Introduction
Bladder cancer is among the most detrimental tumors
of the urinary system, particularly in China (1). A total of ~70% of patients belong to the
non muscle-invasive bladder cancer group and <50% of patients
will suffer from recurrence; furthermore, ~20% will progress to the
muscle-invasive bladder cancer type (2). The incidence and mortality rates of
patients with bladder cancer have markedly increased in recent
years (3). Numerous therapeutic
methods for bladder cancer intervention have been investigated,
including chemotherapy, radiotherapy and surgery; however, the
overall survival (OS) rate remains poor (4). Therefore, identifying novel biomarkers
for an early diagnosis and effective prognosis, as well as
establishing the underlying mechanisms for bladder cancer
progression, is critically important for increasing the survival
rate among patients with bladder cancer.
The occurrence and development of bladder cancer has
been ascribed to multiple mechanisms. For example, frequent
inactivation of tumor suppressor pathways, including the p53
signaling pathway, contributes to bladder cancer progression
(5). Similarly, aberrant activation
of oncogenic pathways, including the Akt and mitogen-activated
protein kinase pathways, has also been reported to increase
motility and invasion in bladder cancer (6). Furthermore, epigenetic alterations,
including loss of heterozygosity, hypermethylation and point
mutations, have also been implicated in the development of
multifocal bladder cancer (7,8). Notably, factors involved in chromosome
abnormalities such as C14orf166 also function as high-risk
biomarkers for bladder cancer progression (9). However, the intrinsic cellular
mechanisms remain largely elusive and further studies are required
to elucidate the mechanisms of bladder cancer development.
Although research regarding small non-coding NRAs
(e.g., microRNAs) has been the focus in the field of molecular
biology, numerous studies have indicated that long non-coding RNAs
(lncRNAs) also exhibit essential roles in biological processes
including proliferation, senescence and apoptosis (10). LncRNAs belong to a class of RNAs that
are >200 nucleotides in length with no protein coding activities
(11). Despite their inability to
encode proteins, lncRNAs can regulate various biological pathways
and are characterized by their complex mechanisms of activity
(12). The aberrant expression of
lncRNAs is critically involved in a variety of diseases,
particularly cancer. Previously, Liu et al (13) identified that lncRNA cartilage
injury-related (lncRNA-CIR) may increase the degradation of
chondrocyte extracellular matrix in osteoarthritis (OA). However,
the precise function of lncRNA-CIR especially in bladder cancer
progression remains unknown.
In the present study, the role of lncRNA-CIR in the
pathogenesis of bladder cancer was examined. It was identified that
lncRNA-CIR was frequently upregulated in bladder cancer. Higher
lncRNA-CIR expression promotes the viability and invasion of
bladder cancer cells. In addition, it was found that patients with
higher lncRNA level were associated with poor overall survival. The
lncRNA-CIR may consistently increase xenograft tumor growth in
vivo. Overall, the results of the present study identified a
novel and oncogenic role of lncRNA-CIR in bladder cancer and may
provide important insights into potential therapeutic interventions
targeting lncRNA-CIR.
Materials and methods
Cell culture and specimen
collection
The present study used bladder cancer T24, SW780,
UBC-40, 5637 and UM-UC-3 cells lines, in addition to the
transformed cell line, SV-HUC-1. The cell lines were all purchased
from the Shanghai Institute of Cell Biology (Shanghai, China) and
were cultured in RPMI-1640 medium supplemented with 5% fetal bovine
serum (FBS), 200 U/ml penicillin and 50 mg/ml streptomycin (all
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and maintained in
an incubator with 5% CO2 at 37°C. Matched fresh bladder
cancer specimens and normal adjacent tissues were collected from 52
patients at The Affiliated Hospital of Guilin Medical College
(Guilin, Guangxi, China) between February 2009 and March 2011.
Following surgical resection, tissues were immediately stored at
−80°C until total RNA was extracted. None of the patients had
received preoperative chemotherapy or radiotherapy prior to
surgery. All patients provided written informed consent, and the
research on human specimens was reviewed and formally approved by
the Ethics Committee of the Affiliated Hospital of Guilin Medical
College.
lncRNA-CIR-knockdown and
-overexpression
The sequences of specific siRNAs targeting
lncRNA-CIR were designed and synthesized by Sigma-Aldrich (Merck
KGaA). The lncRNA-CIR fragment obtained by RNA extraction and
RT-qPCR from bladder cancer cell lines, tissues and normal
adjacent tissues was amplified by PCR as described in next section.
The reaction conditions were: Initial denaturation at 95°C for 2
min, followed by 35 cycles of denaturation at 95°C for 15 sec,
annealing at 60°C for 30 sec and extension at 72°C for 45 sec. The
amplified products were extracted and purified using Qiaquick gel
extraction kit (Qiagen, Valencia, CA, USA), and then digested with
Hind III and Xho I. Following this, the products were cloned into
the pcDNA3.1 vector. The correct constructs were verified by DNA
sequencing and were used to transfect T24 and SW780 cells. The
constructs were sequenced using standard procedures of dye
terminator chemistry on a 3700 Sequencer (Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Sequences were
compared to sequences in Gene Bank using the Blast program (version
2.5.0; https://blast.ncbi.nlm.nih.gov/Blast.cgi). All siRNAs
against lncRNA-CIR were designed and synthesized by Sigma-Aldrich;
Merck KGaA. All transfections were performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol. Briefly, cells were cultured with RPMI-1640 medium in a
24-well plate for 24 h at 37°C. Prior to transfection, the medium
was replaced with 200 µl/well of medium without antibiotics and the
cells were cultured for 24 h at 37°C. The DNA or RNA interference
Lipofectamine® 2000 complex was prepared by mixing for
30 min, and then 100 µl of the complex was added to each well. The
cells were cultured for 24 h at 37°C with normal RPMI-1640 medium
and then subject to additional assays. The si-CIR sequences used
were: si-CIR#1, sense: 5′-GCGCUGAACGGACUGA-3′, antisense:
5′-GACAGUCCGUUCAGC=3′; si-CIR#2, sense: 5′-CGUCAACAAGGAGCA-3′,
anti-sense: 5′-AUGCUCCUUGUUG-3′, si-CIR#3: sense:
5′-CGAGAACUGCGUGGCA-3′, anti-sense: 5′-AUGCCACGCAGUUCUC-3′.
