Introduction
Ovarian cancer is often diagnosed when it is at an
advanced stage. The overall 5-year survival rate of patients with
ovarian cancer is ~40% (1) and
ovarian cancer is one of the leading causes of gynecological
cancer-related mortality. The exact cause of epithelial ovarian
cancer has not yet been determined. It has been suggested that
gonadotropins, including follicle-stimulating hormone (FSH) and
luteinizing hormone (LH) serve an important role in the development
of ovarian cancer (2), although their
underlying mechanisms of action remain unknown (3). Ovarian epithelial cancer is usually
characterized by elevated levels of FSH and LH, particularly in
post-menopausal women or in women receiving treatment to induce
ovulation (4–7). Furthermore, the results of
epidemiological studies have revealed that reduced exposure to, or
lower levels of gonadotropins are associated with a decreased risk
of ovarian cancer (4–7). Lower levels of gonadotropins may be
induced following multiple pregnancies, breast-feeding, the use of
oral contraceptives and during estrogen replacement therapy
(8,9).
Compared with FSH, the effect of LH on ovarian cancer is
contentious. It has been reported that there is no association
between LH and ovarian cancer cell proliferation (10); furthermore, studies have demonstrated
that LH may inhibit or stimulate the progression of ovarian cancer
(11–16).
Cisplatin has been widely used to treat various
solid malignancies, including ovarian cancer, with a consistent
rate of initial responses (17).
Following the binding of cisplatin to DNA, unrepairable DNA lesions
are generated, the DNA damage response is activated and
mitochondrial apoptosis or proliferative arrest is subsequently
induced (17). However, resistance to
cisplatin readily develops and this may compromise its anti-tumor
effect, resulting in therapeutic failure (17). Therefore, it is important to
understand the underlying mechanisms of cisplatin resistance. It
has been demonstrated that the development of cisplatin resistance
is a complicated process that arises at diverse stages of
DNA-targeting (17).
LH may inhibit cisplatin-induced apoptosis in
vitro (18). Therefore, the
present study used epithelial ovarian cancer cells to determine
whether LH impairs the in vivo anti-tumor effect of
cisplatin in xenograft nude mice, at least in part, by inhibiting
the pro-apoptotic activity of cisplatin.
Materials and methods
Reagents and antibodies
LH was purchased from Merck KGaA (Darmstadt,
Germany) and cisplatin was purchased from Selleck Chemicals LLC.
(Houston, TX, USA). The antibodies used in the immunohistochemistry
assay were anti-ki67 (cat. no. MA5-14520; Neomarkers, Inc.,
Waltham, MA, USA) and anti-cleaved caspase-3 (cat. no. 9661S; Cell
Signaling Technology, Inc., Danvers, MA, USA).
Cell lines and culture conditions
The highly metastatic human ovarian cancer cell
lines, HeyA8 and SKOV3ip1, were used as described previously
(19). Cells were purchased from the
MD Anderson Characterized Cell Line Core Facility (Houston, TX,
USA). Cells were cultured in RPMI 1640 medium supplemented with 10%
fetal bovine serum (both purchased from Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and 0.5% gentamicin, maintained
on plastic and incubated at 37°C in a mixture of 5% CO2
and 95% air. Tumor cells were free of pathogenic murine viruses and
mycoplasma. The cells were maintained at 37°C in a mixture of 5%
CO2 and 95% air for <10 weeks following recovery from
a frozen stock.
Animals
A total of 80 age-matched 8–10-week-old female
athymic nude mice (NCr-nu) weighing 15.7–20.3 g were purchased from
the Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China).
The mice were housed under specific pathogen-free conditions at the
animal facility of Shanghai Medical School, Fudan University
(Shanghai, China). All mice were bred in a specific pathogen-free
animal facility and were housed 4 per cage bedded with heat-treated
chipped hardwood which was changed weekly. The facility used a 12 h
light/dark cycle, and a standardized room temperature and humidity
(30–70%). Sterile pelleted food and water were freely available.
Animal care and experiments were approved by the Institutional
Animal Care and Use Committee at Shanghai Medical School, Fudan
University and all procedures complied with the Guide for the Care
and Use of Laboratory Animals published by the US National
Institutes of Health (20).
Orthotopic tumor implantation and
treatment
Sub-confluent cultures of HeyA8 and SKOV3ip1 cells
were harvested and suspended in Hank's balanced salt solution
medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Cell viability was determined to be >95% using trypan blue
exclusion (19). Suspended cells were
intraperitoneally implanted into mice at a concentration of
2.5×105 cells/0.2 ml for HeyA8 cells or
1.0×106 cells/0.2 ml for SKOV3ip1 cells. The 40 mice
were randomly divided into 4 groups (n=10/group) 7 days following
tumor implantation. One group, which served as a control, received
injections of PBS (equal volume, intraperitoneal, once a week), one
group received LH alone (3 U/day, subcutaneous), one group received
cisplatin alone (2.5 mg/kg/week, intraperitoneal) and one group
received LH (3 U/day, subcutaneous) combined with cisplatin (2.5
mg/kg/week, intraperitoneal). It has been demonstrated that
low-doses (3 U/day, subcutaneously) of FSH induce an improved
effect compared with high-doses (10 U/day, subcutaneous) (21). Therefore, 3 U/day LH was administered
to mice undergoing treatment with LH in the present study.
