Introduction
Esophageal squamous cell carcinoma (ESCC) has poor
prognosis in comparison with other gastrointestinal cancer.
Especially, prognosis of advanced or recurrent ESCC is extremely
poor (1) and main standard treatment
of it is chemotherapy. However, chemotherapy for esophageal cancer
is not extremely effective and safe at the present time. Clinical
useful biomarker of ESCC will be detected on the molecular
biological experiments and then development of more effective and
safe therapies are expected.
The Wnt signaling pathway is pivotal in
embryogenesis and development and aberrant activation of canonical
Wnt signaling is also widely implicated in a number of human
disease such as cancer (2,3). β-catenin, which is a central molecule in
the canonical Wnt pathway, translocates from a free cytosolic form
to the nucleus and then plays a role as a cofactor with T-cell
factor/lymphoid enhancer factor in order to trigger gene
transcription (4–6). Recently it was suggested that abnormal
expression of some key components of canonical Wnt signaling
pathway, such as β-catenin, GSK-3β, Axin, APC and some ligands of
this pathway, was associated with the development and progression
of gastroenterological cancer such as colon cancer (7,8),
hepatocellular carcinoma (9,10) and so on. There is also a study which
suggested that Wnt2 which was secreted by tumor fibroblast and
activated the canonical Wnt signaling pathway leaded to poor
prognosis of ESCC (11). Wnt3a is one
of typical ligands of the canonical Wnt signaling pathway. It has
been regarded as an activator of the canonical Wnt signaling
pathway including nuclear accumulation of β-catenin. Several
studies suggested that high expression of Wnt3a in many kinds of
tumors was correlated with a worse clinical outcome (12,13).
Moreover, it was reported that Wnt3a regulated self-renewal of
prostate cancer cells with stem cell characteristics (14). However, the clinical significance of
Wnt3a expression in ESCC is not clear. We thought that canonical
Wnt signaling, including Wnt3a, might be a key component of cancer
progression or chemoresistance, and we hypothesized that Wnt3a
expression might be a prognostic factor of ESCC because of its
roles in chemoresistance and tumor progression.
The aim of this study is to investigate the
relationship between Wnt3a expression in ESCC tumor and prognosis
in patients with ESCC.
Materials and methods
Patient selection
Wnt3a expression was evaluated in resected specimens
from 139 patients with pathological stages I–III thoracic ESCC who
underwent thoracic esophagectomy with three field lymphadenectomy
without neoadjuvant therapy in Tokai University Hospital from 2007
to 2009. Staging was defined as following UICC TNM classification
7th edition. Adjuvant chemotherapy was performed for patients with
pathological stage III ESCC in principle, however elder patients,
patients with renal dysfunction or patients who rejected it did not
perform it. Adjuvant chemotherapy consisted of a combination
5-fluorouracil (5-FU) and cisplatin, and 800 mg/m2 5-FU
was given by continuous intravenous infusion on day 1–5 and 80
mg/m2 cisplatin by intravenous infusion. This regimen
consisted of two cycles of chemotherapy separated by a 3-week
interval. The important clinicopathological information of each
patient was collected from the medical records. The present study
was approved by the Ethics Committee of Tokai University, School of
Medicine (Kanagawa, Japan). The need for written informed consent
was waived due to the retrospective, non-interventional nature of
the present study.
Immnohistochemical staining
Resected specimens were fixed in 10% formaldehyde at
4°C for 10 h. Paraffin block sections of ESCC were deparaffinised.
For antigen retrieval, the sections were boiled at 120°C in citrate
buffer (pH 6.0) for 5 min and peroxidase was quenched with methanol
and 3% H2O2 at room temperature for 10 min.
These were blocked with 5% normal goat serum for 10 min and
incubated with mouse anti-Wnt3a monoclonal antibody (dilution 1:50;
Abcam, Cambridge, MA, USA) overnight at 4°C. Additionally, these
sections were incubated with peroxidase-labeled anti-rabbit
antibody (Histofine Simple Stain Max PO; Nichirei, Tokyo, Japan)
for 30 min at room temperature. For the negative control reaction,
phosphate-buffered saline was used instead of the primary
antibody.