RNA extraction and RT-qPCR
Total RNAs were isolated from T24 and SW780 cell
lines and human specimens with TRIzol® reagent (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Following cell lysis, 0.5 ml chloroform (Sigma-Aldrich; Merck KGaA)
was added and cells were maintained at 20°C for 5 min.
Centrifugation was performed at 15,000 × g at 4°C for 10 min. The
aqueous phase was transferred to another tube and 0.2 ml
isopropanol per 1 ml TRIzol was added. Following incubation for 15
min at 20°C, centrifugation was conducted at 10,000 × g at 4°C for
10 min. The RNA pellet was washed with 70% ethanol and the RNAs
were dissolved using 0.05 ml fresh water and incubated for 15 min.
Isolated RNA was reverse-transcribed with Improm-II reverse
transcription kit (Promega Corporation, Madison, WI, USA) according
to the manufacturer's protocol. GAPDH was used as the control.
Reactions were performed using the ABI PRISM® 7000
Sequence Detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
expression of lncRNA-CIR was calculated by the 2−ΔΔCq
method (14). The quantitative
real-time PCR was performed using TaqMan Non-coding RNA Assays
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The reactions
were initially denatured at 95°C for 2 min, followed by 35 cycles
of denaturation at 95°C for 15 sec, annealing at 60°C for 30 sec,
and 72°C for 45 sec. The experiments were performed a minimum of
three times. The primer sequences were as follows: lncRNA-CIR
sense, 5′-GCGAGATGTTTACG-3′ and anti-sense, 5′-CTGATGGAGAGAT-3′;
and GAP DH sense, 5′-GGTAGTCTGTCGAGTT-3′ and anti-sense,
5′-ATGGTCGCTGAATCGAT-3′.
Transwell invasion assay
The invasion assay was performed using Transwell
plates (pore size, 8 µm) with a Boyden chamber (Sigma-Aldrich;
Merck KGaA). Transfected cells were washed twice with
serum-containing RPMI-1640 medium (Sigma-Aldrich; Merck KGaA). A
total of 1×105 T24 and SW780 cells were seeded onto the
Transwell apparatus. Each insert was preloaded with Matrigel (50
µg; Sigma-Aldrich; Merck KGaA). Cells were suspended in 100 µl
RPMI-1640 serum-free medium (Sigma-Aldrich; Merck KGaA) and placed
in the top chambers. RPMI-1640 medium (100 µl; Sigma-Aldrich; Merck
KGaA) containing 10% fetal calf serum (Sigma-Aldrich; Merck KGaA)
was added to the bottom chambers. The chambers were incubated for
24 h at 37°C with 5% FBS in the lower chamber. Cells on the upper
layer were removed by cotton buds and then washed with PBS. The
cells that had invaded into the lower chambers were fixed with
methanol for 15 min (Sigma-Aldrich; Merck KGaA) and stained with 5%
crystal violet for 30 min at 20°C (Sigma-Aldrich; Merck KGaA). The
results were visualized by light microscopy (DFC500; Leica
Microsystems GmbH, Wetzlar, Germany) and the final values represent
the mean from three fields on the membrane. The results were
visualized at magnification, ×100. Experiments were performed in
triplicate.
Cell viability assay
Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was used to evaluate cell
viability. Following transfection with pcDNA, si-NC, pcDNA-CIR or
si-CIR for 36 h, T24 and SW780 cells were re-suspended and seeded
into a 96-well plate at a density of ~1×104 cell/ml.
CCK-8 solution (25 ml/well) was then added and the plate was
incubated for 3 h at 37°C. The viability was monitored once a day
for 5 days. The optical density at 490 nm was used to determine
cell viability with the Spectramax M5 microplate monitor (Molecular
Devices, LLC, Sunnyvale, CA, USA) according to the manufacturer's
protocol.
In vivo implantation assay
T24 cells transfected with pcDNA, si-NC, pcDNA-CIR
or si-CIR for 36 h were continuously cultured in RPMI 1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc.) for an additional 24 h
at 20°C. The cells were then resuspended and ~1×106
cells were injected subcutaneously into nude mice. A total of 40
mice (BALB/C; female; age, 4–5 weeks; mean weight, 15.6 g) were
used. Mice were housed at ~20°C, 55–60% humidity, with a light-dark
cycle of 12 h. Ad libitum access to food and water was
provided. The nude mice were obtained from the Model Animal
Research Center (Nanjing, China). The Ethics Committee of
Affiliated Hospital of Guilin Medical College approved the animal
experiments. The volumes (length × width × height) of the tumors
in vivo were recorded by external caliper every 3 days for
30 days. Animals were sacrificed when tumor volumes reached 2,000
mm3, or when animals lost >20% of initial body
weight. After 30 days post-injection, all mice were sacrificed by
an overdose of sodium pentobarbital (4%, 300 mg/kg with
intraperitoneal injection; catalog no. 1507002; Sigma-Aldrich;
Merck KGaA) and the implants were immunostained with Ki-67
(Sigma-Aldrich; Merck KGaA). Following antigen retrieval in the
supplied solution, the primary antibody against Ki-67 (catalog no.
