRC
cell lines compared with the normal colon epithelial cell line. The
EZH2 expression in the siRNA-transfected HCT-116 was lower compared
with the cell line without transfection. In addition, the cell
proliferation rate of the CRC cell lines and normal colon
epithelial cell line used in the present study was analyzed.
Furthermore, the cell proliferation rate of CRC cell lines was
higher compared with the normal colon epithelial cell line and the
siRNA-transfected CRC cell line, which indicated that EZH2
overexpression may promote cell proliferation. Furthermore, the
specified mutation in the tyrosine 641 of EZH2 was introduced using
CRISPR/Cas9-based genome editing system. Consistent with the
previous results (10), it was shown
that the EZH2 Y641F, Y641N, Y641S, Y641C and Y641H mutations may
increase the activity of EZH2. These in vitro results
indicated that EZH2 may perform an important role in the
development and progression of CRC.
Finally, the expression of EZH2 was investigated in
patients with CRC and its role on the prognosis of patients with
CRC was examined. Initially, it was verified that high EZH2
expression was observed in patients with CRC through examining the
EZH2 expression in tumor tissues obtained from the patients with
CRC using RT-qPCR, western blot analysis and immunohistochemistry
approaches. These findings were consistent with the results
obtained from analysis of EZH2 expression in CRC cell lines and
normal colon epithelial cell line. Furthermore, the association of
EZH2 expression with other clinical characteristics collected from
the patients with CRC was analyzed and the results indicated EZH2
overexpression was associated with tumor stage, tumor size,
histological differentiation and lymph node metastasis, which
suggest that EZH2 overexpression is associated with tumor
progression. In addition, the 5-year survival rate comparisons
between the patients with CRC with high EZH2 expression and those
with low expression revealed that the patients with high EZH2
expression had a shorter survival estimate, which provide further
support that EZH2 overexpression is associated with poor outcomes
in patients with CRC. Multivariate analyses revealed that EZH2
overexpression was an independent factor for prediction of
prognosis of patients with CRC. Additionally, increased EZH2
expression, tumor size, tumor stage, lymph node metastases and
histological differentiation are also considered to be independent
factors for poor prognosis of CRC.
In conclusion, the in vitro and in
vivo studies in the present study demonstrated that EZH2 was
overexpressed in patients with CRC. The overexpression of EZH2 was
an independent biomarker for the poor outcome of patients with CRC.
The results indicated that EZH2 has potential value as a
therapeutic target in patients with CRC in the future. However,
more in vitro and in vivo studies are required to
identify the downstream target genes in CRC to improve the
understanding of the biological role of EZH2 in CRC.
Acknowledgements
The present study was funded by the National Nature
Science Foundation of China (grant no. 81272556).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stewart BW and Wild CP: Colorectal
cancerWorld Cancer Report 2014. IARC Publications; pp. 392–402.
2014
|
|
4
|
Laiyemo AO, Doubeni C, Pinsky PF, Doria
Rose VP, Bresalier R, Lamerato LE, Crawford ED, Kvale P, Fouad M,
Hickey T, et al: Race and colorectal cancer disparities:
Health-care utilization vs different cancer susceptibilities. J
Natl Cancer Inst. 102:538–546. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Hees F, Saini SD, Lansdorp-Vogelaar I,
Vijan S, Meester RG, de Koning HJ, Zauber AG and van Ballegooijen
M: Personalizing colonoscopy screening for elderly individuals
based on screening history, cancer risk, and comorbidity status
could increase cost effectiveness. Gastroenterology. 149:1425–1437.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma W, Yu Q, Jiang J, Du X, Huang L, Zhao L
and Zhou QI: miR-517a is an independent prognostic marker and
contributes to cell migration and invasion in human colorectal
cancer. Oncol Lett. 11:2583–2589. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tonini T, D'Andrilli G, Fucito A, Gaspa L
and Bagella L: Importance of Ezh2 polycomb protein in tumorigenesis
process interfering with the pathway of growth suppressive key
elements. J Cell Physiol. 214:295–300. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Margueron R and Reinberg D: The Polycomb
complex PRC2 and its mark in life. Nature. 469:343–349. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan J, Ng SB, Tay JL, Lin B, Koh TL, Tan
J, Selvarajan V, Liu SC, Bi C, Wang S, et al: EZH2 overexpression
in natural killer/T-cell lymphoma confers growth advantage
independently of histone methyltransferase activity. Blood.
121:4512–4520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sneeringer CJ, Scott MP, Kuntz KW, Knutson
SK, Pollock RM, Richon VM and Copeland RA: Coordinated activities
of wild-type plus mutant EZH2 drive tumor-associated
hypertrimethylation of lysine 27 on histone H3 (H3K27) in human
B-cell lymphomas. Proc Natl Acad Sci USA. 107:pp. 20980–20985.
2010; View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Orkin SH and Hochedlinger K: Chromatin
connections to pluripotency and cellular reprogramming. Cell.