During tumor progression, mice in the control and LH
groups became weaker and moribund. These mice exhibited increased
tumor load, decreased food consumption, decreased activity, and
increased size of the ascites. Most mice exhibited some of these
symptoms, however, mice in the control and LH groups exhibited the
most severe symptoms. Seeing as mice in these two groups did not
receive cisplatin chemotherapy, this was in line with expectations
(18). When most mice in the control
and LH groups presented with symptoms and became moribund, all the
mice in the experiment were sacrificed immediately. HeyA8-implanted
mice were sacrificed following 3 weeks treatment and
SKOVip1-implanted mice were sacrificed following 5 weeks treatment.
Prior to sacrifice, mice underwent inhalation anesthesia with 2%
isoflurane (Ruiwode Lifescience Co. Shenzhen, China.). Following
collection of ~1 ml blood via a cardiac puncture under anesthesia,
mice were sacrificed. Body and tumor weight and the number of tumor
nodules were recorded. Additionally, ascites were collected, blood
was collected via cardiac puncture and all other samples were
collected following sacrifice of mice on day 28 for HeyA8-implanted
mice and on day 42 for SKOVip1-implanted mice.
Prior to immunohistochemical (IHC) staining, tumor
tissues were fixed in formalin (cat. no. 50-00-0; Sinopharm
Chemical Reagent Co., Ltd., Shanghai, China) at room temperature
for ≥24 h and embedded in paraffin. Tissues were then cut into 4-µM
thick sections.
Measurement of serum LH levels
Blood samples were collected from the inferior vena
cava and allowed to clot for 2 h at room temperature prior to
centrifugation for 20 min at 2,000 × g. Sera were then collected
for LH level measurements using a Luteinizing Hormone Human ELISA
kit (cat. no. EHLH) (Thermo Fisher Scientific, Inc.).
IHC staining for ki67 and cleaved
caspase-3
Paraffin sections were deparaffinized and
rehydrated, then antigen retrieval was performed using EZ antigen
retrieval 3 solution (BioGenex Laboratories, San Ramon, CA, USA).
Sections were then blocked with 3% hydrogen peroxide in methanol
and 4% fish gelatin at room temperature for 30 min. Sections were
then incubated with rabbit anti-ki67 (1:200) or rabbit anti-cleaved