P6834; dilution, 1:1,000; Sigma-Aldrich; Merck KGaA) was added and
incubated for 30 min at 20°C. The image was visualized using a
CX31-LV320 light microscope at magnification, ×100 (Olympus
Corporation, Tokyo, Japan).
Statistical analysis
All statistical results are shown as the mean ±
standard deviation. Statistical significance was determined by
Mann-Whitney test using SPSS version 16.0 (SPSS, Inc., Chicago, IL,
USA) and P<0.05 was used to indicate a statistically significant
difference. One-way ANOVA was used to determine the statistical
significance across multiple groups followed by Least Significant
Difference post hoc comparison. Kaplan-Meier curves were evaluated
by log-rank test. The contingency table for clinicopathological
features was determined by Fisher's exact test.
Results
lncRNA-CIR is upregulated in bladder
cancer tissues and cells
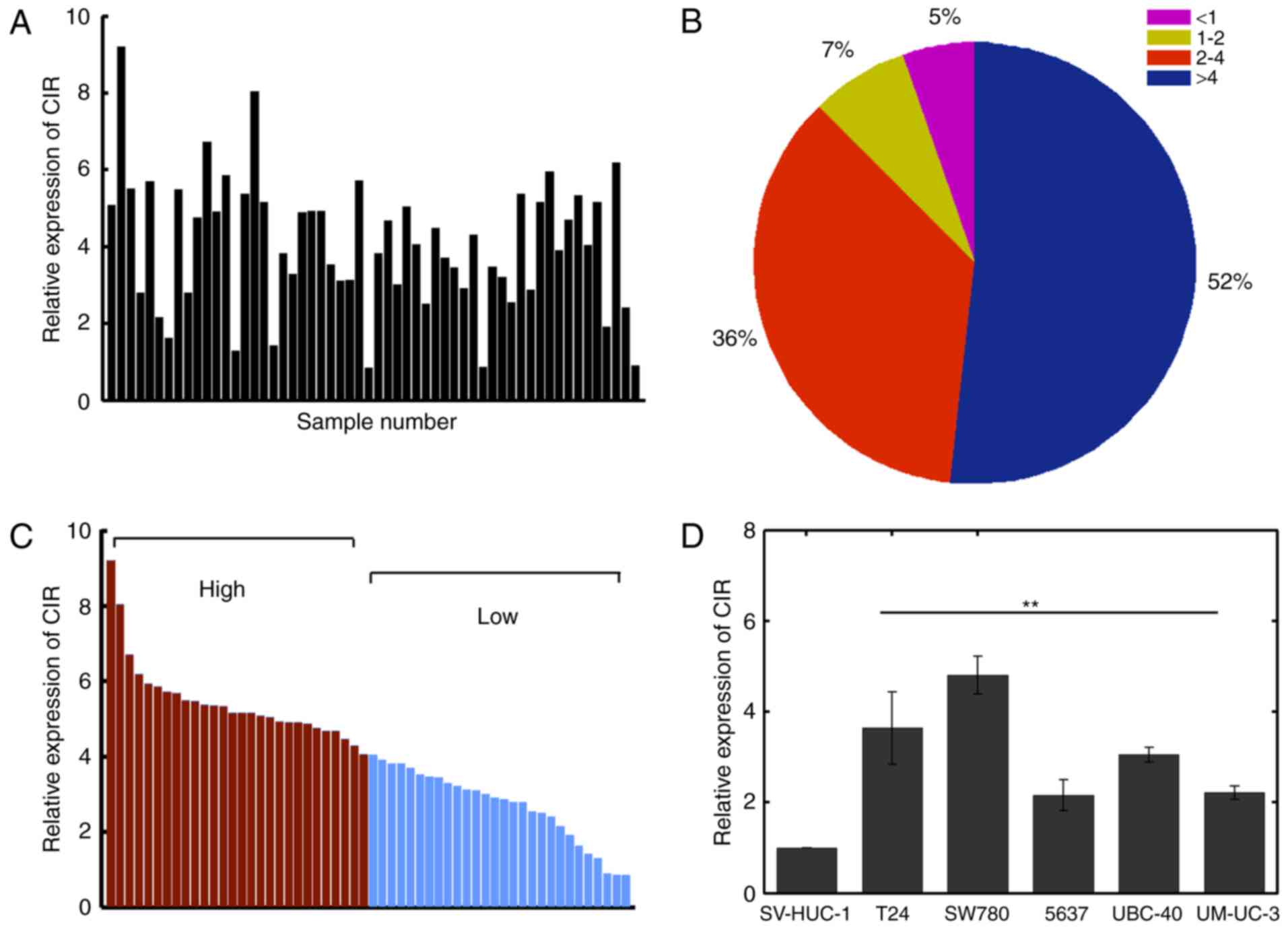
RT-qPCR was used to evaluate the expression of
lncRNA-CIR in bladder cancer tissues and corresponding normal
adjacent tissues. The relative expression of lncRNA-CIR was
quantified by dividing the value in bladder cancer tissues by the
associated value in normal adjacent tissues. The results
demonstrated that lncRNA-CIR was significantly upregulated in
bladder cancer tissues (Fig. 1A).
