Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
sixth most common type of cancer worldwide, representing 90% of all
head and neck cancers (1–3). Despite improvements in therapeutic
interventions over the last 20 years, in 2010 the 5-year survival
rate was ~50% (4,5). According to the literature, from
1975–2010, the morbidity and mortality of patients with HNSCC
remained at a high level, with >650,000 novel HNSCC cases
diagnosed annually worldwide (6). To
date, a series of biomarkers associated with HNSCC including p16,
p53, epidermal growth factor receptor and vascular endothelial
growth factor have been identified (7), which have proven to be beneficial in
directing diagnosis, prognosis and therapy for this disease.
However, these are insufficient to accurately define the
pathogenesis of HNSCC. Therefore, there is an urgent requirement to
explore and identify novel molecular biomarkers that are associated
with HNSCC, as potential therapeutic targets.
Cytoplasmic polyadenylation element-binding protein
(CPEB) is a type of sequence-specific highly conserved RNA-binding
protein (8,9), which regulates the translational
activation and cytoplasmic polyadenylation of target mRNAs
(10). The CPEB family of proteins
all confer a similar structure, including highly variable N-termini
and relatively conservative C-termini, consisting of two RNA
recognition motifs and a zinc finger domain essential for RNA
binding (11–13). The family of CPEBs, which are widely
expressed in vertebrates, are composed of four family members
(CPEB1-CPEB4), of which CPEB1 differs from CPEB2-CPEB4 in terms of
binding specificity and regulatory domains (11,14,15).
Several previous studies have demonstrated that CPEBs are
associated with various biological processes, including cell cycle
progression (16), development
(17,18), cellular senescence (19) and malignant tumor progression
(20). The direct link between the
aberrant expression of CPEBs and tumorigenesis has been previously
observed in glioblastomas and pancreatic ductal adenocarcinomas
(PDA) (11).
CPEB4 belongs to the CPEB family, and functions to
directly mediate translation and polyadenylation (11). A previous study reported that CPEB4
was abundantly expressed in glioblastomas and PDA, and influenced
the acceleration of tumor proliferation, vascularization and
invasion (21). Several previous
studies researched the association between CPEB4 and various types
of cancer, and further validated the crucial involvement of CPEB4
in tumorigenesis. Taken together, these results highlighted the
probability that CPEB4-mediated abnormal regulation of downstream
target gene expression may be a common mechanism in malignant
tumors (21,22). To the best of our knowledge, the
biological functions and clinical significance of CPEB4 expression
in HNSCC has not been previously reported. The aim of the present
study was to investigate the potential function of CPEB4 in the
tumorigenesis of HNSCC, and identify the potential underlying
molecular mechanisms involved.
Materials and methods
Datasets retrieved
The Gene Expression Omnibus (GEO) database,
established and maintained by the National Center for Biotechnology
Information, is an international public functional genomics data
repository (23). A total of six
microarray datasets were downloaded from the GEO repository for
data analysis, including GSE33205 (24), GSE59102 (25), GSE58911 (26), GSE51985 (27), GSE39366 (28) and GSE25093 (29). The CPEB4 expression data series
originated from tumor tissues or adjacent non-neoplastic tissues of
human HNSCC, benign lesions of the head and neck, and normal
tissues from non-HNSCC. In the six datasets, the association
between CPEB4 expression and the occurrence of HNSCC was analyzed
independently.
Tissue microarray (TMA)
The TMA was obtained from US Biomax, Inc.
(Rockville, MD, USA; cat no. HN803b). The TMA was composed of 7
nasal carcinoma, 31 laryngeal carcinoma, 32 tongue carcinoma and 10
normal tongue tissues, from subjects ranging between 18 and 90
years of age, with a median age of 53.4 years. The samples were
obtained from 18 women and 62 men. Detailed information of the TMA
is summarized in Table I. The present
study was approved by the appropriate Ethical Committees of Renmin
Hospital of Wuhan University.
 | Table I.Characteristics of the head and neck
squamous cell carcinoma tissue microarray. |
Table I.
Characteristics of the head and neck
squamous cell carcinoma tissue microarray.
| Patient
characteristic | n (%) |
|---|
| Localization |
|
|
Tongue | 42 (52.5) |
|
Larynx | 31 (38.6) |
|
Nose | 7 (8.9) |
| T-stage |
|
| T1 | 5 (8.3) |
| T2 | 30 (50.0) |
| T3 | 17 (28.3) |
| T4 | 8 (13.3) |
| N-stage |
|
| N0 | 42 (68.9) |
| N1 | 16 (26.2) |
| N2 | 3 (5.0) |
| Grade |
|
| I | 12 (16.7) |
| II | 36 (50.0) |
|
III | 24 (33.3) |
| NAT | 2 (2.5) |
| Normal | 9 (11.2) |
Immunohistochemical (IHC)
staining
IHC analysis of CPEB4 protein expression in the TMA
was performed, using the two-step staining method. TMA slides were
dried for 2 h at 60°C, dewaxed in pure xylene at room temperature
three times for 15 min, and rehydrated through a graded series of
alcohols. Antigen retrieval was performed on TMA slides, which were
incubated in sodium citrate buffer (pH 6.0) at 100°C for 15 min in
the pressure cooker. Slides were immersed in 0.3% hydrogen peroxide
at room temperature for 10 min to block endogenous peroxidase
activity. Non-specific binding was blocked with normal goat serum
(50 µl) (cat. no. KIT-9706; Fuzhou Maixin Biotech, Co., Ltd.,
Fuzhou, China) at room temperature for 10 min. Subsequently, the
slides were incubated with primary antibodies against CPEB4 (1:200;
cat. no. HPA038394; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
overnight at 4°C. TMA slides were rinsed twice in PBS at room
temperature for 5 min each time, prior to incubation with the
secondary antibody (poly-horseradish peroxidase-conjugated goat
anti-rabbit IgG; cat. no. KIT-9706; 1:200, Maixin Biotech, Co.,
Ltd, Fuzhou, China) for 30 min at room temperature. TMA sections
were stained with 3,3-diaminobenzidine at room temperature for 5
min to detect the antigen, and the cell nucleus was counterstained
with Mayer's hematoxylin at room temperature for 3 min. Slides were
dehydrated with an ascending series of alcohols, prior to
mounting.