caspase-3 (1:100) overnight at 4°C. Following washing with PBS,
sections were incubated with horseradish peroxidase-conjugated goat
anti-rabbit immunoglobulin G (cat. no. 111-005-045; dilution,
1:200; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA,
USA) for 60 min at room temperature. Sections were visualized with
a 3,3′-diaminobenzidine kit (Vector Laboratories, Inc.),
counterstained with hematoxylin at room temperature for 10 sec,
dehydrated and mounted in Richard-Allan Scientific™
Cytoseal™ XYL (Thermo Fisher Scientific, Inc.). Antibody
staining in the tissue sections was observed using a light
microscope (×40 magnification).
The count of ki67- or cleaved caspase-3-positive
cells was independently performed by two experienced pathologists.
Briefly, each entire slide was evaluated and five fields were
randomly visualized at a magnification of ×200. Subsequently, the
average proportion of positively stained tumor cells was calculated
based on the results from the five fields using ImageJ software
(version 1.31; National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
All data are presented as the mean ± standard error
of the mean. For in vivo therapy experiments, 10 mice were
used in each group, which enabled the detection of a 50% reduction
in tumor size (ß error=0.2). Continuous variables were compared
using two-tailed Student's t-tests (for 2 groups) or one-way
analysis of variance followed by Tukey's test (>2 groups) if the
data were normally distributed. For non-parametric distributions,
the Mann-Whitney U or the Kruskal-Wallis test were used. P<0.05
was considered to indicate a statistically significant
difference.
Results
LH impairs the in vivo anti-tumor
effect of cisplatin
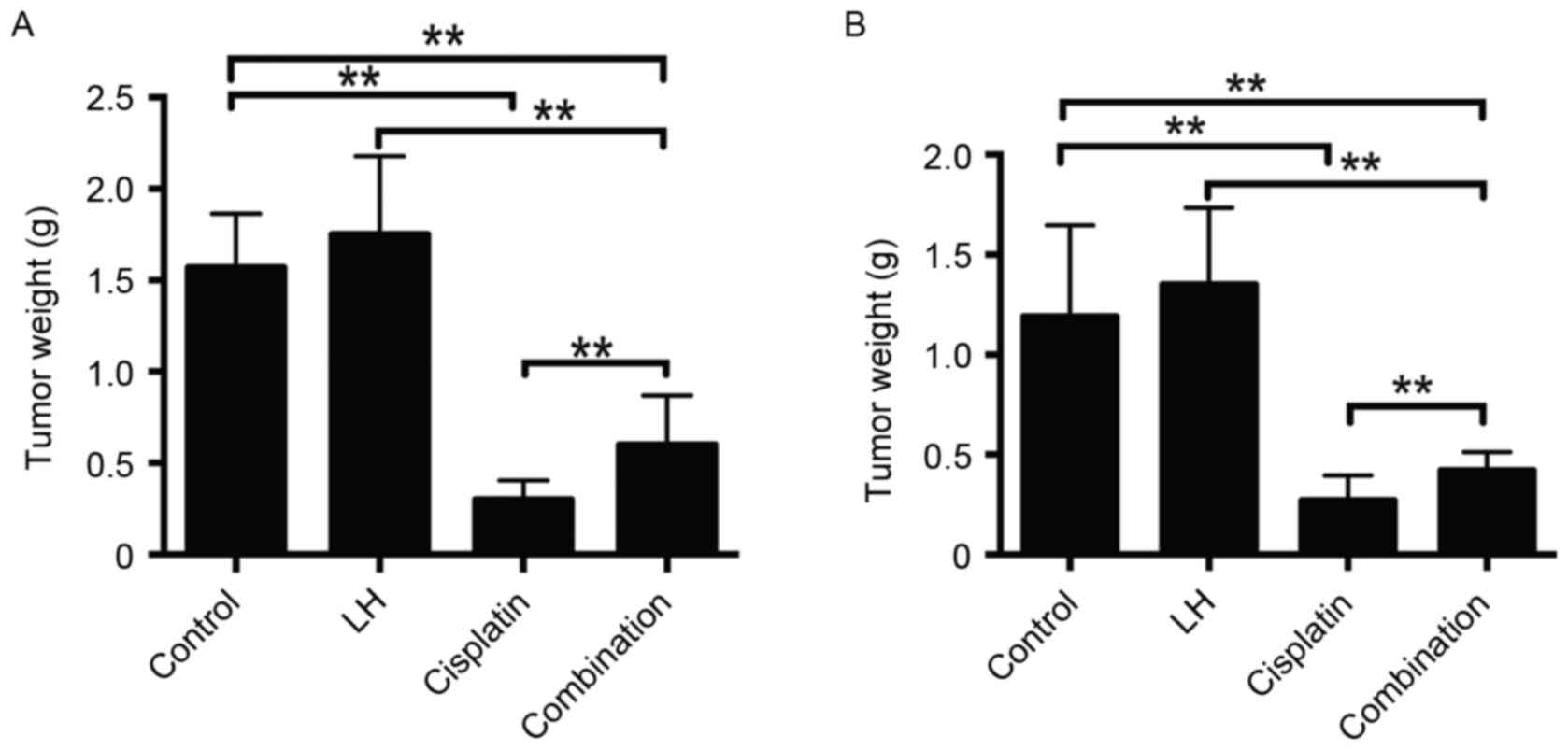
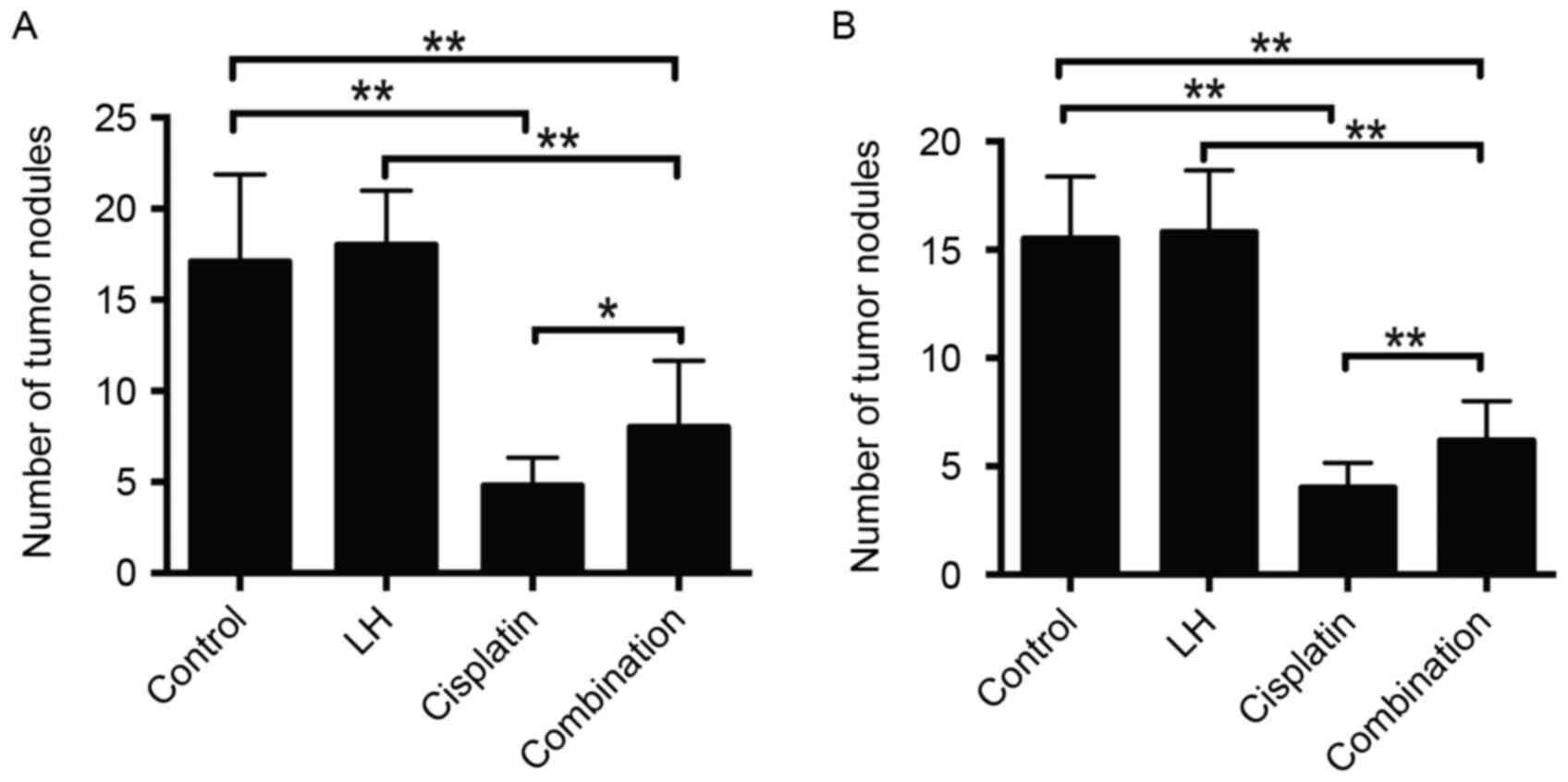
In the HeyA8- and SKOV3ip1- implanted mice,
cisplatin alone but not LH alone treatment significantly reduced
tumor weight and nodule number compared with the control group
(Figs. 1 and 2). Although the treatment of cisplatin
combined with LH still suppressed tumor weight and nodule number
compared with the control group, the addition of LH significantly
compromised the anti-tumor effect compared with cisplatin alone
treatment (Figs. 1 and 2).