Notably, >80% of tissues were overexpressed in lncRNA-CIR by
>2-fold increase (Fig. 1B). The
lncRNA-CIR expression was markedly associated with metastasis and
tumor size (P=0.005 and P=0.004 respectively; Table I). However, lncRNA-CIR expression was
not significantly associated with age, sex or pathological grade
(Table I). The samples were
classified into two groups based on the median value of expression
(4.0489; Fig. 1C). The expression of
lncRNA-CIR was further examined in bladder cancer cell lines and
the level of lncRNA-CIR was demonstrated to be consistently
significantly increased in cancer cell lines compared with that in
normal cells (Fig. 1D). These results
suggested lncRNA-CIR was overexpressed in bladder cancer cells and
specimens. The T24 and SW780 cell lines were selected for further
analysis as they displayed the highest lncRNA-CIR expression among
the bladder cancer cell lines examined.
 | Table I.Association between CIR and
clinicopathological features. |
Table I.
Association between CIR and
clinicopathological features.
|
|
| CIR expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
factor | n | Low, n (%) | High, n (%) | P-values |
|---|
| Age, years |
|
|
|
|
|
<60 | 30 | 14 (46.7) | 16 (53.3) | 0.779 |
| ≥60 | 22 | 12 (54.5) | 10 (45.5) |
|
| Sex |
|
|
|
|
| Male | 24 | 11 (45.8) | 13 (54.2) | 0.391 |
|
Female | 28 | 15 (53.6) | 13 (46.4) |
|
| Metastasis |
|
|
|
|
|
Absent | 23 | 17 (73.9) | 6
(26.1) | 0.005a |
|
Present | 29 | 9
(31.0) | 20 (69.0) |
|
| Tumor size, cm |
|
|
|
|
|
<3 | 21 | 16 (76.2) | 5
(23.8) | 0.004a |
| ≥3 | 31 | 10 (32.3) | 21 (67.7) |
|
| Pathological
grade |
|
|
|
|
| Low | 27 | 11 (40.7) | 16 (59.3) | 0.267 |
|
High | 25 | 15 (60.0) | 10 (40.0) |
|
lncRNA-CIR levels are associated with
poor survival
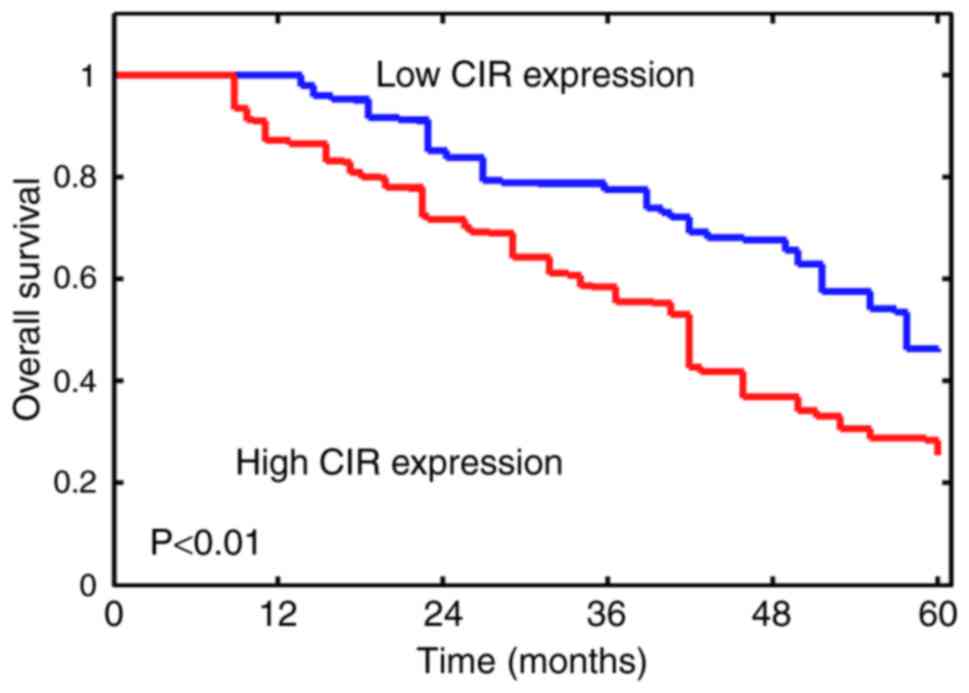
Next, whether lncRNA-CIR may dictate survival of
patients with bladder cancer was investigated. The samples were
divided into low and high CIR expression using the median value of
4.0489 as the cutoff. The results demonstrated that lower
lncRNA-CIR expression was significantly associated with improved
survival (P<0.01; Fig. 2).
Furthermore, the difference in OS rate between the two groups
generally increased during the follow-up period (Fig. 2). These results suggested that higher
lncRNA-CIR levels were associated with poor overall survival among
patients with bladder cancer.
lncRNA-CIR promotes malignant
phenotypes in bladder cancer cell lines
To further explore the potential oncogenic effect of
lncRNA-CIR in vitro, a series of in vitro experiments
were performed in the T24 and SW780 bladder cancer cell lines and
lncRNA-CIR was demonstrated to be either knocked down or
overexpressed in T24 and SW780 cells. The knockdown and
overexpression efficiency were verified and the si-RNA-mediated
knockdown and pcDNA-mediated transfection was demonstrated to
significantly alter the expression of lncRNA-CIR compared with that
in the corresponding control group (Fig.
3A). si-CIR#1 displayed the most significant effect to knock
down CIR expression in the two cells lines (Fig. 3A). Therefore, si-CIR#1 was used for
further analysis (abbreviated as si-CIR thereafter). CCK-8 assay
was used to evaluate the viability of T24 and SW780 cells.
Overexpressing lncRNA-CIR was demonstrated to significantly
increase the viability of T24 cells (Fig.