Assessment of IHC staining
The majority of the CPEB4 protein is located in the
cytoplasm, but enters the cell nucleus in response to the lack of
oxygen and glucose (30). The TMA
slides were scored by two independent investigators using a
two-index scoring system, which considers the staining intensity
and proportion of tumor cells stained. A 4-point intensity scoring
system was graded as follows: Grade 0, negative staining; grade 1,
weak staining, light yellow; grade 2, moderate staining,
yellow-brown; and grade 3, strong staining, brown. According to the
percentage of positive cancer cells, the proportion score was
divided into four levels: 0–25% positive tumor cells, 26–50%
positive tumor cells, 51–75% positive tumor cells and 76–100%
positive tumor cells. Finally, the total scores from each stained
area were calculated as a composite expression score (CES; range
0–12) for further statistical analysis, using the following
formula; CES=intensity × proportion. The CES for tumors was defined
as negative (score=0), weak positive (score=1–4), positive
(score=5–8) and strong positive (score=9–12).
Statistical analysis
Statistical evaluation was performed using SPSS
(version 16.0; SPSS, Inc., Chicago, IL, USA). All gene expression
data downloaded from GEO were inputted into Microsoft Excel.
Analysis of variance was used to compare differences among the
groups, with a Tukey's multiple comparison test performed following
ANOVA. An unpaired Student's t-test was used for comparisons
between two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
CPEB4 gene expression in HNSCC and
normal tissues
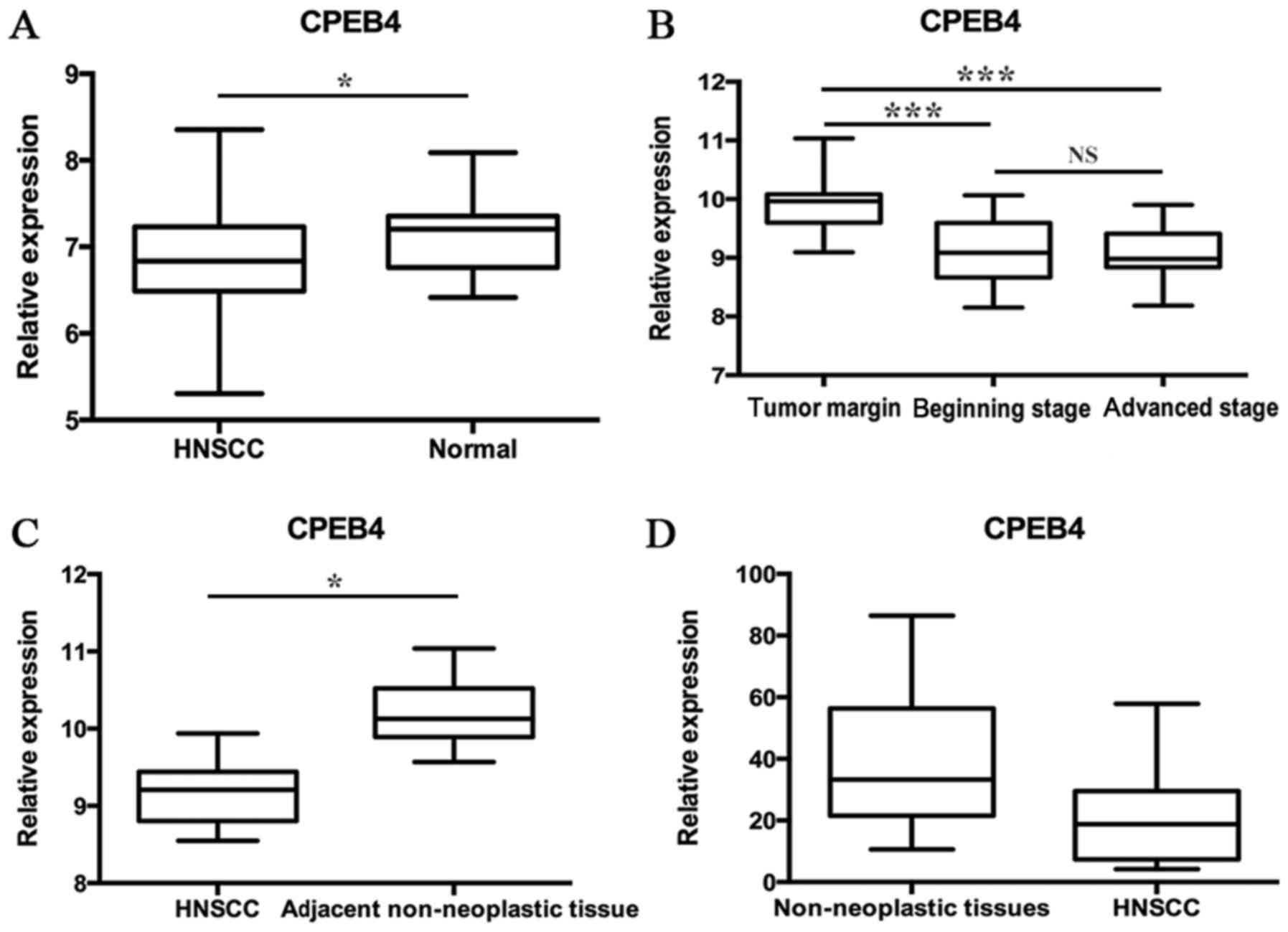
The GSE33205 data consisted of 44 HNSCC tumor
samples and 25 normal mucosal samples, of which the normal samples
were taken from uvulopalatopharyngoplasty. The statistical analyses