The maximum total tumor weight in SKOV3ip1-implanted
nude mice was 2.0 g and in HeyA8-implanted nude mice, it was 2.4 g.
The maximum number of tumors in SKOV3ip1-implanted nude mice was 21
and that in HeyA8-implanted nude mice was 25. The longest diameter
of a single tumor was ~1 cm.
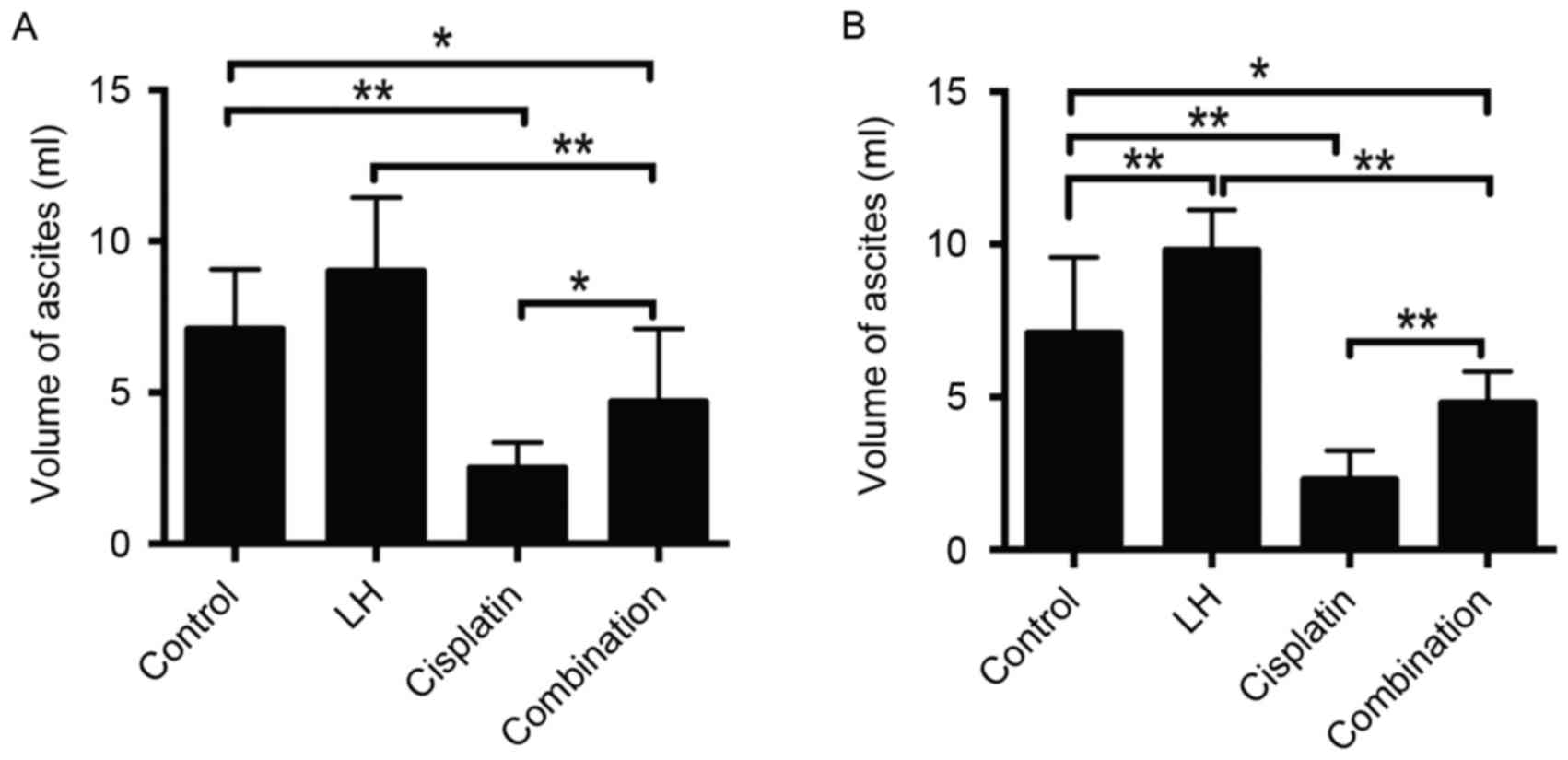
The volume of ascites may also represent the
orthotopic tumor growth of ovarian cancer (22). The maximum volume of ascites observed
in the current study was 12 ml. Treatment with cisplatin alone
significantly reduced the volume of ascites compared with the
control group in HeyA8- and SKOV3ip1-implanted nude mice (Fig. 3). Additionally, treatment with LH
alone slightly increased the volume of ascites in HeyA8-implanted
mice (Fig. 3A) and significantly
increased the volume of ascites in SKOV3ip1-implanted mice
(Fig. 3B). Combined treatment of
cisplatin with LH significantly impaired the anti-tumor effect of
cisplatin, indicated by the significant increase of ascite volume
in the group receiving combination treatment compared with the
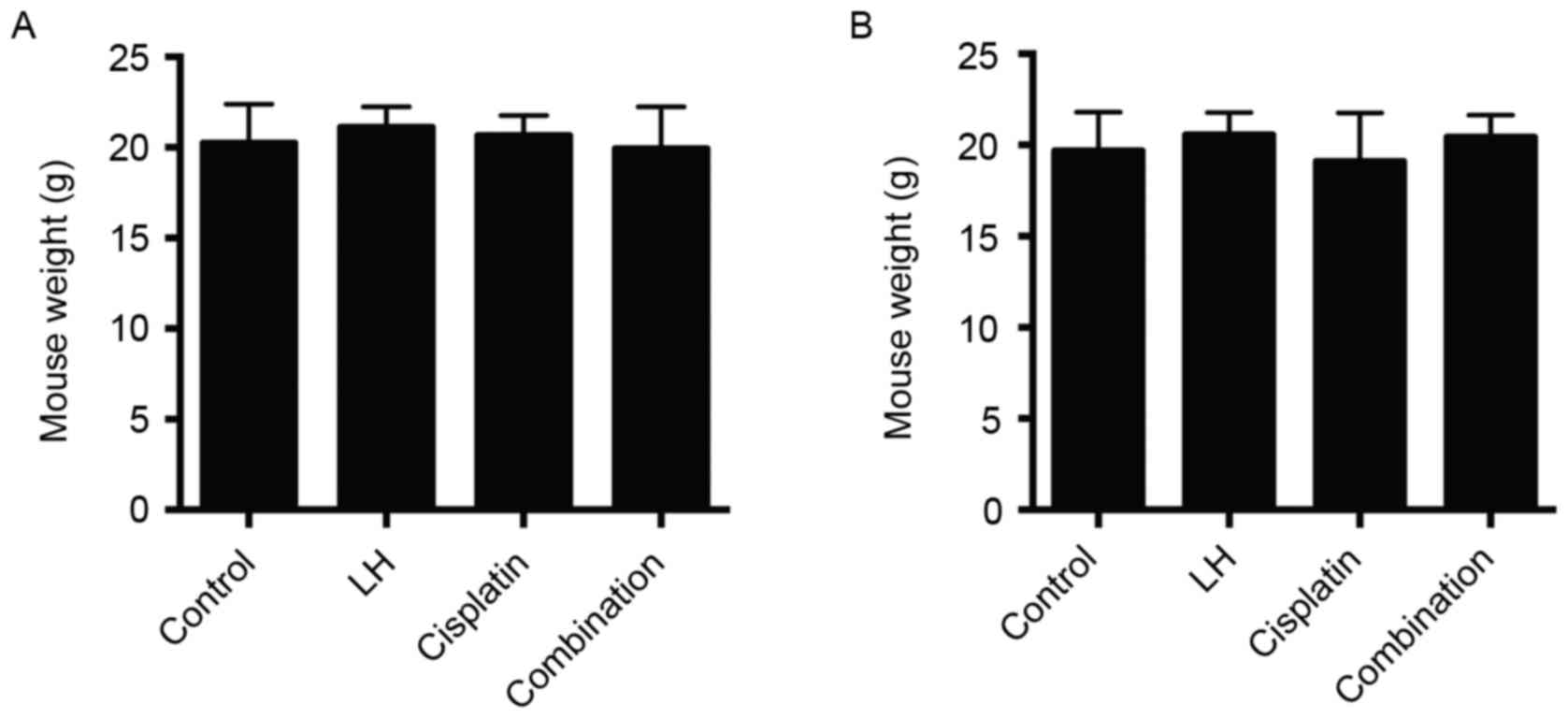
group receiving treatment with cisplatin alone (Fig. 3). In addition, no differences were in
mouse weights were observed among all four groups (Fig. 4). These results indicate that LH may
impair the in vivo anti-tumor effect of cisplatin in nude
mice implanted with epithelial ovarian cancer cells.
LH compromises the pro-apoptotic
effect of cisplatin
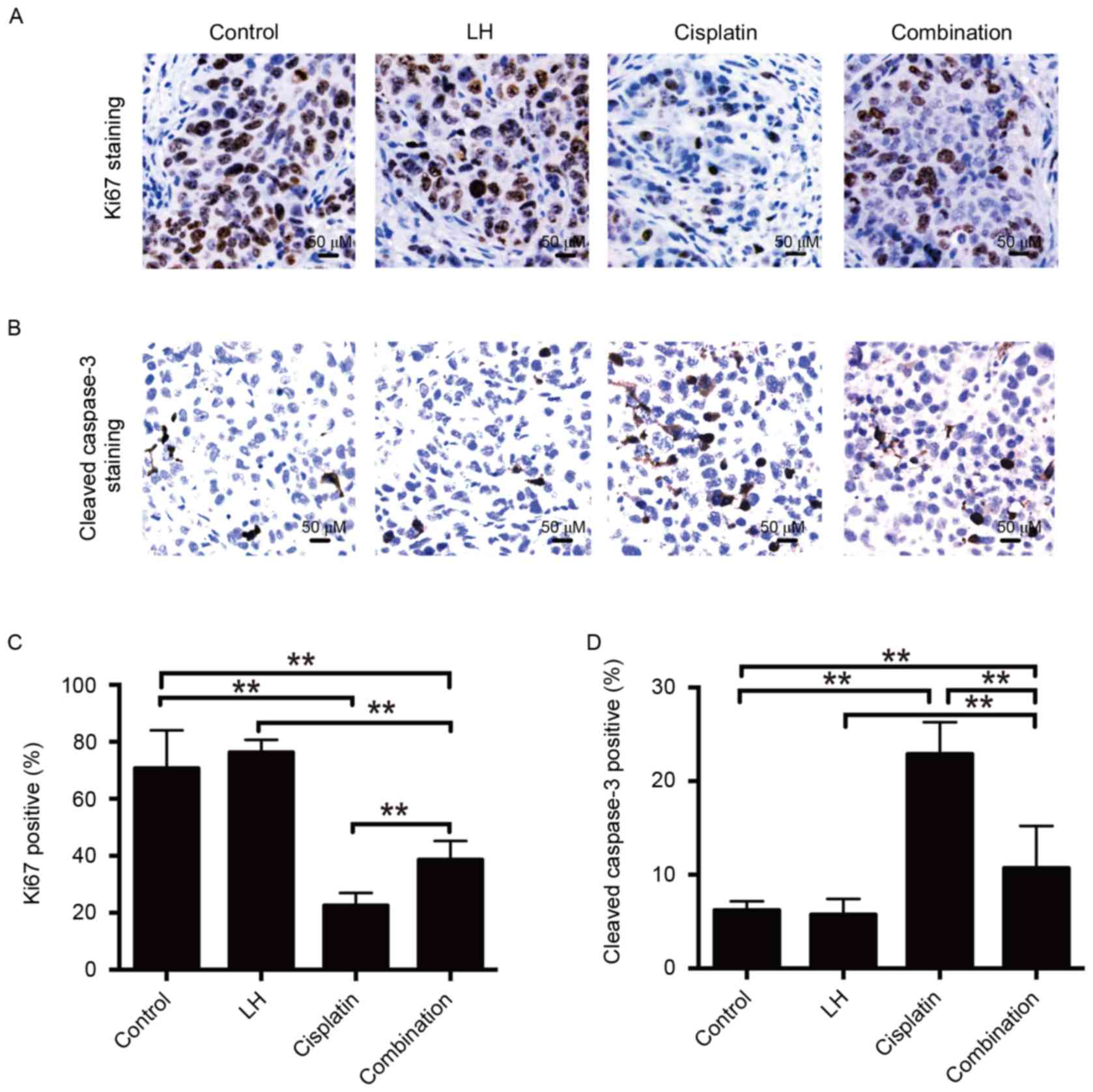
The nuclear protein ki67 has been used as a
biomarker for cell proliferation (23). Staining of proliferative and apoptotic
cells and the quantitative analysis are depicted in Fig. 5. It was observed that the number of
ki67-positive cells was significantly decreased in the cisplatin
and combination treatment groups compared with the control group in