3B). Decreasing lncRNA-CIR by si-RNA instead markedly inhibited
the T24 cell viability compared with that in corresponding controls
(Fig. 3B). Similar results were
obtained in SW780 cells (Fig. 3C).
The invasion was further investigated and the present study
identified that overexpression of lncRNA-CIR could significantly
upregulate the invasive cell numbers of bladder cancer cells, while
decreasing lncRNA-CIR expression displayed the opposite effect
compared with that in the corresponding control group (Fig. 3D and E). These results indicated that
lncRNA-CIR promotes the viability and invasion of bladder cancer
cell lines in vitro.
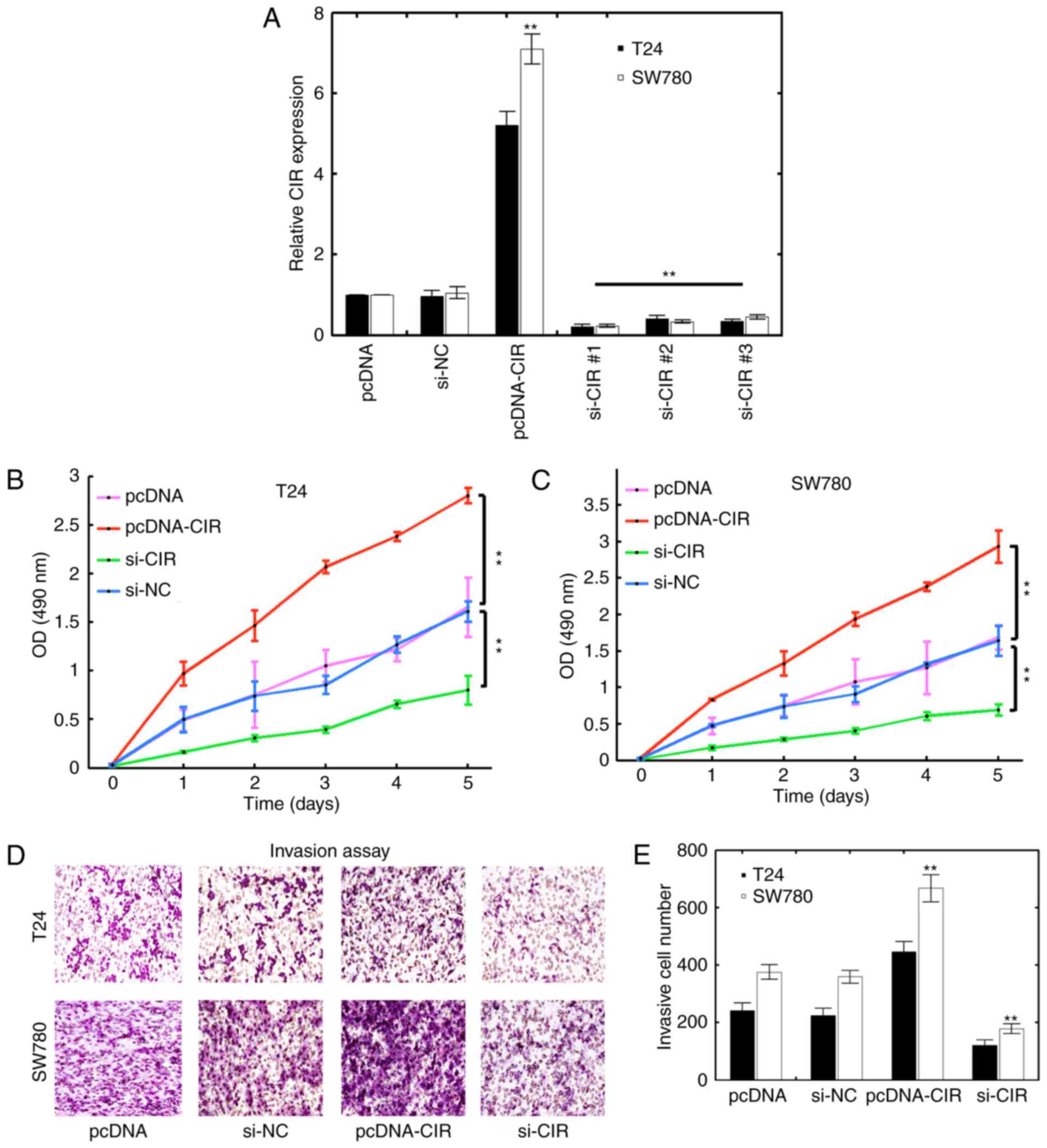 | Figure 3.Inhibitory effect of lncRNA-CIR on
bladder cancer cell lines. (A) The T24 and SW780 cells were
transfected with pcDNA, pcDNA-CIR plasmid, si-NC or three si-CIR
synthetics (si-CIR#1, si-CIR#2 and si-CIR#3). lncRNA-CIR expression
was then quantified using qPCR. One-way analysis of variance was
used among si-NC group and the different si-CIR groups
(**P<0.01, the si-CIR groups covered by the black line vs the
si-NC group). The Mann-Whitney test was used for comparison between
the pcDNA and pcDNA-CIR groups. **P<0.01, pcRNA-CIR vs. pcDNA.