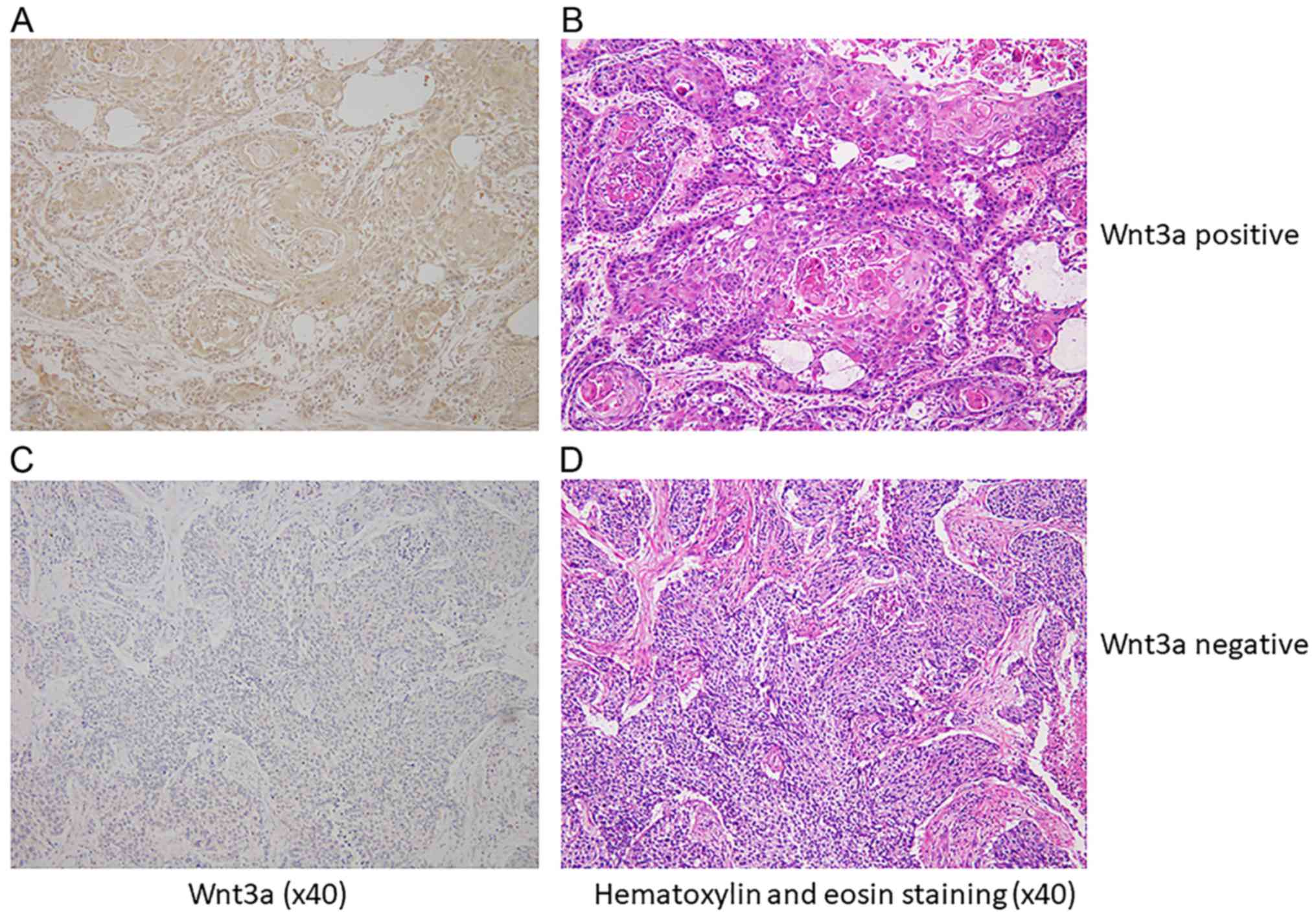
Positive expression of Wnt3a was detected when brown
granules were identified in the cytoplasm and more than 10% of the
cancer cells in each sections were immunoreactive to Wnt3a
(15). Non-cancerous tissues in the
resected specimens were used as a negative control. The results of
immnohistochemical staining were assigned by two independent
investigators without knowledge of clinicopathological data. One
hundred thirty nine patients with ESCC were divided to two groups
according to expression of Wnt3a in tumor tissue. Two groups were
compared about clinicopathological findings and prognosis, and we
investigated the influence of Wnt3a expression on ESCC.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 24; SPSS Inc., Chicago, IL, USA). The difference
of clinicopathological findings between Wnt3a-positive and
Wnt3a-negative groups was analyzed by Chi-square test and Student's
t-test. Cox's proportional hazard regression model was used to
analyze the independent prognostic factors with univariate and
multivariate analysis. Survival rates was calculated using
Kaplan-Meier method and these of two groups were compared by the
log rank test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Immnohistologically, 68 cases (49%) were
Wnt3a-positive in cytoplasm of cancer cells (Wnt3a-positive group),
and 71 cases (51%) were negative (Wnt3a-negative group) (Fig. 1). Background data of
clinicophathological features in both group was shown in Table I. There was no significant difference
between two groups in age, sex, location of tumor and size of
tumor. In Wnt3a-positive group, the number of patients with poor
differentiated carcinoma (P=0.028) and T2 or deeper of pT (P=0.019)
than these in negative group. There was no significant correlation
between lympatic invasion, venous invasion, pN, pStage and Wnt3a
expression. There was not significant difference of recurrent rate
between two groups. There was not also difference of recurrent
patterns, however frequency of recurrence at distant organs in
Wnt3a-positive group tended to be higher than that in negative
group (P=0.051) Median follow-up period of Wnt3a-positive group was
37 months and that of Wnt3a-negative group was 44 months, and there
was no difference between two groups (P=0.172). Multivariate
analysis by Cox proportional hazard model showed the relationship
between pN (HR=3.539, P=0.001), venous invasion (HR=2.798,
P=0.012), Wnt3a expression (HR=1.691, P=0.046) and overall survival
(OS) (Table II), and the
relationship between only pN (HR=1.658, P<0.001) and disease
free survival (DFS). These parameters were identified as
independent prognostic factors for ESCC. On the other hand, serum
SCC antigen level, which was a former indicator of progression of
ESCC as one of typical tumor marker, was not related to
prognosis.
 | Table I.Comparison of clinicopathological
factors between Wnt3a-positive and Wnt3a-negative groups. A
Chi-square test was performed comparing all subgroups in each
factor. |
Table I.