were made between tumors and normal tissues (P<0.05; Fig. 1A). The GSE59102 data included 29 tumor
specimens and 13 margin samples, which were derived from patients
suffering surgical procedures of laryngeal squamous cell carcinoma
(LSCC). Results indicated that the CPEB4 gene expression
level was lower in early- and advanced-stage LSCC tumor tissues
compared with the tumor margin (P<0.0001), whereas no
significant difference between early- and advanced-stage LSCC tumor
tissues was identified (Fig. 1B). The
GSE58911 data consisted of 15 paired normal and tumor samples
obtained from patients who were diagnosed with HNSCC (oropharynx,
hypopharynx and larynx). Prior to therapy, the samples taken from a
site at a distance from the tumor tissues and from the tumor site
were used for normal samples and tumor samples, respectively. The
statistical analyses were made between normal and tumor samples
(P<0.01; Fig. 1C). The GSE51985
data originated from 10 patients undergoing surgery for LSCC. The
cancer tissues were compared with corresponding adjacent
non-neoplastic tissues. The statistical analyses were made between
cancer tissues and the corresponding adjacent non-neoplastic
tissues (P=0.0577; Fig. 1D). These
data indicated that, in the majority of cases, CPEB4 expression was
significantly downregulated in HNSCC samples compared with normal
corresponding tissue samples.
Associations between CPEB4 gene
expression and pathological grading
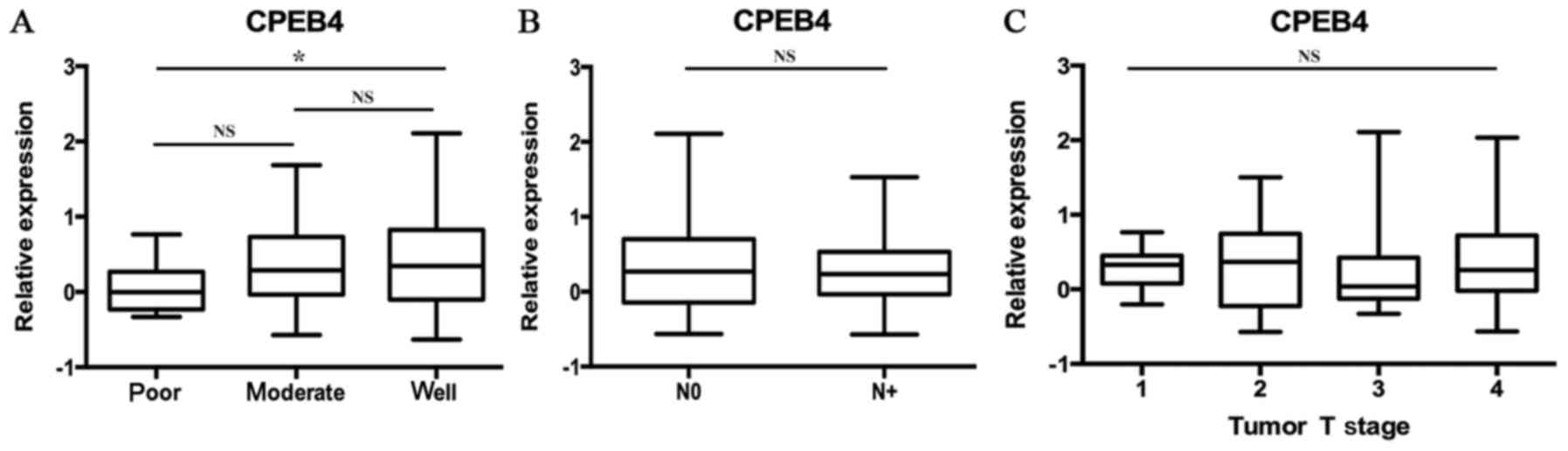
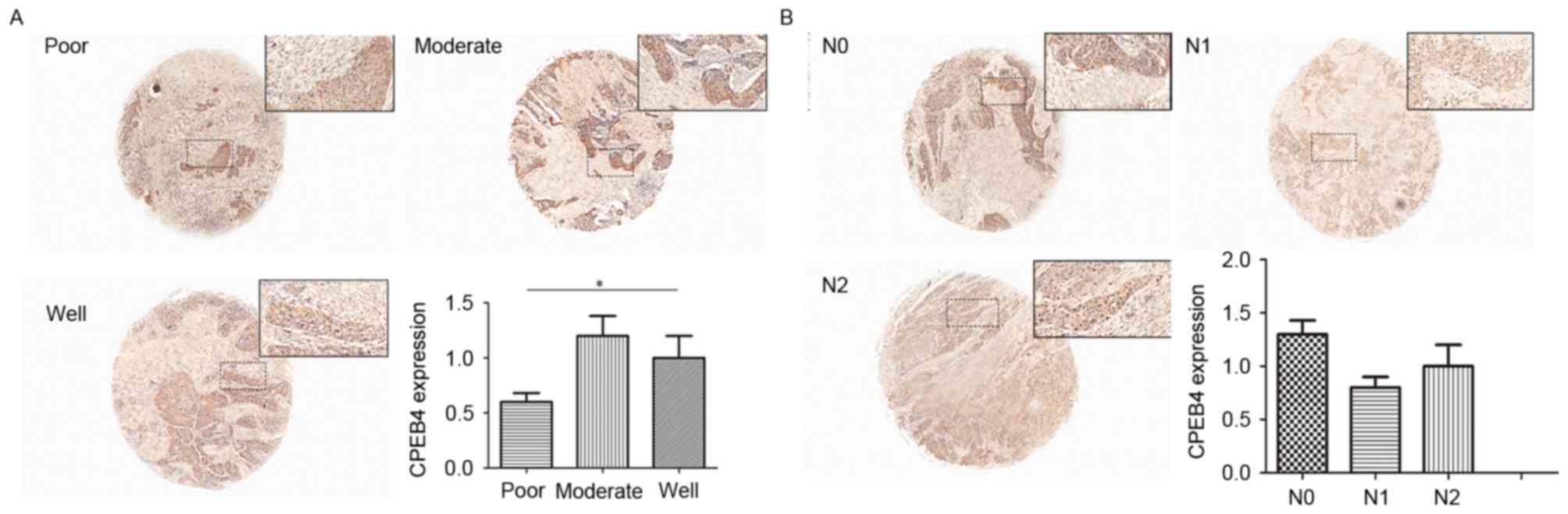
The data of GSE39366 included a total of 138 HNSCC
specimens. According to tumor differentiation, these samples were
divided into three grades: Poorly, moderately and
well-differentiated. The statistical analyses were made between
these three grades (P<0.05; Fig.
