Introduction
In western countries, prostate cancer is the most
common type of non-malignant skin tumor and is the leading cause of
cancer-associated mortality (1).
Castration remains the standard method for the treatment of
prostate cancer, particularly metastatic prostate cancer, however
it cannot successfully treat this disease (2). It is predicted that ~80% of patients
with prostate cancer experience a recurrence of clinical symptoms
or a change to the volume of prostate cancer following the removal
of the androgen (3). However,
following a median treatment period of 18–24 months, almost all
patients enter the hormone independent phase of prostate cancer
(4).
Cofilin is a type of actin-binding protein that
exists in eukaryotes, and has a low molecular weight (5). The Cofilin-1 gene is located on hormone
11q13 and is expressed in various non-muscular tissues,
particularly in the liver and brain (6). The progression of tumor cells occurs in
a complexed micro-environment through migration (7), by invasion into pseudopods. During the
invasion and metastasis of tumor cells, Cofilin-1 performs an
essential role in the remodeling of the actin skeleton (8). A previous study suggested that
expression levels of Cofilin-1 and changes to cellular activities
have been identified in tissues of oral squamous cell carcinoma,
renal cell carcinoma and ovarian cancer as well as in in
vitro cultured carcinoma cell lines (9).
Ursolic acid (UA) is a pentacyclic triterpenoid,
with the chemical name, molecular formula and molecular weight of
(3β)-3-Hydroxy-urs-12-en-28-oic acid,
C30H48O3 and 456, respectively
(10). UA has a wide distribution,
and exists in the form of dissociation or glycoside in Sambucus
chinensis, Folium eriobotryae, bearberry, glossy privet
fruits, plantain herbs, hawthorn, selfheal and Oldenlandia
diffusa (11). UA has low
toxicity and few side effects, and has various pharmacological
activities including anti-hepatitic, anti-tumor, anti-inflammatory,
anti-viral and reducing blood lipids (12–14).
Additional investigation into the effect of UA on the apoptosis of
prostate cancer and its possible signal transduction pathway may
provide a potential drug target for the clinical treatment of
patients with prostate cancer.
Materials and methods
Cell culture
Human prostate cancer LNCaP cells were cultured in
RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.), 100 g/ml streptomycin
and 100 U/ml penicillin and maintained at a high humidity, at 37°C
under 5% CO2.
Cell viability analysis
Cell viability was measured using an MTT assay
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). LNCaP cells were
seeded in 96-well plates at 1×104 cells/well and
incubated with varying concentrations of ursolic acid (0–80 µM,
purity ≥98.5%; Sigma-Aldrich; Merck KGaA), and incubated for 24, 48
or 72 h at 37°C. Cells incubated with 0 µM ursolic acid were used
as controls. Following the incubation, 50 µl of the MTT assay was
added into each well and the cells were incubated for an additional
4 h at 37°C. Following a 10-min wash with DMSO, the absorbance was
detected at a wavelength of 490 nm using a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Western blot analysis
LNCaP cells were seeded in 6-well plates at a
density of 1×106 cells/well (n=3), incubated with
varying concentrations of ursolic acid (0–80 µM) and incubated for
48 h at 37°C. The LNCaP cells were prepared using a Proteo JET
cytoplasmic protein extraction kit (Fermentas; Thermo Fisher
Scientific, Inc.). Protein concentration was measured using
bicinchoninic acid (BCA; Beyotime Institute of Biotechnology,
Haimen, China). Proteins (50 µg per lane) were loaded onto 10–12%
SDS-PAGE for separation by electrophoresis, transferred onto
polyvinylidene difluoride (PVDF) membranes, and blocked using TBST
(and 0.1% Tween-20) containing 5% non-fat milk. The PVDF membranes
were then incubated overnight at 4°C with the following primary
antibodies: Anti-ROCK (dilution, 1:1,000; cat. no. sc-33779);
anti-phosphorylation-PTEN (dilution, 1:1,000; cat. no. sc-101789);
anti-Cofilin-1 (dilution, 1:1,000; cat. no. sc-33779);
anti-Cytochrome c (dilution, 1:3,000; cat. no. sc-7159); and
β-actin (dilution, 1:1,000; cat. no. sc-7210) (all from Santa Cruz
Biotechnology, Inc. Dallas, TX, USA). PVDF membranes were
subsequently incubated with secondary antibody (1:2,000; cat. no.
sc-2004; Santa Cruz Biotechnology, Inc. Dallas, TX, USA) for 1 h at
37°C and were visualized using BeyoECL Plusenhanced
chemiluminescence (P0018, Beyotime Institute of Biotechnology) and
analyzed using Image_Lab_3.0 (Bio-Rad Laboratories, Inc.).