Comparison of clinicopathological
factors between Wnt3a-positive and Wnt3a-negative groups. A
Chi-square test was performed comparing all subgroups in each
factor.
| Parameters | Total (n=139) | Wnt3a-positive
(n=68) | Wnt3a-negative
(n=71) | P-value |
|---|
| Age (years;
mean) | 64.3 | 65.4 | 63.3 | 0.741 |
| Sex |
|
|
| 0.907 |
| Male | 115 | 56 | 59 |
|
|
Female | 24 | 12 | 12 |
|
| Location of
tumor |
|
|
| 0.435 |
|
Upper | 19 | 9 | 10 |
|
|
Middle | 78 | 35 | 43 |
|
|
Lower | 42 | 24 | 18 |
|
| Size of
tumor (mm; mean) | 52.8 | 53.2 | 52.4 | 0.163 |
| Serum SCC level
(ng/ml; mean) |
|
|
| 0.834 |
| ≥1.5 | 28 | 13 | 15 |
|
|
<1.5 | 111 | 55 | 56 |
|
| Differentiation |
|
|
| 0.028 |
| Well | 43 | 19 | 24 |
|
|
Moderate | 63 | 27 | 36 |
|
|
Poorly | 33 | 22 | 11 |
|
| Lymphatic
invasion |
|
|
| 0.780 |
|
Positive | 109 | 54 | 55 |
|
|
Negative | 30 | 14 | 16 |
|
| Venous
invasion |
|
|
| 0.172 |
|
Positive | 101 | 53 | 48 |
|
|
Negative | 38 | 15 | 23 |
|
| INF |
|
|
| 0.240 |
| a | 5 | 4 | 1 |
|
| b | 111 | 51 | 60 |
|
| c | 23 | 13 | 10 |
|
| pT |
|
|
| 0.019 |
| 1 | 60 | 23 | 37 |
|
| 2 | 22 | 9 | 13 |
|
| 3 | 57 | 36 | 21 |
|
| pN |
|
|
| 0.475 |
| 0 | 47 | 21 | 26 |
|
| 1 | 92 | 47 | 45 |
|
| pStage |
|
|
| 0.322 |
| I | 32 | 12 | 20 |
|
| II | 50 | 24 | 26 |
|
|
III | 57 | 32 | 25 |
|
| Adjuvant
chemotherapy |
|
|
| 0.607 |
| + | 80 | 41 | 39 |
|
| − | 59 | 27 | 32 |
|
 | Table II.Univariate and multivariate survival
analysis of overall survival for ESCC patients by Cox's
proportional hazard model. |
Table II.
Univariate and multivariate survival
analysis of overall survival for ESCC patients by Cox's
proportional hazard model.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameters | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
| ≥65 vs.
≤64 | 1.484 | 0.892–2.468 | 0.128 |
|
|
|
| Sex |
|
|
|
|
|
|
| Male
vs. female | 1.098 | 0.557–2.168 | 0.787 |
|
|
|
| Location of
tumor |
|
|
|
|
|
|
| Upper
vs. middle and lower | 1.545 | 0.781–3.053 | 0.211 |
|
|
|
| Size of tumor |
|
|
|
|
|
|
| ≥49 vs.
≤48 | 1.651 | 0.997–2.736 | 0.051 |
|
|
|
| Serum SCC
level |
|
|
|
|
|
|
| ≥1.5
vs. <1.5 | 1.118 | 0.605–2.066 | 0.723 |
|
|
|
| Adjuvant
chemotherapy |
|
|
|
|
|
|
| Done
vs. not done | 1.390 | 0.819–2.357 | 0.222 |
|
|
|
|
Differentiation |
|
|
|
|
|
|
| Poorly
vs. well, moderate | 2.012 | 1.191–3.397 | 0.009 |
|
|
|
| Infiltrative growth
pattern |
|
|
|
|
|
|
| c vs.
a, b | 1.357 | 0.721–2.556 | 0.344 |
|
|
|
| Lymphatic
invasion |
|
|
|
|
|
|
|
Positive vs. negative | 3.126 | 1.252–7.808 | 0.015 |
|
|
|
| Venous
invasion |
|
|
|
|
|
|
|
Positive vs. negative | 3.424 | 1.557–7.532 | 0.002 | 2.798 | 1.259–6.215 | 0.012 |
| pT |
|
|
|
|
|
|
| 3 vs.
1, 2 | 2.534 | 1.446–4.442 | 0.001 |
|
|
|
| pN |
|
|
|
|
|
|
| 1 vs.
0 | 3.833 | 1.884–7.798 | <0.001 | 3.539 | 1.728–7.248 | 0.001 |
| Wnt3a
expression |
|
|
|
|
|
|
|
Positive vs. negative | 1.906 | 1.141–3.185 | 0.014 | 1.691 | 1.009–2.836 | 0.046 |
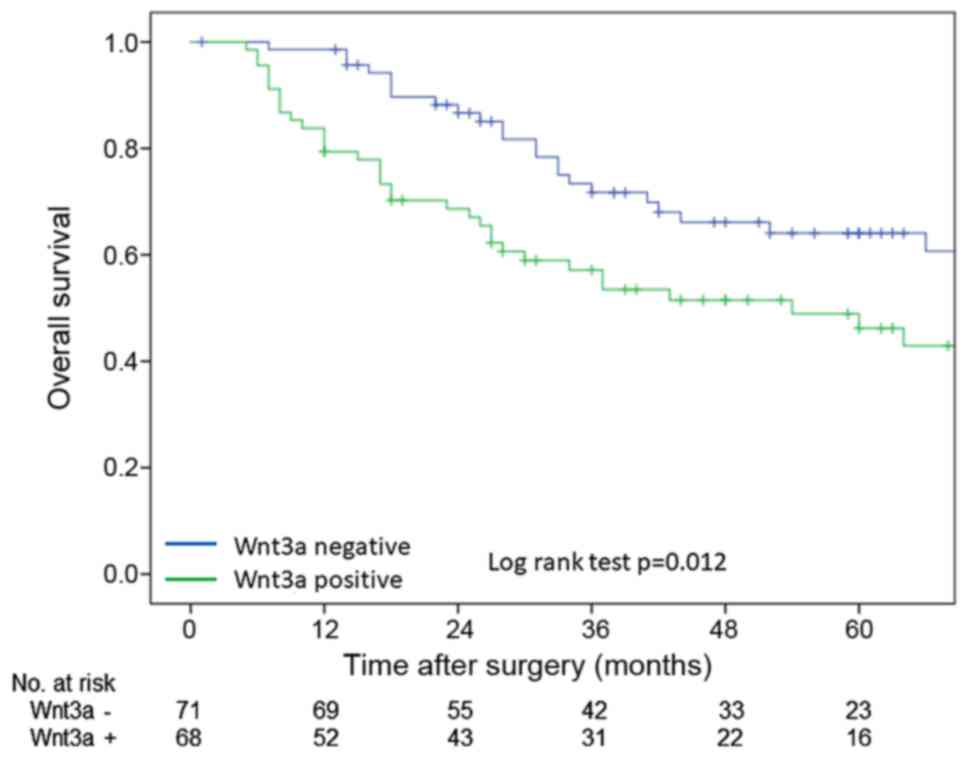
OS rate which were calculated by Kaplan-Meier method
were poorer in Wnt3a-positive group than these in Wnt3a-negative
group in log-rank test (P=0.012) (Fig.