SKOV3ip1-implanted mice (Fig. 5C). No
significant differences were observed between the LH and control
groups. Furthermore, combination treatment with cisplatin and LH
resulted in increased ki67 expression compared to treatment with
cisplatin alone (Fig. 5A and C),
indicating that LH may compromise the anti-tumor activity of
cisplatin.
Apoptosis is primary mechanism by which cisplatin
induces cell death (24). Therefore,
the current study investigated whether the impairment of cisplatin
anti-tumor activity by LH results from the inhibition of LH on the
cisplatin-mediated pro-apoptotic effect. The results of IHC
staining demonstrated that combination treatment of cisplatin and
LH led to fewer apoptotic cells determined by staining for cleaved
caspase-3, compared with the group treated with cisplatin alone.
However, combination treatment still induced more apoptosis than
the control group (Fig. 5B and D). No
significant differences were identified between the LH and control
groups. These results indicate that LH impairs the in vivo
anti-tumor effect of cisplatin, at least in part, by inhibiting the
pro-apoptotic activity of cisplatin.
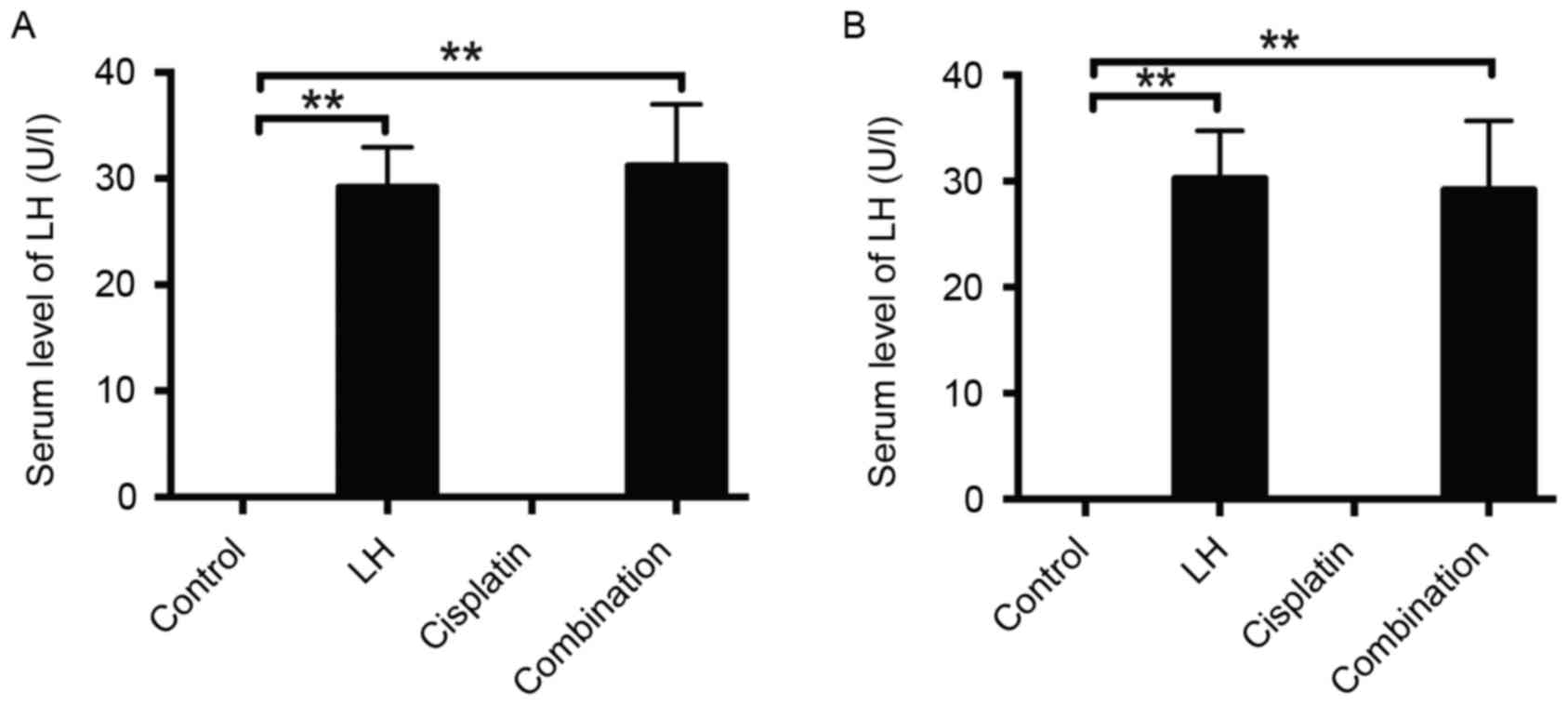
Serum levels of LH in the xenografted
nude mice
In order to confirm the validity of the LH
injection, the serum levels of LH were measured. As expected, LH
was undetectable in the sera of control and cisplatin-treated mice,
whereas the concentration of LH in the mouse sera of the groups
treated with LH alone or LH combined with cisplatin was ~30 U/l in
the HeyA8- and SKOV3ip1-implanted mice (Fig. 6).
Discussion
Ovarian cancer often occurs in postmenopausal women
and is characterized by high gonadotropin levels (~40 U/l).
Therefore, gonadotropins including FSH and LH have been regarded as
probable risk factors for the development of ovarian cancer. FSH