Flow cytometric analysis of
apoptosis
An Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) kit (BD Biosciences, Franklin Lakes,
NJ, USA) was used to measure apoptosis. LNCaP cells were seeded in
6-well plates at a density of 1×106 cells/well,
incubated with varying concentrations of ursolic acid (0–80 µM) and
incubated for 48 h. The cells were collected, washed twice with
cold PBS (Shanghai Yantuo Biological Technology Co., Ltd.,
Shanghai, China), and resuspended in 400 µl of binding buffer. A
total of 5 µl of Annexin V-FITC was added into each well and cells
were incubated for 10 min at 4°C in the dark. A total of 10 µl of
PI was then added and cells were cultured for an additional 5 min
in the dark. Apoptosis was analysis using FACSCalibur 7.6.1 flow
cytometry (BD Biosciences).
Analysis of caspase-3 and caspase-9
protease activity
Caspase-3 and caspase-9 protease activity was
measured using Caspase 3 Activity Assay Kit and Caspase 9 Activity
Assay Kit (Promega Corporation, Madison, WI, USA). LNCaP cells were
seeded in 96-well plates at 1×104 cells/well and
incubated with varying concentrations of UA (0–80 µM) for 48 h at
37°C. Subsequently, 100 µl of Caspase-Glo 3 or Caspase-Glo 9
reagent was added to each well and incubated at room temperature
for 2 h. Caspase-3 and caspase-9 protease activity was measured
using a TD 20/20 luminometer (Promega Corporation).
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using the statistical software SPSS version 11.0
(SPSS, Inc., Chicago, IL, USA). Statistical analysis was performed
using a one way analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
Ursolic acid suppresses cell
proliferation of prostate cancer
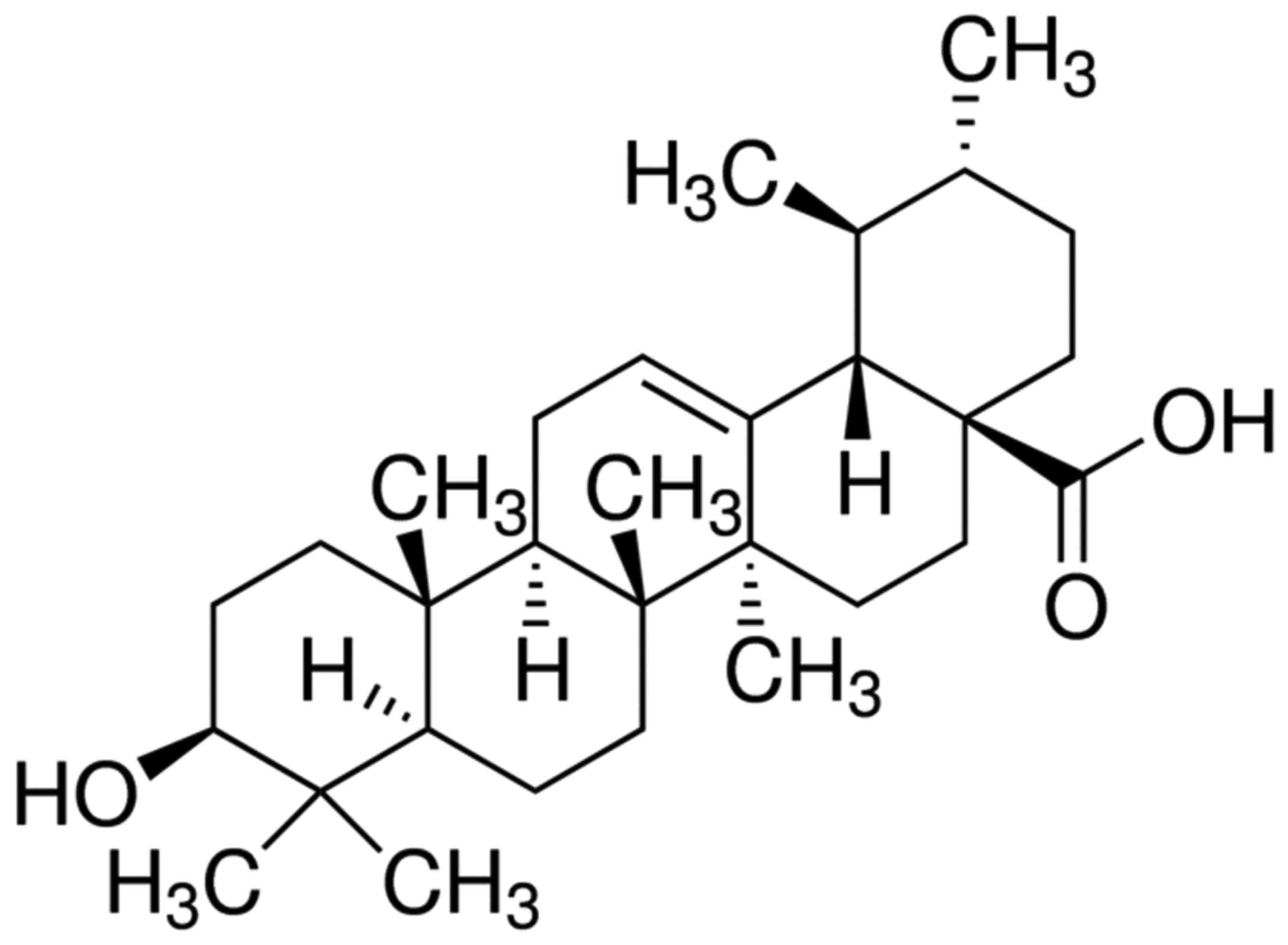
The chemical structure of ursolic acid is shown in
Fig. 1. The present study evaluated
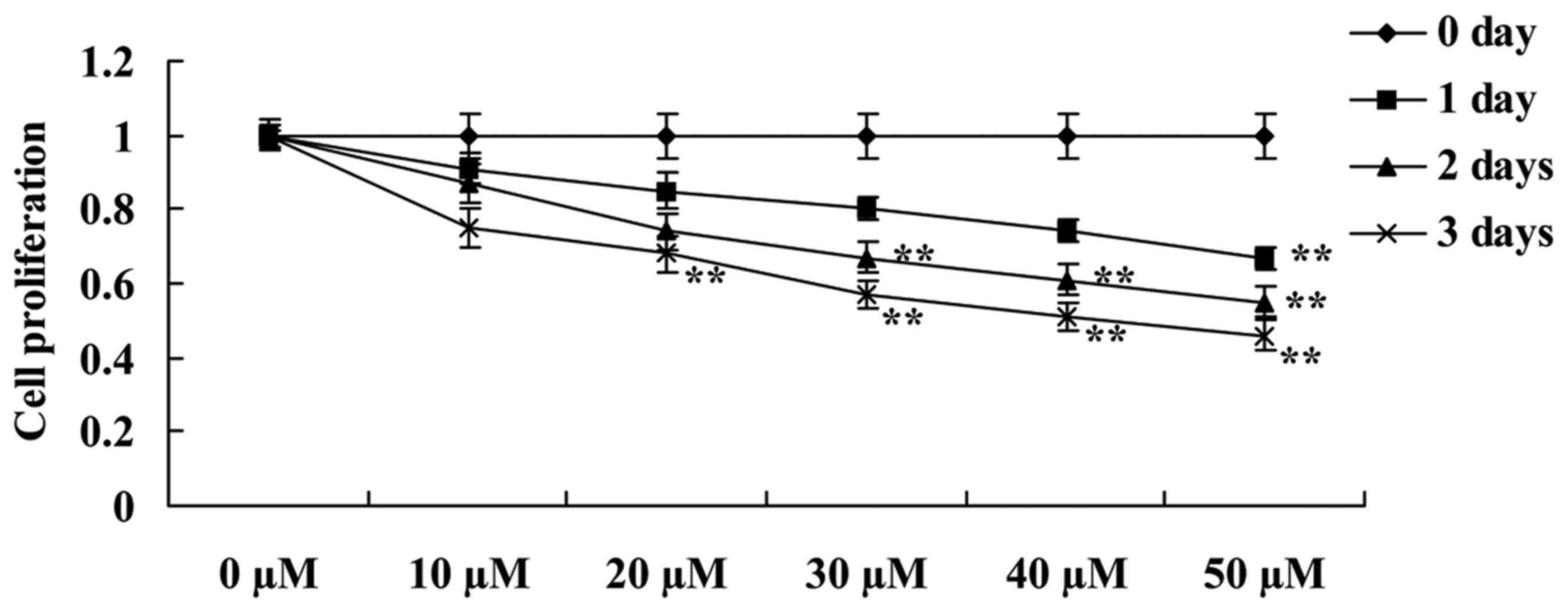
whether UA suppresses the cell proliferation of LNCaP prostate
cancer using an MTT assay. When compared with 0 µM, ursolic acid
caused a decrease in cell proliferation of LNCaP cells in a time-
and dose-dependent manner (Fig. 2).