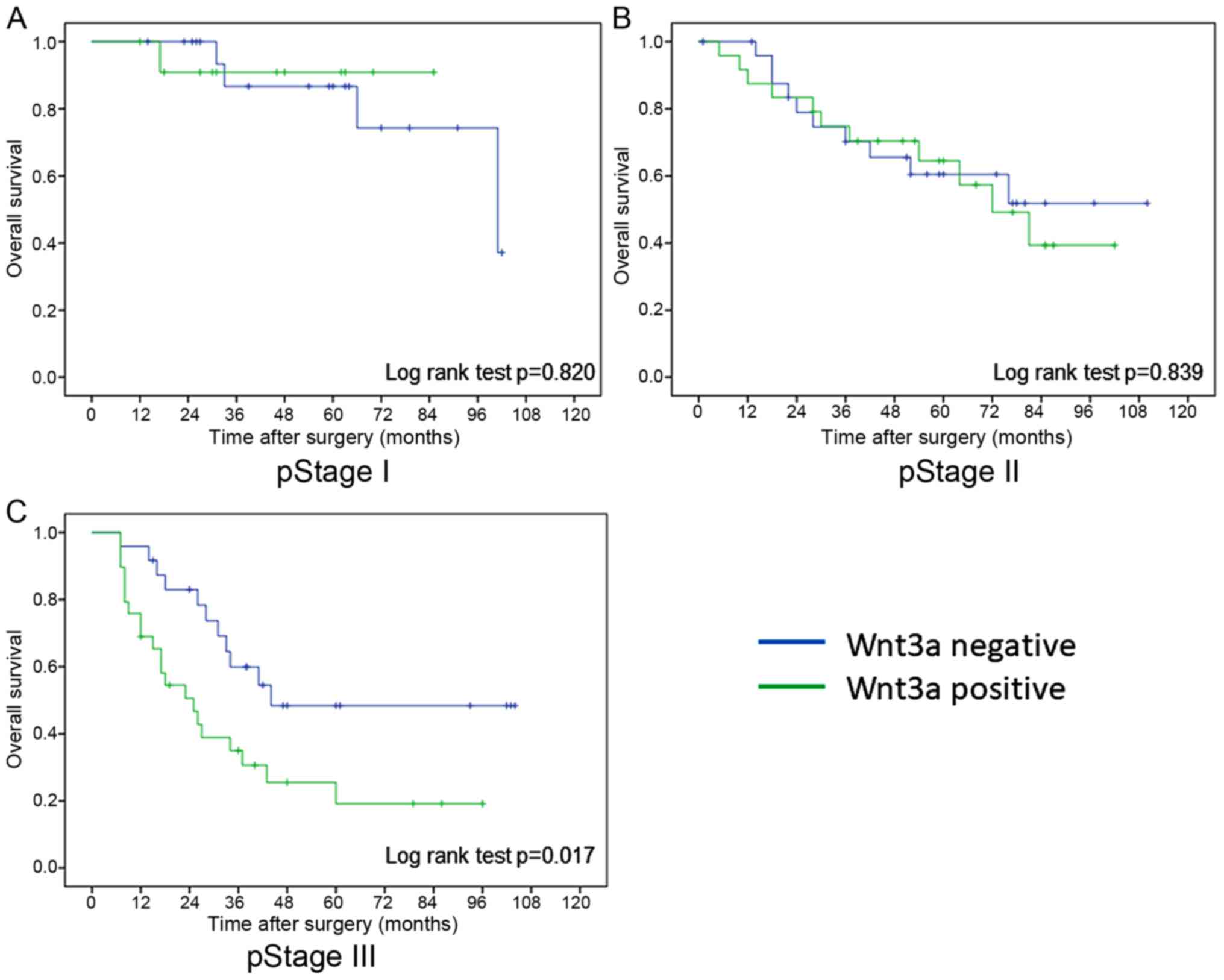
2). In pathological stages I and II, there was no difference in
overall survival rate between Wnt3a-positive and Wnt3a-negative
groups, however overall survival rate of Wnt3a-positive group was
significantly worse than that of Wnt3a-negative group in
pathological stage III (log rank test; P=0.017) (Fig. 3). On the other hand, there was no
significant difference in DFS rate between two groups in all
pathological stage. However, prognosis of Wnt3a-positive group
tended to be worse than that of Wnt3a-negative group in
pathological stage III (log rank test; P=0.051).
Next, pathological Stage III ESCC cases were focused
on in order to exclude the influence of T factor on progression. In
pathological stage III ESCC, background data of
clinicophathological features in both group was shown in Table III. There was no significant
different factor between two groups. Multivariate analysis by Cox
proportional hazard model showed that Wnt3a expression (HR=2.531,
P=0.012) were only independent prognostic factors (Table IV).
 | Table III.Comparison of clinicopathological
factors between Wnt3a-positive and Wnt3a-negative groups in
pathological stage III ESCC. |
Table III.