The cell viability of (B) T24 and (C) SW780 cells was measured
using Cell Counting Kit-8. The T24 and SW780 cells were transfected
with pcDNA, si-NC, pcDNA-CIR or si-CIR. The Mann-Whitney test was
used across the same time points. **P<0.01. (D) Transwell
invasion assay results for T24 and SW780 cells transfected with
pcDNA, si-NC, pcDNA-CIR or si-CIR. (E) Quantification results for
(D). **P<0.01, pcDNA-CIR vs. pcDNA group and si-CIR group vs.
si-NC group. si, small interfering; NC, negative control; OD,
optical density; CIR, cartilage injury-related. |
LncRNA-CIR increases xenograft tumor
proliferation and growth
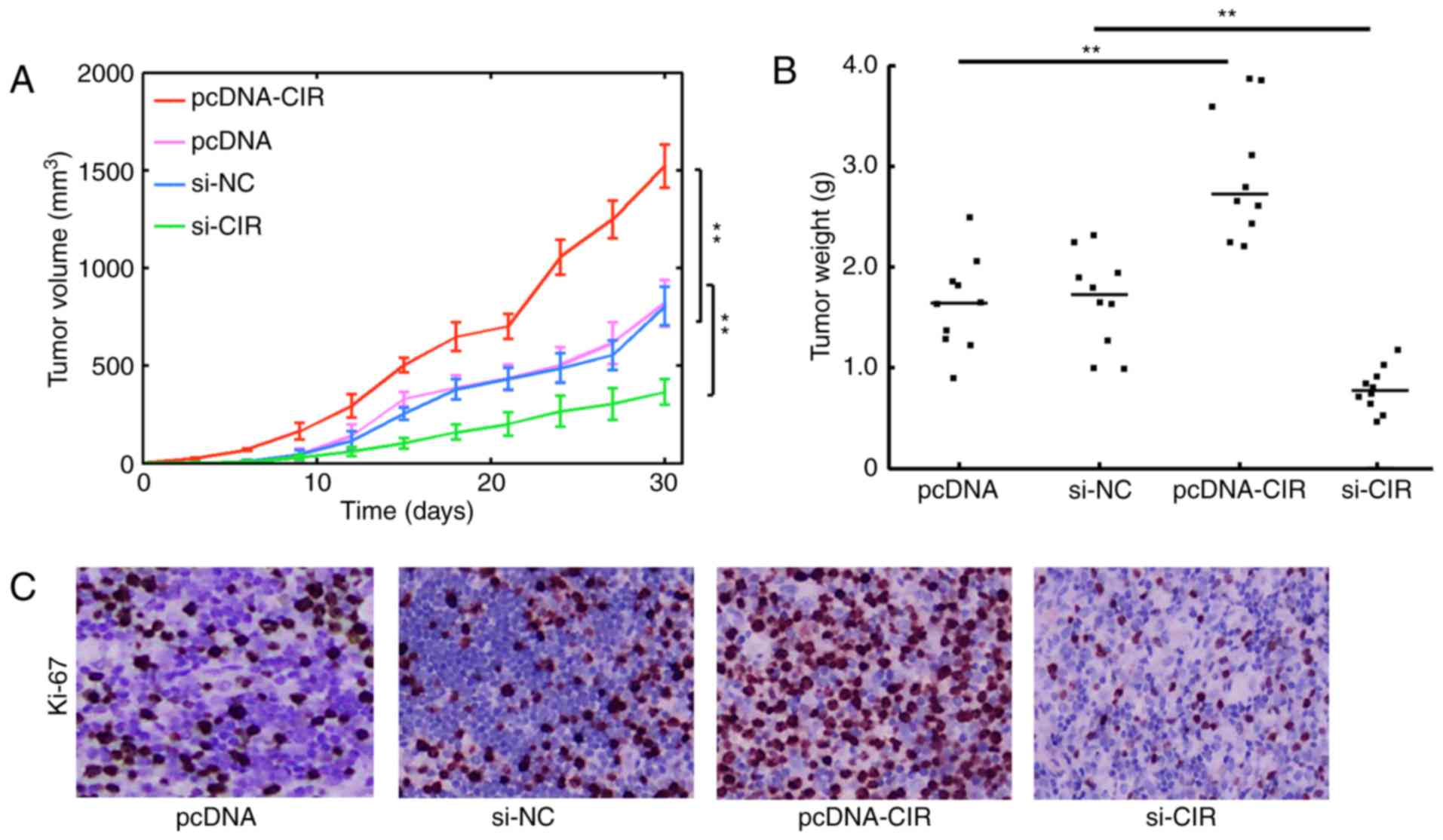
To further ascertain whether lncRNA-CIR exhibited a
tumorigenic effect in vivo, tumor implantation assays were
performed. Approximately 1×106 T24 cells were
subcutaneously injected into nude mice and the tumor volume was
monitored every 3 days. It was observed that lncRNA-CIR
overexpression substantially promoted tumor growth (Fig. 4A). However, reducing lncRNA-CIR
expression markedly inhibited tumor growth compared with that in
the control group (Fig. 4A). The
solid tumors were resected and weighed at 30 days. The tumor weight
in the lncRNA-CIR transfection group was significantly higher than
that in the control group (Fig. 4B).
Reduction in lncRNA-CIR expression by si-RNA-mediated knockdown was
demonstrated to consistently decrease tumor weight (Fig. 4B). The Ki-67 staining indicative of
cell proliferation also exhibited enhanced staining in the
pcDNA-CIR transfection group, while the proliferation was
significantly weakened with si-CIR-mediated knockdown compared with
that in the control group (Fig. 4C).
These results demonstrated that lncRNA-CIR can support tumor growth
and further implied an oncogenic role of lncRNA-CIR in
vivo.