2A). The results indicated that decreased CPEB4 expression was
associated with increasing pathological grading. Statistically
significant differences were observed for CPEB4 expression between
poor and well tumor grades (P<0.05). The CPEB4 gene
expression level was lower in poorly differentiated HNSCC tissues
compared with well-differentiated HNSCC tissues (P<0.05),
whereas no significant difference in CPEB4 expression between
distinct tumor grades was identified.
Associations between CPEB4 gene
expression and N-stage
Statistical analyses of the expression of the
CPEB4 gene were made between different N-stages of HNSCC,
from the data originating from the dataset GSE39366 (P>0.05;
Fig. 2B). No statistically
significant associations were identified between CPEB4 gene
expression and N-stages of HNSCC.
Associations between CPEB4 gene
expression and T-stage
Statistical analyses of CPEB4 gene expression
were made between different T-stages of HNSCC, using the data
obtained from the dataset GSE39366 (P>0.05; Fig. 2C). No statistically significant
associations were identified between CPEB4 gene expression
and T-stages of HNSCC.
Analysis of CPEB4 gene methylation in
HNSCC and normal tissues
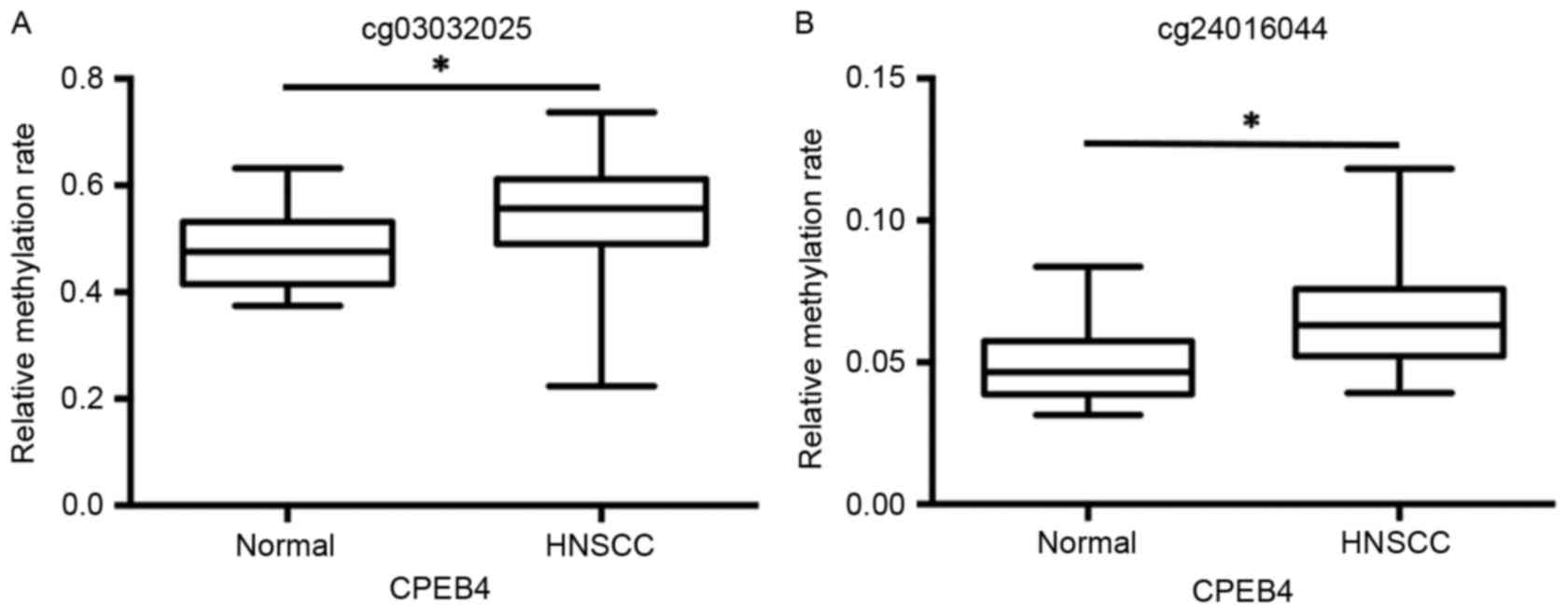
The data from the GSE25093 dataset was collected
from 91 fresh-frozen HNSCC tumor tissues and 18 fresh-frozen normal
samples drawn from the larynx, pharynx and oral cavity. The data
from the cg03032025 and cg24016044 datasets revealed that
CPEB4 methylation was significantly increased in HNSCC tumor
samples and compared with normal tissue samples (P<0.01;
Fig. 3A and B).