Comparison of clinicopathological
factors between Wnt3a-positive and Wnt3a-negative groups in
pathological stage III ESCC.
| Parameters | Total (n=57) | Wnt3a-positive
(n=32) | Wnt3a-negative
(n=25) | P-value |
|---|
| Age (years;
mean) | 64.3 | 66.1 | 61.9 | 0.063 |
| Sex |
|
|
| 0.520 |
|
Male | 45 | 24 | 21 |
|
|
Female | 12 | 8 | 4 |
|
| Location of
tumor |
|
|
| 0.972 |
|
Upper | 5 | 3 | 2 |
|
|
Middle | 30 | 17 | 13 |
|
|
Lower | 22 | 12 | 10 |
|
| Size of
tumor (mean) | 55.5 | 58.1 | 52.2 | 0.292 |
| Serum SCC level
(ng/ml; mean) |
|
|
| 0.225 |
|
| 15 | 6 | 9 |
|
|
| 42 | 26 | 16 |
|
|
Differentiation |
|
|
| 0.265 |
|
Well | 18 | 8 | 10 |
|
|
Moderate | 19 | 10 | 9 |
|
|
Poorly | 20 | 14 | 6 |
|
| Lymphatic
invasion |
|
|
| 1.000 |
|
Positive | 55 | 31 | 24 |
|
|
Negative | 2 | 1 | 1 |
|
| Venous
invasion |
|
|
| 0.309 |
|
Positive | 53 | 31 | 22 |
|
|
Negative | 4 | 1 | 3 |
|
| INF |
|
|
| 0.589 |
| a | 1 | 1 | 0 |
|
| b | 43 | 23 | 20 |
|
| c | 13 | 8 | 5 |
|
| Adjuvant
chemotherapy |
|
|
| 0.141 |
| + | 42 | 21 | 21 |
|
| − | 15 | 11 | 4 |
|
 | Table IV.Univariate and multivariate survival
analysis of overall survival for pathological stage III ESCC
patients by Cox's proportional hazard model. |
Table IV.
Univariate and multivariate survival
analysis of overall survival for pathological stage III ESCC
patients by Cox's proportional hazard model.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameters | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
| ≥65 vs.
≤64 | 1.384 | 0.708–2.706 | 0.342 |
|
|
|
| Sex |
|
|
|
|
|
|
| Male
vs. female | 0.851 | 0.371–1.953 | 0.703 |
|
|
|
| Location of
tumor |
|
|
|
|
|
|
| Upper
vs. middle and lower | 2.298 | 0.796–6.633 | 0.124 |
|
|
|
| Size of tumor |
|
|
|
|
|
|
| ≥56 vs.
≤55 | 1.875 | 0.957–3.675 | 0.067 | 1.775 | 0.894–3.524 | 0.101 |
| Serum SCC
level |
|
|
|
|
|
|
| ≥1.5
vs. <1.5 | 0.859 | 0.390–1.892 | 0.706 |
|
|
|
| Adjuvant
chemotherapy |
|
|
|
|
|
|
| Done
vs. not done | 0.783 | 0.374–1.638 | 0.515 |
|
|
|
|
Differentiation |
|
|
|
|
|
|
| Poorly
vs. well, moderate | 1.454 | 0.743–2.843 | 0.274 |
|
|
|
| Infiltrative growth
pattern |
|
|
|
|
|
|
| c vs.
a, b | 1.003 | 0.455–2.214 | 0.994 |
|
|
|
| Lymphatic
invasion |
|
|
|
|
|
|
|
Positive vs. negative | 1.052 | 0.143–7.718 | 0.960 |
|
|
|
| Venous
invasion |
|
|
|
|
|
|
|
Positive vs. negative | 2.912 | 0.397–21.345 | 0.293 |
|
|
|
| Wnt3a
expression |
|
|
|
|
|
|
|
Positive vs. negative | 2.719 | 1.324–5.585 | 0.006 | 2.531 | 1.230–5.206 | 0.012 |
In order to investigate the influence of Wnt3a
expression on the response of chemotherapy and chemoradiotherapy
after recurrence, all patients were classified into two groups,
between patients with recurrence during follow-up period
(recurrence group; n=61) and patients without recurrence (no
recurrence group; n=78). All of patients with recurrence underwent
chemotherapy or chemoradiotherapy after diagnosis of recurrence.