has been identified to serve a function in the development and
progression of ovarian cancer in vitro and in vivo
(21,25). However, previous studies have
identified that LH is able to inhibit apoptosis and facilitate
angiogenesis in vitro (26–28), and
therefore the effect of LH in vivo is worth
investigating.
In the present study, two epithelial ovarian cancer
cell lines, HeyA8 and SKOV3ip1, were implanted into nude mice and
the effect of exogenous LH was detected on the cisplatin anti-tumor
activity. ELISA was employed to verify the LH serum level ~30 U/l
in mice treated with LH, which is comparable to the LH level in
patients with post-menopausal ovarian cancer (29). Cisplatin is capable of inducing
apoptosis in ovarian cancer cells (30,31). In
the present study, cisplatin significantly inhibited the growth of
ovarian tumors, number of tumor nodules and volume of ascites. In
addition, it was revealed that treatment with LH alone exhibited a
minimal effect on tumor weights and the number of tumor nodules,
although it increased the volumes of ascites. The in vivo
anti-tumor effect of cisplatin was significantly impaired when
administered in combination with LH, which was determined by the
increase of growth of ovarian tumors, the number of tumor nodules
and the volume of ascites compared with the group that underwent
treatment with cisplatin alone.
It was observed that the number of apoptotic cells
with cleaved caspase-3-positive was significantly reduced following
combination treatment with LH and cisplatin, and that the number of
proliferative cells with ki67-positive was increased compared with
the group that received treatment with cisplatin alone. However,
the present study did not investigate the underlying antitumor
mechanisms responsible for the effect of LH on impairing cisplatin