145:835–850. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bracken AP, Pasini D, Capra M, Prosperini
E, Colli E and Helin K: EZH2 is downstream of the pRB-E2F pathway,
essential for proliferation and amplified in cancer. EMBO J.
22:5323–5335. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Herrera-Merchan A, Arranz L, Ligos JM, de
Molina A, Dominguez O and Gonzalez S: Ectopic expression of the
histone methyltransferase Ezh2 in haematopoietic stem cells causes
myeloproliferative disease. Nat Commun. 3:6232012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kleer CG, Cao Q, Varambally S, Shen R, Ota
I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, et al: EZH2
is a marker of aggressive breast cancer and promotes neoplastic
transformation of breast epithelial cells. Proc Natl Acad Sci USA.
100:pp. 11606–11611. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Varambally S, Dhanasekaran SM, Zhou M,
Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt
RG, Otte AP, et al: The polycomb group protein EZH2 is involved in
progression of prostate cancer. Nature. 419:624–629. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sudo T, Utsunomiya T, Mimori K, Nagahara
H, Ogawa K, Inoue H, Wakiyama S, Fujita H, Shirouzu K and Mori M:
Clinicopathological significance of EZH2 mRNA expression in
patients with hepatocellular carcinoma. Br J Cancer. 92:1754–1758.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bachmann IM, Halvorsen OJ, Collett K,
Stefansson IM, Straume O, Haukaas SA, Salvesen HB, Otte AP and
Akslen LA: EZH2 expression is associated with high proliferation
rate and aggressive tumor subgroups in cutaneous melanoma and
cancers of the endometrium, prostate, and breast. J Clin Oncol.
24:268–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weikert S, Christoph F, Köllermann J,
Müller M, Schrader M, Miller K and Krause H: Expression levels of
the EZH2 polycomb transcriptional repressor correlate with
aggressiveness and invasive potential of bladder carcinomas. Int J
Mol Med. 16:349–353. 2005.PubMed/NCBI
|
|
19
|
Zingg D, Debbache J, Schaefer SM, Tuncer
E, Frommel SC, Cheng P, Arenas-Ramirez N, Haeusel J, Zhang Y and
Bonalli M: The epigenetic modifier EZH2 controls melanoma growth
and metastasis through silencing of distinct tumor suppressors. Nat
Commun. 6:60512015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang CJ, Yang JY, Xia W, Chen CT, Xie X,
Chao CH, Woodward WA, Hsu JM, Hortobagyi GN and Hung MC: EZH2
promotes expansion of breast tumor initiating cells through
activation of RAF1-β-catenin signaling. Cancer Cell. 19:86–100.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Landen CN Jr, Chavez-Reyes A, Bucana C,
Schmandt R, Deavers MT, Lopez-Berestein G and Sood AK: Therapeutic
EphA2 gene targeting in vivo using neutral liposomal small
interfering RNA delivery. Cancer Res. 65:6910–6918. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang W, Bikard D, Cox D, Zhang F and
Marraffini LA: RNA-guided editing of bacterial genomes using
CRISPR-Cas systems. Nat Biotechnol. 31:233–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wittekind C, Compton CC, Greene FL and
Sobin LH: TNM residual tumor classification revisited. Cancer.
94:2511–2516. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuang Y, Lu F, Guo J, Xu H, Wang Q, Xu C,
Zeng L and Yi S: Histone demethylase KDM2B upregulates histone
methyltransferase EZH2 expression and contributes to the
progression of ovarian cancer in vitro and in vivo. Onco Targets
Ther. 10:3131–3144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kirk JS, Sachaarchuch K, Dalimov Z,
Lasorsa E, Ku S, Ramakrishnan S, Hu Q, Azabdaftari G, Wang J, Pili
R and Ellis L: Top2a identifies and provides epigenetic rationale
for novel combination therapeutic strategies for aggressive
prostate cancer. Oncotarget. 6:3136–3146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fillmore CM, Xu C, Desai PT, Berry JM,
Rowbotham SP, Lin YJ, Zhang H, Marquez VE, Hammerman PS, Wong KK
and Kim CF: EZH2 inhibition sensitizes BRG1 and EGFR mutant lung
tumours to TopoII inhibitors. Nature. 520:239–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McCabe MT, Ott HM, Ganji G, Korenchuk S,
Thompson C, Van Aller GS, Liu Y, Graves AP, Della Pietra A III,
Diaz E, et al: EZH2 inhibition as a therapeutic strategy for
lymphoma with EZH2-activating mutations. Nature. 492:108–112. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qi W, Chan H, Teng L, Li L, Chuai S, Zhang
R, Zeng J, Li M, Fan H, Lin Y, et al: Selective inhibition of Ezh2
by a small molecule inhibitor blocks tumor cells proliferation.
Proc Natl Acad Sci USA. 109:pp. 21360–21365. 2012; View Article : Google Scholar : PubMed/NCBI
|