Background data of clinicophathological features in both group was
no significant different in all factors. Patients in recurrence
group were classified into Wnt3a-positive (n=35) and Wnt3a-negative
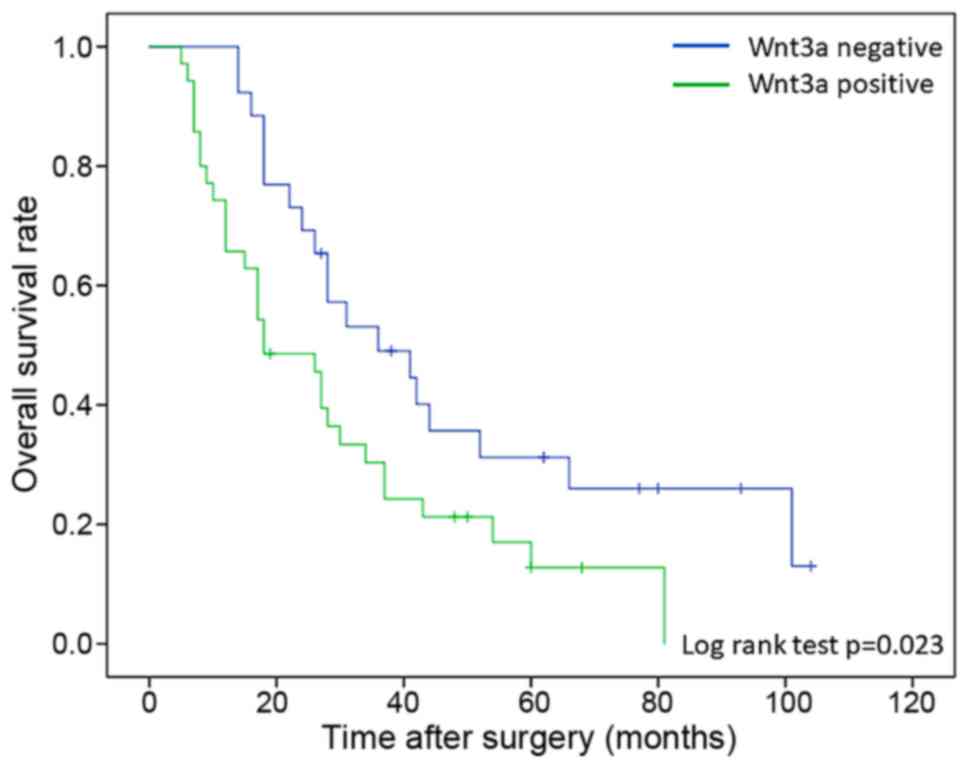
groups (n=26). In comparison with overall survival rate between two
groups, Wnt3a-positive patients were significant poor prognosis
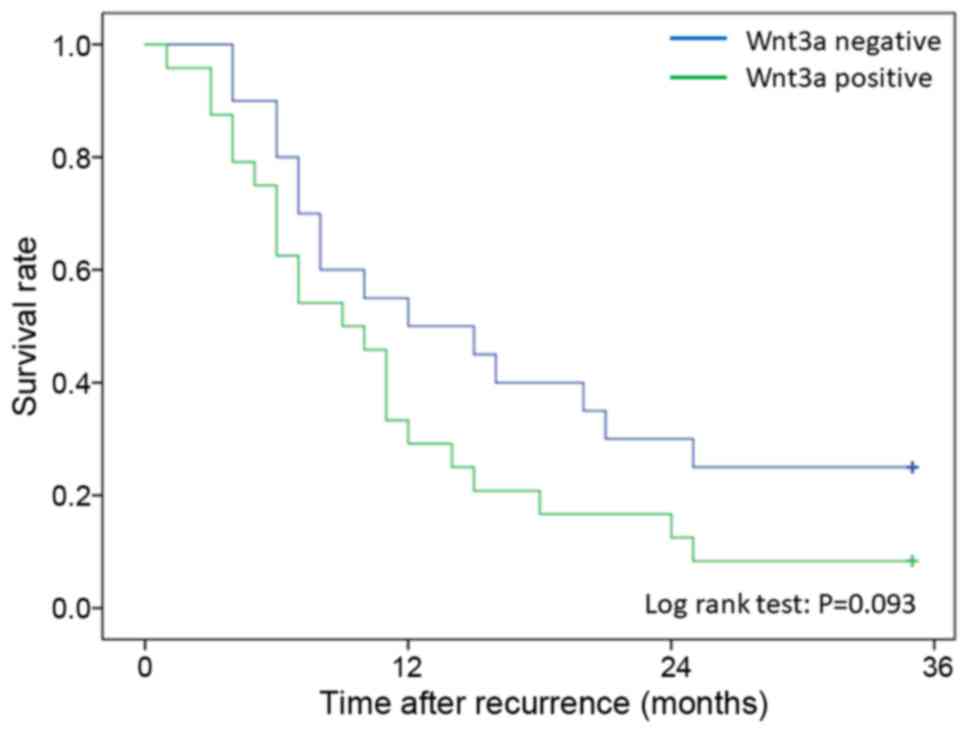
than negative patients (log-rank test; P=0.023) (Fig. 4). Moreover, survival after recurrence
was compared with Wnt3a expression only in patients who received
chemotherapy after recurrence. Twenty-eight patients with
recurrence were Wnt3a-positive, and 25 patients were
Wnt3a-negative. No significant difference in survival after
recurrence was seen between the Wnt3a-positive and the
Wnt3a-negative patients (P=0.093); however, the Wnt3a-positive
patients tended to have a poorer survival outcome than the
Wnt3a-negative patients (Fig. 5).
Discussion
In this study, it was suggested that expression of
Wnt3a in cytoplasm of ESCC was a poor prognostic factor of ESCC
after lymph node metastasis and venous invasion. In pathological
stages I and II, there was no difference in prognosis between
Wnt3a-positive and Wnt3a-negative groups, however prognosis of
Wnt3a-positive group was significantly worse than that of
Wnt3a-negative group in pathological stage III. Among patients with
recurrence in pathological stage III, overall survival rate of
Wnt3a-positive group was significant lower than that in
Wnt3a-negative group.
Wnt3a is one of regular ligands of canonical Wnt
signaling pathway. Several studies suggested that development and
progression of glioblastoma (16),
prostate cancer (12), breast cancer
(17), or malignant mesothelioma
(18) was related to the activity of
canonical Wnt signaling pathway, and Wnt3a expression in cancer
cells of colorectal cancer (19) or
hepatocellular carcinoma (20) was
associated with poor prognosis. There was also some study which
suggested that the activity of canonical Wnt signaling pathway was
related to development and prognosis of head and neck squamous cell
carcinoma (21). Some study suggested
that the activity of canonical Wnt signaling pathway, β-catenin and
TCF-4, which were regular factors of canonical Wnt signaling
pathway, was related to prognosis of ESCC (11,22,23). There
was also a study which suggested that expression of dickkopf-1,
which was one of inhibitor of canonical Wnt signaling pathway, was
associated with poor prognosis of ESCC (15). Relationship between ESCC and canonical
Wnt signaling is still controversial. Epithelial-mesenchymal
transition (EMT), which was proposed as one of the important
mechanism of tumor growth and metastasis, was a concept of the
transition from epithelial cells to mesenchymal cells. According to
progression of cancer, cancer cells seems to acquire the ability of
invasion and metastasis by EMT (24).