CPEB4 protein expression in the HNSCC
TMA
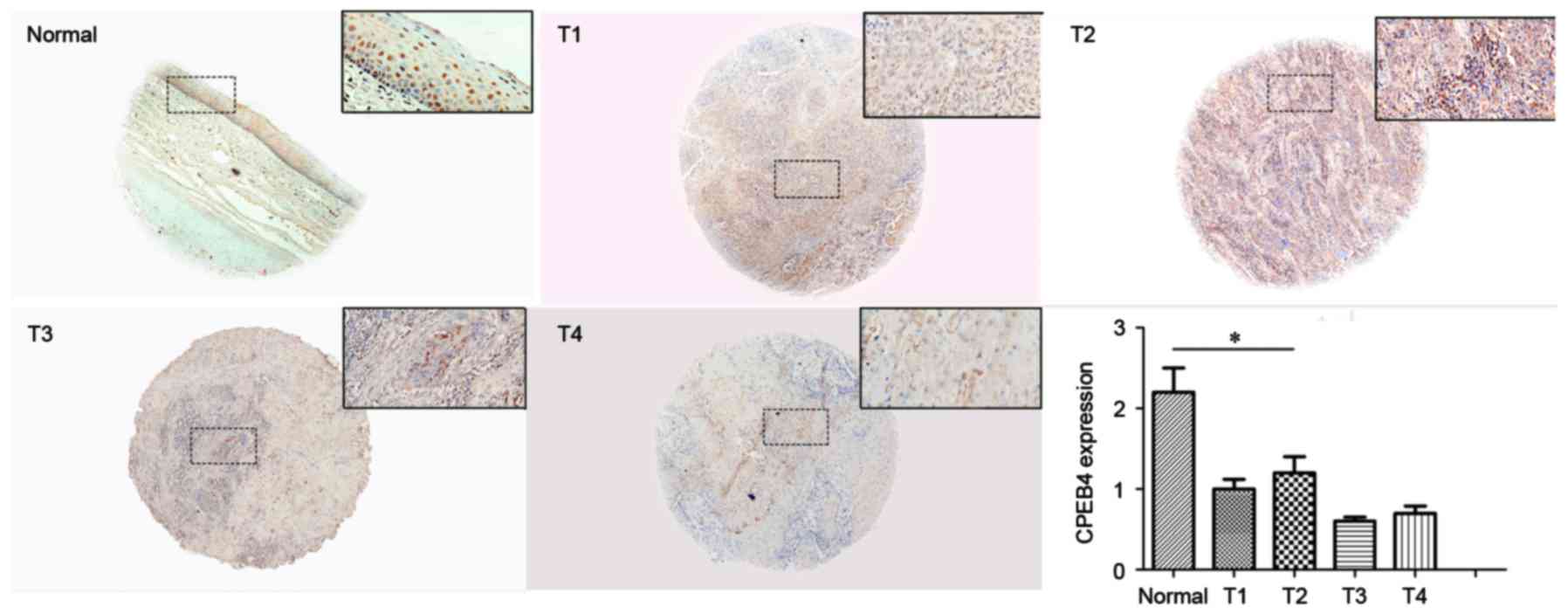
To further verify CPEB4 expression in HNSCC, IHC
staining was performed to examine CPEB4 protein in the TMA. Each
TMA section was analyzed by two individual index parameters, i.e.,
proportion and intensity, which were transformed into a CES for
assessment. CPEB4 protein expression in HNSCC tissue and normal
tissue is presented in Fig. 4. The
immunostaining indicated that CPEB4 protein was significantly
decreased in tumor tissues compared with normal tissues
(P<0.05). This provides further evidence that CPEB4 was
frequently downregulated in HNSCC, which is comparable with the
outcome of CPEB4 gene expression, obtained from the GEO
dataset.
Differences in CPEB4 protein between
different tumor grades in the TMA
The range of tumor grades (poor to well) represents
increasing deterioration of histological differentiation, ranging
from well differentiated to poorly differentiated. CES scores were
calculated for each tumor grade for statistical analysis. The
results from the present study demonstrated that CES for poorly
differentiated tumors was significantly decreased compared with
that of moderately differentiated tumors (P<0.05; Fig. 5A). Furthermore, moderately
differentiated tumors had a decreased CES score compared with that
of the well differentiated tumors (P<0.05; Fig. 5A). These data demonstrated that a
significant decrease in expression of CPEB4 protein was associated
with the poorest tumor differentiation.
Differences in CPEB4 protein
expression between different T-stages in TMA
CES scores for each T-stage were calculated for
statistical analysis. No significant differences for CPEB4 protein
were observed between different T-stages (P>0.05; Fig. 4), which demonstrated that no
significant associations between CPEB4 and T-stage of HNSCC were
identified. The outcome was comparable with that for the analysis
from the GEO dataset.
Differences in CPEB4 protein between
different N-stages in TMA
The same method as that for T-stage was used for
N-stage. However, there was no significant difference in CPEB4
protein expression between N-stages (P>0.05; Fig. 5B), indicating that CPEB4 was not
significantly associated with the N-stage of HNSCC. This result was
consistent with the conclusion from the GEO dataset.