Discussion
Due to the increasing progress in sequencing
technology, lncRNAs have been demonstrated to be associated with
the pathology of various diseases, including cancer (15). Significant advances in lncRNA research
have greatly enriched the level of understanding regarding lncRNA
profiles. An increasing number of studies have implied that lncRNAs
can exert a diverse range of functions in various types of tumors
(16,17). For example, lncRNAs may assist in
classifying subtypes in glioma, colon and breast cancer (17). BRAF-activated LncRNA may suppress
tumor development in papillary thyroid cancer (18). The lncRNA Gm15290 may instead promote
invasion and proliferation of lung cancer via interactions with
miR-615-5p (19). However, the
mechanisms by which lncRNAs are involved in bladder cancer
progression have not been fully identified. In the present study,
an unexpected function of lncRNA-CIR in the development of bladder
cancer was identified. lncRNA-CIR expression was demonstrated to be
frequently upregulated in bladder cancer tissues and cell lines
when compared with that in normal tissues and cell lines.
Meanwhile, increasing lncRNA-CIR expression was also associated
with poor survival and the malignant phenotypes of bladder cancer
cells, including advanced proliferation, migration and invasion.
The in vivo oncogenic effect for lncRNA-CIR was also
confirmed, suggesting that lncRNA-CIR may exhibit a tumorigenic
role in the present study.
A number of lncRNAs have been demonstrated to
actively participate in bladder cancer development. For example,
the lncRNA H19 is an imprinted gene and is highly associated with
the risk of bladder cancer progression (20). Furthermore, lncRNA-UCA1 promotes
bladder cancer cell invasion and migration by mediating the miR-145
pathway (21). The lncRNA-ABHD11-AS1
instead functions as a tumor suppressor lncRNA and can suppress
malignant characteristics in bladder cancer (22). A well-known lncRNA, cancer
susceptibility 2 (non-protein coding), was also shown to inhibit
the migration and proliferation of bladder cancer cells in an in
vitro study (23). The role of
lncRNA-CIR, however, has never previously been investigated in
bladder cancer, and therefore, to the best of our knowledge, the
present study has presented for the first time the tumorigenic
function of lncRNA-CIR in bladder cancer.
A limited number of studies have focused on
lncRNA-CIR, particularly in cancer-associated research. A study by
Liu et al (13) previously
identified that lncRNA-CIR can specifically promote the degradation
of chondrocyte extracellular matrix in osteoarthritis (OA). In OA
cartilage, the expression of lncRNA-CIR is highly expressed among
the 152 differentially expressed lncRNAs and regulates the
expression of matrix metalloproteinase 13 and a disintegrin and
metalloproteinase with thrombospondin motifs 5 (13). Silencing lncRNA-CIR may elevate the
induction of collagen and aggrecan, while overexpressing lncRNA-CIR
consistently raises the level of matrix-degrading enzymes (13). However, in the field of tumor biology,
the implications of lncRNA-CIR have never been revealed. Hence, the
results of the present study may broaden the understanding of
lncRNAs in cancer research.
A number of the functions of lncRNAs have not yet
been comprehensively identified. It has been argued that the RNA
products from pseudogenes can induce mRNA degradation via the
coding copies, which leads to the production of small RNAs
(24). The long antisense transcripts
generated from the pseudogenes can interact with the spliced
counterparts and form double-stranded RNAs, which will be cleaved
by Dicer (24). Therefore, the mRNAs
that can encode proteins may instead promote the formation of
RNA-induced silencing complex to consume additional copies of mRNAs
and decrease the expression of protein-coding genes (25).
The lncRNA-CIR is, however, a pseudogene product of
vimentin (13) and may possibly serve
as an siRNA to downregulate vimentin expression. Vimentin is known
as an important factor in cell stiffness, the disruption of which
may induce weakened cellular integrity and promote cell migration
or invasion (26). Hence, we
hypothesize that lncRNA-CIR may inhibit the expression of vimentin
through the aforementioned mechanism and extracellular matrix
degradation to promote the invasion of bladder cancer cells.
Whether lncRNA-CIR also presents oncogenic roles in other types of
tumor requires an in-depth investigation in the future.
There are also certain limitations in the present
study. The clinical materials assessed were from Chinese patients
only; lncRNA-CIR must be verified as a diagnostic and prognostic
biomarker in different populations prior clinical usage. Additional
multicenter studies that include diverse ethnic populations are
required. In addition, the detailed molecular mechanism of
lncRNA-CIR in bladder cancer was not identified. Additional studies
are required to unravel the underlying mechanisms of
lncRNA-CIR-mediated bladder cancer progression. Whether lncRNA-CIR
serves a role in other types of cancer remains a focus for future
studies.
In summary, the results of the present study suggest
a tumorigenic role for lncRNA-CIR in bladder cancer. The
involvement of lncRNA-CIR in bladder cancer progression may further
extend the notion that many ncRNAs are critical modulators in
diverse biological processes. Deciphering the complex codes in
lncRNA fields may assist in unraveling the intrinsic complexity in
tumor development and identifying effective therapeutics targeting
important nodes in cancer intervention.
References
|
1
|
Kolodziej A, Krajewski W, Matuszewski M
and Tupikowski K: Review of current optical diagnostic techniques
for non-muscle-invasive bladder cancer. Cent European J Urol.
69:150–156. 2016.PubMed/NCBI
|
|
2
|
D'Costa JJ, Goldsmith JC, Wilson JS, Bryan
RT and Ward DG: A systematic review of the diagnostic and
prognostic value of urinary protein biomarkers in urothelial
bladder cancer. Bladder Cancer. 2:301–317. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistic, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Madka V, Mohammed A, Li Q, Zhang Y,
Biddick L, Patlolla JM, Lightfoot S, Towner RA, Wu XR, Steele VE,
et al: Targeting mTOR and p53 signaling inhibits muscle invasive
bladder cancer in vivo. Cancer Prev Res (Phila). 9:53–62. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Metalli D, Lovat F, Tripodi F, Genua M, Xu
SQ, Spinelli M, Alberghina L, Vanoni M, Baffa R, Gomella LG, et al:
The insulin-like growth factor receptor I promotes motility and
invasion of bladder cancer cells through Akt- and mitogen-activated
protein kinase-dependent activation of paxillin. Am J Pathol.