Several studies already suggested that Wnt signaling pathway
closely related to EMT (25,26), and EMT related factors would be
prognostic factors of ESCC (27,28). These
studies support the hypothesis that the canonical Wnt signaling
pathway activates the progression and metastasis of ESCC through
the EMT; whether Wnt3a expression might also be correlated with the
progression and metastasis of ESCC should be investigated.
In Japan, neoadjuvant chemotherapy (NAC) is a
standard treatment for clinical stages II or III ESCC based on a
clinical phase III trial in the country (29,30). Depth
of invasion, lymph node metastasis and lower level of preoperative
serum albumin were poor prognostic factors for patients who
performed NAC (31), therefore
additional adjuvant therapy seems to be needed for them in order to
improve the prognosis of high risk cases. However, contents of
adjuvant therapy was still controversial. Our results suggested
that Wnt3a is correlated with a poor prognosis in patients with
stage III ESCC and that the prognosis of patients with recurrence
is poorer in patients with Wnt3a expression than in patients
without Wnt3a expression. These results suspected that patients
with expression of Wnt3a may have poor response of treatment after
recurrence. Although a significant correlation between survival
after recurrence and Wnt3a expression was not seen, the correlation
between the canonical Wnt signaling pathway, including Wnt3a, and
the chemoresistance of ESCC is worth investigating. Several studies
suggested that canonical Wnt signaling played a role in
chemotherapy and radiation resistance of various malignancies
(32–34). Advanced ESCC with positive expression
of Wnt3a might have malignant potential or chemoresistance which
leaded to poor prognosis and Wnt3a also may be a target of adjuvant
therapy for high risk cases who performed NAC.
Expression of Wnt3a in ESCC was more associated with
the depth of invasion and histological differentiation of main
tumor than with lymph node metastasis and vessel invasion. Some
studies suggested that Wnt3a expression was also related to
histological differentiation of colon cancer (19) and hepatocellular carcinoma (20). These results suggested that upper
stream factor of canonical Wnt signaling pathway, such as Wnt3a,
seemed to be more associated with local development and
differentiation of tumor than with migration and metastasis, and
then it would be identified as a poor prognostic factor of
ESCC.
The limitations of this study were that it was a
retrospective study and the small number of patients who were
enrolled in present study, and it was investigated about Wnt3a
alone which was one of factors related to canonical Wnt signaling
pathway. Generally, cell signaling pathway was activated by the
interaction of a lot of factors. Therefore, the role of Wnt3a in
the progression of ESCC was not clear in this study. In order to
investigate the mechanism of ESCC progression, a lot of factors,
investigation in the cell cultures and animal models will be needed
in the future.
To our knowledge, this study was a first study which
suggested that Wnt3a seemed to be a prognostic factor of ESCC. It
is expected that relationship between ESCC and canonical Wnt
signaling pathway will be clear based on this study and development
of novel treatment for ESCC with poor prognosis will progress in
the future.
References
|
1
|
Tachimori Y, Ozawa S, Numasaki H,
Fujishiro M, Matsubara H, Oyama T, Shinoda M, Toh Y, Udagawa H, Uno
T, et al: Comprehensive regitry of esophageal cancer in Japan,
2009. Esophagus. 13:110–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kikuchi A, Yamamoto H and Sato A:
Selective activation mechanisms of Wnt signaling pathways. Trends
Cell Biol. 19:119–129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duchartre Y, Kim YM and Kahn M: The Wnt
signaling pathway in cancer. Crit Rev Oncol Hematol. 99:141–149.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Behrens J, von Kries JP, Kühl M, Brihn L,
Wedlich D, Grosschedl R and Birchmeier W: Functional interaction of
beta-catenin with the transcription factor LEF-1. Nature.
382:638–642. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Molenaar M, van de Wetering M, Oosterwegel
M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destrée O and
Clevers H: XTcf-3 transcription factor mediates
beta-catenin-induced axis formation in Xenopus embryos. Cell.
86:391–399. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nelson WJ and Nusse R: Convergence of Wnt,
beta-catenin, and cadherin pathways. Science. 303:1483–1487. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Firestein R, Bass AJ, Kim SY, Dunn IF,
Silver SJ, Guney I, Freed E, Ligon AH, Vena N, Ogino S, et al: CDK8
is a colorectal cancer oncogene that regulates beta-catenin
activity. Nature. 455:547–551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fritzmann J, Morkel M, Besser D, Budczies
J, Kosel F, Brembeck FH, Stein U, Fichtner I, Schlaq PM and
Birchmeier W: A colorectal cancer expression profile that includes
transforming growth factor beta inhibitor BAMBI predicts metastatic
potential. Gastroenterology. 137:165–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takigawa Y and Brown AM: Wnt signaling in
liver cancer. Curr Drug Targets. 9:1013–1024. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang S, Li J, He F and Wang XM: Abnormal
nuclear expression of Pygopus-2 in human primary hepatocellular
carcinoma correlates with a poor prognosis. Histopathology.
67:176–184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fu L, Zhang C, Zhang LY, Dong SS, Lu LH,
Chen J, Dai Y, Li Y, Kong KL, Kwong DL and Guan XY: Wnt2 secreted
by tumour fibroblasts promotes tumour progression in oesophageal
cancer by activating of the Wnt/β-catenin signaling pathway. Gut.