Discussion
Ortiz-Zapater et al (21) initially demonstrated the direct link
between CPEB4 expression and cancer etiology, and suggested that
overexpression may be a common mechanism for regulating the
reprogramming of gene expression involved in cancer progression.
Subsequently, a number of studies have been performed that
demonstrated that the aberrant expression of CPEB4 is significantly
associated with the clinical prognosis of patients with malignant
tumors (22,31–33).
In the present study, CPEB4 expression was
investigated between various types of cancer and corresponding
normal tissues. Notably in previous studies, the expression of
CPEB4 protein was increased in several malignant tumors, including
metastatic prostate cancer (34),
colorectal cancer (CRC) (31),
astrocytic tumors (32), invasive
ductal breast carcinoma (IDC) (22)
and glioma (33). In contrast,
another study reported the opposite outcome in that CPEB4 protein
was downregulated in hepatocellular carcinoma and non-small cell
lung cancer (35). However, to date,
there is limited knowledge concerning the association between CPEB4
abnormalities and HNSCC. In order to investigate CPEB4 gene
expression levels in HNSCC, four independent GEO datasets,
including GSE33205, GSE59102, GSE58911 and GSE51985, were analyzed.
The analysis of CPEB4 gene expression between cancer and
normal samples identified statistical differences from three
datasets, in which P-values were confirmed to be <0.05. The
results of the present study confirmed that CPEB4 expression was
downregulated in the majority of cases of HNSCC compared with
normal tissues, based on the results obtained from GEO
datasets.
In addition, an HNSCC TMA was used to examine CPEB4
protein expression to further verify the gene expression results
from GEO datasets. The results from the present study confirmed
that CPEB4 protein expression was significantly decreased in cancer
tissues compared with normal corresponding tissues, consistent with
the outcomes from the GEO datasets. Taken together, the results of
the present study confirmed that CPEB4 was consistently
downregulated in HNSCC tissues, which may be associated with the
progression of tumorigenesis. Therefore, CPEB4 may function
as a tumor suppressor gene in HNSCC.
According to previous studies, there has been
controversy surrounding CPEB4 expression levels in various
malignant tumors (22,31,35,36).
Therefore, we speculate that the discrepancies between the results
of the present study and those of previous studies may be due to
the different pathological patterns of cancers. For example, CPEB4
expression was upregulated in adenoma but downregulated in solid
tumors. In different cancer cells, CPEB4 binds to various
downstream genes and regulates the reprogramming of these, acting
either as a cancer suppressor gene or an oncogene and affecting
tumor development in several ways (22,31,32,34).
Several previous studies have been performed to
investigate the associations between CPEB4 and clinicopathological
parameters, including age, sex, T-stage and N-stage (22,31,32). The
positive association between CPEB4 expression and T-stage (tumor
size) or N-stage (lymph node status) of cancer was demonstrated in
astrocytic tumors, IDC and CRC (22,31,32).
However, the statistical analysis from the GSE39366 dataset
revealed that CPEB4 expression was not significantly associated
with T-stages or N-stages in HNSCC. Furthermore, the data from the
TMA which explored the association between CPEB4 protein expression
and T/N-stages, revealed an outcome comparable with that from the
GEO dataset. Therefore, it was concluded that there was no
association between CPEB4 and T/N-stages in HNSCC. The inconsistent
conclusion between the results from the present study and those of
previous studies may be caused by several factors, including tumor
microenvironment, location, histological origin and histological
type.
The association between CPEB4 and histological
grading has been identified in several previous studies, indicating
that CPEB4 expression was positively associated with differing
tumor grades (22,32,33). In
the present study, the analysis of the GEO datasets suggested that
downregulated CPEB4 expression further decreased in association
with increasing histological grades of HNSCC. The differences in
CPEB4 protein between different tumor grades were statistically
significant. In addition, it was observed that CPEB4 protein levels
were lower in high-grade HNSCC compared with that in low-grade
HNSCC in TMA. Furthermore, the significant differences between
different pathological grades supported our previous conclusions
from the GEO dataset. In summary, it was confirmed that CPEB4
expression was negatively correlated with pathological grading, and
CPEB4 may represent a valuable marker for HNSCC prognosis.
Currently, the molecular mechanisms underlying the
contribution of CPEB4 to cancer progression have not been
elucidated. According to previous studies, CPEB4 targets specific
genes that are associated with tumorigenesis. For example, CPEB4 us
able to regulate the activation of genes including Ras-associated
molecules, cyclins, cell signaling components, apoptosis-related
molecules, chromatin remodeling proteins, metabolic enzymes, and
genes involved in migration and metastasis, indicating a
significant effect on tumor development in several capacities
(21,37).
There are three suggested mechanisms responsible for
CPEB4-mediated malignant formation in cancer. CPEB4 may be
critically involved in regulating several downstream signaling
pathways relevant to proliferation, apoptosis and the cell cycle
(31,34). Zhong et al (31) reported that knockdown of CPEB4 in CRC
cells may contribute to the downregulation of B-cell lymphoma (Bcl)
extra-large expression and upregulation of Bcl-2-associated X
protein, apoptosis regulation, resulting in the promotion of cell
apoptosis and the inhibition of cell proliferation. Xu and Liu
(34) demonstrated that CPEB4
accelerated the development of metastasis and invasion through the
transforming growth factor-β signaling pathway. In addition, the
expression of vital genes involved in tumorigenesis were modulated
by CPEB4. In pancreatic carcinoma, enhanced CPEB4 expression
accelerated the progression of tumor malignancy by increasing the
expression level of tissue plasminogen activator mRNA which is an
essential component for PDA (21).