in vivo.
Collectively, results of the present study indicate
that LH weakens the anti-tumor effect of cisplatin in vivo,
and LH may contribute to the development of drug resistance to
cisplatin in ovarian cancer. LH-antagonists may be used in the near
future to reverse the effect of LH and to aid in the treatment of
ovarian cancer (32).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stadel BV: Letter: The etiology and
prevention of ovarian cancer. Am J Obstet Gynecol. 123:772–774.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mertens-Walker I, Baxter RC and Marsh DJ:
Gonadotropin signalling in epithelial ovarian cancer. Cancer Lett.
324:152–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Venn A, Watson L, Bruinsma F, Giles G and
Healy D: Risk of cancer after use of fertility drugs with in-vitro
fertilisation. Lancet. 354:1586–1590. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brekelmans CT: Risk factors and risk
reduction of breast and ovarian cancer. Curr Opin Obstet Gynecol.
15:63–68. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Holschneider CH and Berek JS: Ovarian
cancer: Epidemiology, biology, and prognostic factors. Semin Surg
Oncol. 19:3–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tavani A, Ricci E, La Vecchia C, Surace M,
Benzi G, Parazzini F and Franceschi S: Influence of menstrual and
reproductive factors on ovarian cancer risk in women with and
without family history of breast or ovarian cancer. Int J
Epidemiol. 29:799–802. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Daly M and Obrams GI: Epidemiology and
risk assessment for ovarian cancer. Semin Oncol. 25:255–264.
1998.PubMed/NCBI
|
|
9
|
Gnagy S, Ming EE, Devesa SS, Hartge P and
Whittemore AS: Declining ovarian cancer rates in U.S. women in
relation to parity and oral contraceptive use. Epidemiology.