60:1635–1643. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Verras M, Brown J, Li X, Nusse R and Sun
Z: Wnt3a growth factor induces androgen receptor-mediated
transcription and enhances cell growth in human prostate cancer
cells. Cancer Res. 64:8860–8866. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao Q, Qin SJ, Fan J, Zhou J, Xiao YS, Xu
Y, Li YW and Tang ZY: Intratumoral balance of regulatory and
cytotoxic T cells is associated with prognosis of hepatocellular
carcinoma after resection. J Clin Oncol. 25:2586–2593. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bisson I and Prowse DM: WNT signaling
regulates self-renewal and differentiation of prostate cancer cells
with stem cell characteristics. Cell Res. 19:683–697. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Makino T, Yamasaki M, Takemasa I, Takeno
A, Nakamura Y, Miyata H, Takiguchi S, Fujiwara Y, Matsuura N, Mori
M and Doki Y: Dickkopf-1 expression as a marker for predicting
clinical outcome in esophageal squamous cell carcinoma. Ann Surg
Oncol. 16:2058–2064. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaur N, Chettiar S, Rathod S, Rath P,
Muzumdar D, Shaikh ML and Shiras A: Wnt3a mediated activation of
Wnt/β-catenin signaling promotes tumor progression in glioblastoma.
Mol Cell Neurosci. 54:44–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
King TD, Suto MJ and Li Y: The
Wnt/β-catenin signaling pathway: A potential therapeutic target in
the treatment of triple negative breast cancer. J Cell Biochem.
113:13–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fox SA, Richards AK, Kusumah I, Perumal V,
Bolitho EM, Mutsaers SE and Dharmarajan AM: Expression profile and
function of Wnt signaling mechanisms in malignant mesothelioma
cells. Biochem Biophys Res Commun. 440:82–87. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi L, Sun B, Liu Z, Cheng R, Li Y and Zhao
X: Wnt3a expression is associated with epithelial-mesenchymal
transition and promotes colon cancer progression. J Exp Clin Cancer
Res. 33:1072014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan LH, Yao M, Cai Y, Gu JJ, Yang XL, Wang
L and Yao DF: Oncogenic Wnt3a expression as an estimable prognostic
marker for hepatocellular carcinoma. World J Gastroenterol.
22:3829–3836. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goto M, Mitra RS, Liu M, Lee J, Henson BS,
Carey T, Bradford C, Prince M, Wang CY, Fearon ER and D'Silva NJ:
Rap1 stabilizes beta-catenin and enhances beta-catenin-dependent
transcription and invasion in squamous cell carcinoma of the head
and neck. Clin Cancer Res. 16:65–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deng F, Zhou K, Cui W, Liu D and Ma Y:
Clinicopathological significance of wnt/β-catenin signaling pathway
in esophageal squamous cell carcinoma. Int J Clin Exp Pathol.
8:3045–3053. 2015.PubMed/NCBI
|
|
23
|
Ishiguro H, Wakasugi T, Terashita Y,
Sakamoto N, Tanaka T, Sagawa H, Okubo T and Takeyama H: Nuclear
expression of TCF4/TCF7L2 is correlated with poor prognosis in
patients with esophageal squamous cell carcinoma. Cell Mol Biol
Lett. 21:52016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thiery JP: Epithelial-mesenchymal
transitions in tumor progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smith BN and Bhowmick NA: Role of EMT in
metastasis and therapy resistance. J Clin Med. 5:E172016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tanaka M, Kijima H, Shimada H, Makuuchi H,
Ozawa S and Inokuchi S: Expression of podoplanin and vimentin is
correlated with prognosis in esophageal squamous cell carcinoma.
Mol Med Rep. 12:4029–4036. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin H, Morohashi S, Sato F, Kudo Y,
Akasaka H, Tsutsumi S, Ogasawara H, Miyamoto K, Wajima N, Kawasaki
H, et al: Vimentin expression of esophageal squamous cell carcinoma
and its aggressive potential for lymph node metastasis. Biomed Res.
31:105–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ando N, Iizuka T, Ide H, Ishida K, Shinoda
M, Nishimaki T, Takiyama W, Watanabe H, Isono K, Aoyama N, et al:
Surgery plus chemotherapy compared with surgery alone for localized
squamous cell carcinoma of the thoracic esophagus: A Japan Clinical
Oncology Group Study-JCOG9204. J Clin Oncol. 21:4592–4596. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ando N, Kato H, Igaki H, Shinoda M, Ozawa
S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, et al: A
randomized trial comparing postoperative adjuvant chemotherapy with
cisplatin and 5-fluorouracil versus preoperative chemotherapy for
localized advanced squamous cell carcinoma of the thoracic
esophagus (JCOG9907). Ann Surg Oncol. 19:68–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yokota T, Ando N, Igaki H, Shinoda M, Kato
K, MIzusawa J, Katayama H, Nakamura K, Fukuda H and Kitagawa Y:
Prognostic factors in patients receiving neoadjuvant 5-fluorouracil
plus cisplatin for advanced esophageal cancer (JCOG9907). Oncology.
89:143–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jamieson CH, Ailles LE, Dylla SJ,
Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating
A, et al: Granulocyte-macrophage progenitors as candidate leukemic
stem cells in blast-crisis CML. N Engl J Med. 351:657–667. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang W, Yan HX, Chen L, Liu Q, He YQ, Yu
LX, Zhang SH, Huang DD, Tang L, Kong XN, et al: Wnt/beta-catenin
signaling contributes to activation of normal and tumorigenic liver
progenitor cells. Cancer Res. 68:4287–4295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Noda T, Nagano H, Takemasa I, Yoshioka S,
Murakami M, Wada H, Kobayashi S, Marubashi S, Takeda Y, Dono K, et
al: Activation of Wnt/beta-catenin signaling pathway induces
chemoresistance to interferon-alpha/5-fluorouracil combination
therapy for hepatocellular carcinoma. Bri J Cancer. 100:1647–1658.
2009. View Article : Google Scholar
|