Furthermore, CPEB4-induced translational control of vimentin may be
responsible for the development of astrocytic tumors (32). Finally, CPEB4 may act as a downstream
target gene of other oncogenes or cancer suppressor genes, and be
involved in promoting invasion and metastasis of tumor cells.
MicroRNA-203, a tumor suppressor, directly targets CPEB4 and
negatively regulates CPEB4 expression to influence the apoptosis
signaling pathway in CRC (31). In
addition, microRNA-550a binds to the 3′-untranslated region of the
CPEB4 gene to negatively regulate CPEB4 expression, leading to
accelerated migration and invasion of liver cancer cells (35). Furthermore, the downregulation of
CPEB4 expression was induced by microRNA-1246 to facilitate
migration and invasion of tumor cells in non-small cell lung
carcinoma (36). It may be speculated
that microRNA-mediated CPEB4 regulation frequently exists in tumor
progression.
However, to the best of our knowledge, the present
study is the first to report on the potential action of CPEB4 in
the tumor progression of HNSCC. The results of the present study
implicated an important function for CPEB4 in HNSCC tumorigenesis,
and further investigation is urgently required. In the present
study, the evidence from the GEO dataset analysis demonstrated that
the methylation status of the CPEB4 gene was significantly
higher in HNSCC compared with normal samples. This is indicative of
an association between the hypermethylation of CPEB4 and the
downregulation of CPEB4 in HNSCC. Thus, is it hypothesized
that CPEB4 hypermethylation may be involved in the
tumorigenesis of HNSCC by downregulating CPEB4 gene
expression. This result warrants further investigation in a
follow-up study to confirm the underlying mechanisms involved.
In conclusion, CPEB4 gene expression was
significantly downregulated in HNSCC, which indicates that
CPEB4 may act as a tumor suppressor gene in HNSCC.
Considering that CPEB4 gene expression was associated with
pathological grading, this indicates the potential value of CPEB4
in directing prognosis for HNSCC. Hypermethylation of the
CPEB4 gene may be responsible for the downregulation of
CPEB4 gene expression in HNSCC, and results in cancer
tumorigenesis. However, further consideration must be given to the
potential of CPEB4 as a therapeutic target for patients with
HNSCC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81372880) and the
Natural Science Foundation of Hubei Province (grant no.
2012FFA045).
References
|
1
|
Howren MB, Christensen AJ, Karnell LH and
Funk GF: Psychological factors associated with head and neck cancer
treatment and survivorship: Evidence and opportunities for
behavioral medicine. J Consult Clin Psychol. 81:299–317. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khan Z, Khan AA, Prasad GB, Khan N, Tiwari
RP and Bisen PS: Growth inhibition and chemo-radiosensitization of
head and neck squamous cell carcinoma (HNSCC) by survivin-siRNA
lentivirus. Radiother Oncol. 118:359–368. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Döbrossy L: Epidemiology of head and neck
cancer: Magnitude of the problem. Cancer Metastasis Rev. 24:9–17.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boeckx C, Op de Beeck K, Wouters A,
Deschoolmeester V, Limame R, Zwaenepoel K, Specenier P, Pauwels P,
Vermorken JB, Peeters M, et al: Overcoming cetuximab resistance in
HNSCC: The role of AURKB and DUSP proteins. Cancer Lett.
354:365–377. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Molinolo AA, Amornphimoltham P, Squarize
CH, Castilho RM, Patel V and Gutkind JS: Dysregulated molecular
networks in head and neck carcinogenesis. Oral Oncol. 45:324–334.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kulasinghe A, Perry C, Jovanovic L, Nelson
C and Punyadeera C: Circulating tumour cells in metastatic head and
neck cancers. Int J Cancer. 136:2515–2523. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thomas GR, Nadiminti H and Regalado J:
Molecular predictors of clinical outcome in patients with head and
neck squamous cell carcinoma. Int J Exp Pathol. 86:347–363. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burns DM, D'Ambrogio A, Nottrott S and
Richter JD: CPEB and two poly(A) polymerases control miR-122
stability and p53 mRNA translation. Nature. 473:105–108. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mendez R and Richter JD: Translational
control by CPEB: A means to the end. Nat Rev Mol Cell Biol.
2:521–529. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Radford HE, Meijer HA and de Moor CH:
Translational control by cytoplasmic polyadenylation in Xenopus
oocytes. Biochim Biophys Acta. 1779:217–229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fernández-Miranda G and Méndez R: The
CPEB-family of proteins, translational control in senescence and
cancer. Ageing Res Rev. 11:460–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hake LE, Mendez R and Richter JD:
Specificity of RNA binding by CPEB: Requirement for RNA recognition
motifs and a novel zinc finger. Mol Cell Biol. 18:685–693. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hake LE and Richter JD: CPEB is a
specificity factor that mediates cytoplasmic polyadenylation during
xenopus oocyte maturation. Cell. 79:617–627. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Theis M, Si K and Kandel ER: Two
previously undescribed members of the mouse CPEB family of genes
and their inducible expression in the principal cell layers of the
hippocampus. Proc Natl Acad Sci USA. 100:pp. 9602–9607. 2003;
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang YS, Kan MC, Lin CL and Richter JD:
CPEB3 and CPEB4 in neurons: Analysis of RNA-binding specificity and
translational control of AMPA receptor GluR2 mRNA. EMBO J.
25:4865–4876. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tay J and Richter JD: Germ cell
differentiation and synaptonemal complex formation are disrupted in
CPEB knockout mice. Dev Cell. 1:201–213. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hafer N, Xu S, Bhat KM and Schedl P: The
drosophila CPEB protein Orb2 has a novel expression pattern and is
important for asymmetric cell division and nervous system function.