176:2997–3006. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muto S, Horie S, Takahashi S, Tomita K and
Kitamura T: Genetic and epigenetic alterations in normal bladder
epithelium in patients with metachronous bladder cancer. Cancer
Res. 60:4021–4025. 2000.PubMed/NCBI
|
|
8
|
van Kessel KE, Van Neste L, Lurkin I,
Zwarthoff EC and Van Criekinge W: Evaluation of an epigenetic
profile for the detection of bladder cancer in patients with
hematuria. J Urol. 195:601–607. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen M, Ye Y, Zou B, Guo S, Zhou F, Lu K,
Liu J, Xu Z, Han H, Liu Z, et al: C14orf166 is a high-risk
biomarker for bladder cancer and promotes bladder cancer cell
proliferation. J Transl Med. 14:552016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hata A and Kashima R: Dysregulation of
microRNA biogenesis machinery in cancer. Crit Rev Biochem Mol Biol.
51:121–134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tani H, Imamachi N, Mizutani R, Imamura K,
Kwon Y, Miyazaki S, Maekawa S, Suzuki Y and Akimitsu N: Genome-wide
analysis of long noncoding RNA turnover. Methods Mol Biol.
1262:305–320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kit OI, Kirichenko EY, Kirichenko YG,
Novikova IA, Selyutina ON and Filippova SY: The long non-coding RNA
associated with cancerogenesis: biological significance and
perspectives of application in diagnostic. Klin Lab Diagn.
61:13–16. 2016.PubMed/NCBI
|
|
13
|
Liu Q, Zhang X, Dai L, Hu X, Zhu J, Li L,
Zhou C and Ao Y: Long noncoding RNA related to cartilage injury
promotes chondrocyte extracellular matrix degradation in
osteoarthritis. Arthritis Rheumatol. 66:969–978. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng N, Wang Y, Zheng M, Yu X, Lin H, Ma
RN, Shi O, Zheng X, Gao M, Yu H, et al: Genome-wide analysis of DNA
methylation and their associations with long noncoding RNA/mRNA
expression in non-small-cell lung cancer. Epigenomics. 2017.(Epub
ahead of print). View Article : Google Scholar
|
|
16
|
Deng H, Zhang J, Shi J, Guo Z, He C, Ding
L, Tang JH and Hou Y: Role of long non-coding RNA in tumor drug
resistance. Tumour Biol. 37:11623–11631. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Flippot R, Malouf GG, Su X, Mouawad R,
Spano JP and Khayat D: Cancer subtypes classification using long
non-coding RNA. Oncotarget. 7:54082–54093. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liao T, Qu N, Shi RL, Guo K, Ma B, Cao YM,
Xiang J, Lu ZW, Zhu YX, Li DS and Ji QH: BRAF-activated LncRNA
functions as a tumor suppressor in papillary thyroid cancer.
Oncotarget. 8:238–247. 2017.PubMed/NCBI
|
|
19
|
Dong Y, Huo X, Sun R, Liu Z, Huang M and
Yang S: LncRNA Gm15290 promotes cell proliferation and invasion in
non-small cell lung cancer through directly interacting with and
suppressing the tumor suppressor miR-615-5p. Oncol Res. 2017.(Epub
ahead of print). View Article : Google Scholar
|
|
20
|
Hua Q, Lv X, Gu X, Chen Y, Chu H, Du M,
Gong W, Wang M and Zhang Z: Genetic variants in lncRNA H19 are
associated with the risk of bladder cancer in a Chinese population.
Mutagenesis. 31:531–538. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen P, Wan D, Zheng D, Zheng Q, Wu F and
Zhi Q: Long non-coding RNA UCA1 promotes the tumorigenesis in
pancreatic cancer. Biomed Pharmacother. 83:1220–1226. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen M, Li J, Zhuang C and Cai Z:
Increased lncRNA ABHD11-AS1 represses the malignant phenotypes of
bladder cancer. Oncotarget. 8:28176–28186. 2017.PubMed/NCBI
|
|
23
|
Pei Z, Du X, Song Y, Fan L, Li F, Gao Y,
Wu R, Chen Y, Li W, Zhou H, et al: Down-regulation of lncRNA CASC2
promotes cell proliferation and metastasis of bladder cancer by
activation of the Wnt/β-catenin signaling pathway. Oncotarget.
8:18145–18153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tam OH, Aravin AA, Stein P, Girard A,
Murchison EP, Cheloufi S, Hodges E, Anger M, Sachidanandam R,
Schultz RM and Hannon GJ: Pseudogene-derived small interfering RNAs
regulate gene expression in mouse oocytes. Nature. 453:534–538.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Watanabe T, Totoki Y, Toyoda A, Kaneda M,
Kuramochi-Miyagawa S, Obata Y, Chiba H, Kohara Y, Kono T, Nakano T,
et al: Endogenous siRNAs from naturally formed dsRNAs regulate
transcripts in mouse oocytes. Nature. 453:539–543. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haudenschild DR, Chen J, Pang N, Steklov
N, Grogan SP, Lotz MK and D'Lima DD: Vimentin contributes to
changes in chondrocyte stiffness in osteoarthritis. J Orthop Res.
29:20–25. 2011. View Article : Google Scholar : PubMed/NCBI
|