11:102–105. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng W, Lu JJ, Luo F, Zheng Y, Feng Yj,
Felix JC, Lauchlan SC and Pike MC: Ovarian epithelial tumor growth
promotion by follicle-stimulating hormone and inhibition of the
effect by luteinizing hormone. Gynecol Oncol. 76:80–88. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kurbacher CM, Jäger W, Kurbacher JA, Bittl
A, Wildt L and Lang N: Influence of human luteinizing hormone on
cell growth and CA 125 secretion of primary epithelial ovarian
carcinomas in vitro. Tumour Biol. 16:374–384. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuroda H, Mandai M, Konishi I, Yura Y,
Tsuruta Y, Hamid AA, Nanbu K, Matsushita K and Mori T: Human
chorionic gonadotropin (hCG) inhibits cisplatin-induced apoptosis
in ovarian cancer cells: Possible role of up-regulation of
insulin-like growth factor-1 by hCG. Int J Cancer. 76:571–578.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tashiro H, Miyazaki K, Okamura H, Iwai A
and Fukumoto M: c-myc over-expression in human primary ovarian
tumours: Its relevance to tumour progression. Int J Cancer.
50:828–833. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tourgeman DE, Lu JJ, Boostanfar R, Amezcua
C, Felix JC and Paulson RJ: Human chorionic gonadotropin suppresses
ovarian epithelial neoplastic cell proliferation in vitro. Fertil
Steril. 78:1096–1099. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi JH, Choi KC, Auersperg N and Leung
PC: Gonadotropins activate proteolysis and increase invasion
through protein kinase A and phosphatidylinositol 3-kinase pathways
in human epithelial ovarian cancer cells. Cancer Res. 66:3912–3920.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tilly JL, Tilly KI, Kenton ML and Johnson
AL: Expression of members of the bcl-2 gene family in the immature
rat ovary: Equine chorionic gonadotropin-mediated inhibition of
granulosa cell apoptosis is associated with decreased bax and
constitutive bcl-2 and bcl-xlong messenger ribonucleic acid levels.
Endocrinology. 136:232–241. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia L, Wen H, Han X, Tang J and Huang Y:
Luteinizing hormone inhibits cisplatin-induced apoptosis in human
epithelial ovarian cancer cells. Oncol Lett. 11:1943–1947. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu C, Shahzad MM, Moreno-Smith M, Lin YG,
Jennings NB, Alle JK, Landen CN, Mangala LS, Armaiz-Pena GN,
Schmandt R, et al: Targeting pericytes with a PDGF-B aptamer in
human ovarian carcinoma models. Cancer Biol Ther. 9:176–182. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guide for the Care and Use of Laboratory
Animals, . National Research Council (US) Committee for the update
of the guide for the care and use of laboratory animals. 8th.
Washington (DC): National Academies Press (US); 2011
|
|
21
|
Huang Y, Jin H, Liu Y, Zhou J, Ding J,
Cheng KW, Yu Y and Feng Y: FSH inhibits ovarian cancer cell
apoptosis by up-regulating survivin and down-regulating PDCD6 and
DR5. Endocr Relat Cancer. 18:13–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garofalo A, Naumova E, Manenti L, Ghilardi
C, Ghisleni G, Caniatti M, Colombo T, Cherrington JM, Scanziani E,
Nicoletti MI and Giavazzi R: The combination of the tyrosine kinase
receptor inhibitor SU6668 with paclitaxel affects ascites formation
and tumor spread in ovarian carcinoma xenografts growing
orthotopically. Clin Cancer Res. 9:3476–3485. 2003.PubMed/NCBI
|
|
23
|
Scholzen T and Gerdes J: The Ki-67
protein: From the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fisher DE: Apoptosis in cancer therapy:
Crossing the threshold. Cell. 78:539–542. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang Y, Hua K, Zhou X, Jin H, Chen X, Lu
X, Yu Y, Zha X and Feng Y: Activation of the PI3K/AKT pathway
mediates FSH-stimulated VEGF expression in ovarian serous
cystadenocarcinoma. Cell Res. 18:780–791. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Z, Liao H, Chen X, Zheng Y, Liu Y,
Tao X, Gu C, Dong L, Duan T, Yang Y, et al: Luteinizing hormone
upregulates survivin and inhibits apoptosis in ovarian epithelial
tumors. Eur J Obstet Gynecol Reprod Biol. 155:69–74. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xia L, Wen H, Han X, Tang J and Huang Y:
Luteinizing hormone inhibits cisplatin-induced apoptosis in human
epithelial ovarian cancer cells. Oncol Lett. 11:1943–1947. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liao H, Zhou Q, Gu Y, Duan T and Feng Y:
Luteinizing hormone facilitates angiogenesis in ovarian epithelial
tumor cells and metformin inhibits the effect through the mTOR
signaling pathway. Oncol Rep. 27:1873–1878. 2012.PubMed/NCBI
|
|
29
|
Rzepka-Górska I, Chudecka-Głaz A and
Kosmowska B: FSH and LH serum/tumor fluid ratios and malignant
tumors of the ovary. Endocr Relat Cancer. 11:315–321. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim JH, Jeong SJ, Kim B, Yun SM, Choi DY
and Kim SH: Melatonin synergistically enhances cisplatin-induced
apoptosis via the dephosphorylation of ERK/p90 ribosomal S6
kinase/heat shock protein 27 in SK-OV-3 cells. J Pineal Res.
52:244–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mabuchi S, Altomare DA, Cheung M, Zhang L,
Poulikakos PI, Hensley HH, Schilder RJ, Ozols RF and Testa JR:
RAD001 inhibits human ovarian cancer cell proliferation, enhances
cisplatin-induced apoptosis, and prolongs survival in an ovarian
cancer model. Clin Cancer Res. 13:4261–4270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tan O and Bukulmez O: Biochemistry,
molecular biology and cell biology of gonadotropin-releasing
hormone antagonists. Curr Opin Obstet Gynecol. 23:238–244. 2011.
View Article : Google Scholar : PubMed/NCBI
|