Genetics. 189:907–921. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang XP and Cooper NG: Characterization of
the transcripts and protein isoforms for cytoplasmic
polyadenylation element binding protein-3 (CPEB3) in the mouse
retina. BMC Mol Biol. 10:1092009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Groisman I, Ivshina M, Marin V, Kennedy
NJ, Davis RJ and Richter JD: Control of cellular senescence by
CPEB. Genes Dev. 20:2701–2712. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hansen CN, Ketabi Z, Rosenstierne MW,
Palle C, Boesen HC and Norrild B: Expression of CPEB, GAPDH and
U6snRNA in cervical and ovarian tissue during cancer development.
APMIS. 117:53–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ortiz-Zapater E, Pineda D, Martínez-Bosch
N, Fernández-Miranda G, Iglesias M, Alameda F, Moreno M, Eliscovich
C, Eyras E, Real FX, et al: Key contribution of CPEB4-mediated
translational control to cancer progression. Nat Med. 18:83–90.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun HT, Wen X, Han T, Liu ZH, Li SB, Wang
JG and Liu XP: Expression of CPEB4 in invasive ductal breast
carcinoma and its prognostic significance. Onco Targets Ther.
8:3499–3506. 2015.PubMed/NCBI
|
|
23
|
Barrett T, Wilhite SE, Ledoux P,
Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH,
Sherman PM, Holko M, et al: NCBI GEO: Archive for functional
genomics data sets-update. Nucleic Acids Res. 41(Database Issue):
D991–D995. 2013.PubMed/NCBI
|
|
24
|
Sun W, Gaykalova DA, Ochs MF, Mambo E,
Arnaoutakis D, Liu Y, Loyo M, Agrawal N, Howard J, Li R, et al:
Activation of the NOTCH pathway in head and neck cancer. Cancer
Res. 74:1091–1104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Barros E Lima, Bueno R, Ramão A,
Pinheiro DG, Alves CP, Kannen V, Jungbluth AA, de Araújo LF, Muys
BR, Fonseca AS, Plaça JR, et al: HOX genes: Potential candidates
for the progression of laryngeal squamous cell carcinoma. Tumour
Biol. 37:15087–15096. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lobert S, Graichen ME, Hamilton RD, Pitman
KT, Garrett MR, Hicks C and Koganti T: Prognostic biomarkers for
HNSCC using quantitative real-time PCR and microarray analysis:
β-tubulin isotypes and the p53 interactome. Cytoskeleton (Hoboken).
71:628–637. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Regla-Nava JA, Nieto-Torres JL,
Jimenez-Guardeño JM, Fernandez-Delgado R, Fett C, Castaño-Rodríguez
C, Perlman S, Enjuanes L and DeDiego ML: Severe acute respiratory
syndrome coronaviruses with mutations in the E protein are
attenuated and promising vaccine candidates. J Virol. 89:3870–3887.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Walter V, Yin X, Wilkerson MD, Cabanski
CR, Zhao N, Du Y, Ang MK, Hayward MC, Salazar AH, Hoadley KA, et
al: Molecular subtypes in head and neck cancer exhibit distinct
patterns of chromosomal gain and loss of canonical cancer genes.
PLoS One. 8:e568232013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Poage GM, Houseman EA, Christensen BC,
Butler RA, Avissar-Whiting M, McClean MD, Waterboer T, Pawlita M,
Marsit CJ and Kelsey KT: Global hypomethylation identifies Loci
targeted for hypermethylation in head and neck cancer. Clin Cancer
Res. 17:3579–3589. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kan MC, Oruganty-Das A, Cooper-Morgan A,
Jin G, Swanger SA, Bassell GJ, Florman H, van Leyen K and Richter
JD: CPEB4 is a cell survival protein retained in the nucleus upon
ischemia or endoplasmic reticulum calcium depletion. Mol Cell Biol.
30:5658–5671. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhong X, Xiao Y, Chen C, Wei X, Hu C, Ling
X and Liu X: MicroRNA-203-mediated posttranscriptional deregulation
of CPEB4 contributes to colorectal cancer progression. Biochem
Biophys Res Commun. 466:206–213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen W, Hu Z, Li XZ, Li JL, Xu XK, Li HG,
Liu Y, Liu BH, Jia WH and Li FC: CPEB4 interacts with Vimentin and
involves in progressive features and poor prognosis of patients
with astrocytic tumors. Tumour Biol. 37:5075–5087. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu W, Yang Y, Xi S, Sai K, Su D, Zhang X,
Lin S and Zeng J: Expression of CPEB4 in human glioma and its
correlations with prognosis. Medicine (Baltimore). 94:e9792015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu H and Liu B: CPEB4 is a candidate
biomarker for defining metastatic cancers and directing
personalized therapies. Med Hypotheses. 81:875–877. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tian Q, Liang L, Ding J, Zha R, Shi H,
Wang Q, Huang S, Guo W, Ge C, Chen T, et al: MicroRNA-550a acts as
a pro-metastatic gene and directly targets cytoplasmic
polyadenylation element-binding protein 4 in hepatocellular
carcinoma. PLoS One. 7:e489582012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang W, Li H and Luo R: The microRNA-1246
promotes metastasis in non-small cell lung cancer by targeting
cytoplasmic polyadenylation element-binding protein 4. Diagn
Pathol. 10:1272015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
D'Ambrogio A, Nagaoka K and Richter JD:
Translational control of cell growth and malignancy by the CPEBs.
Nat Rev Cancer. 13:283–290. 2013. View Article : Google Scholar : PubMed/NCBI
|