The decrease in cell proliferation following treatments with: 50 µM
of ursolic acid for 1 day; 30–50 µM ursolic acid for 2 days; and
20–50 µM of ursolic acid for 3 days were statistically significant
(Fig. 2).
Ursolic acid activates ROCK of
prostate cancer
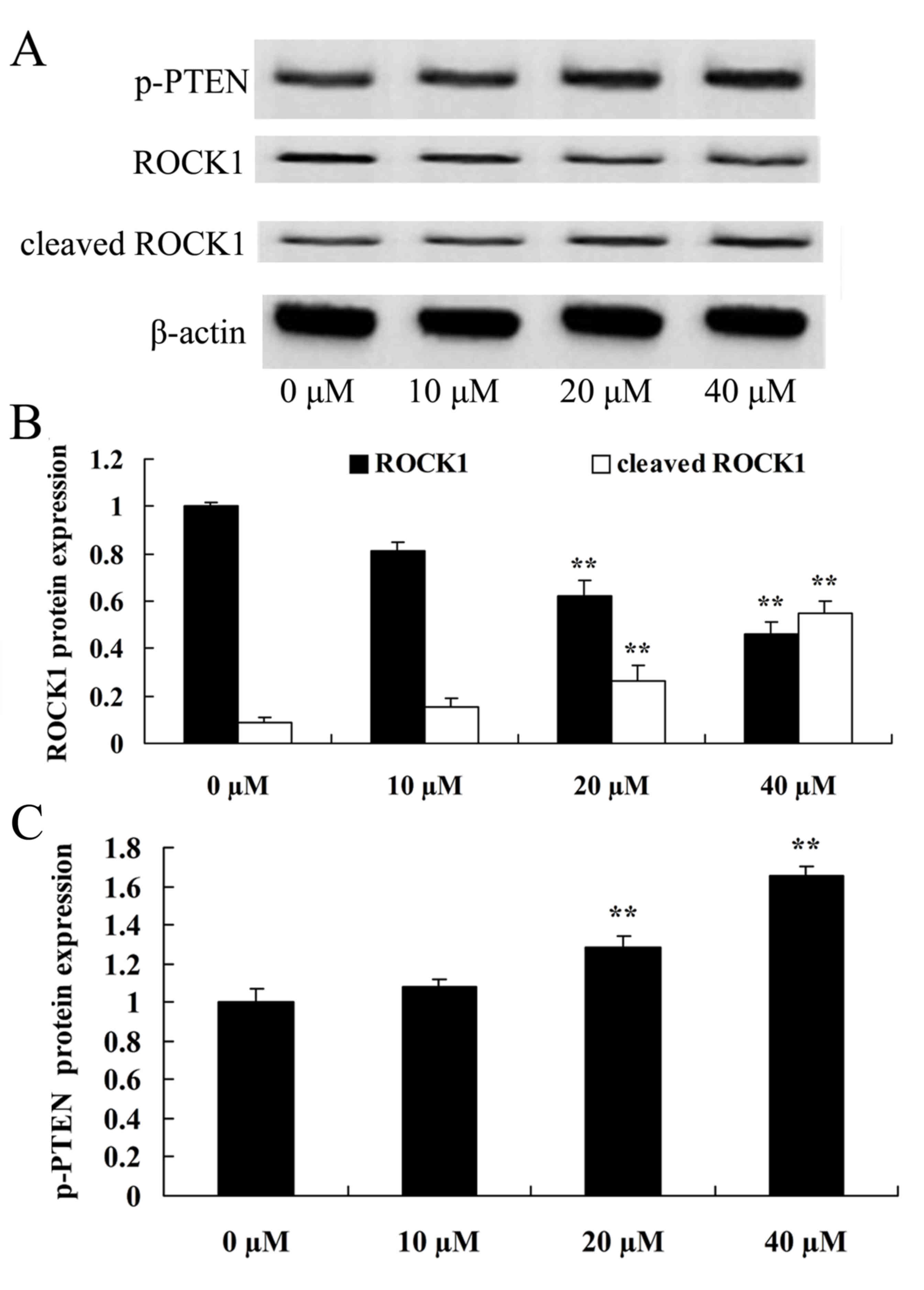
To additionally investigate the effect of UA on the
ROCK pathway of prostate cancer, the present study examined the
protein expression of ROCK and cleaved ROCK in LNCaP cells. In
comparison with the control group (0 µM ursolic acid), ROCK protein
expression in LNCaP cells was significantly reduced and cleaved
ROCK protein expression was significantly elevated in the 20 and 40
µM ursolic acid treatment groups (Fig.
3).
Ursolic acid activates PTEN of
prostate cancer
To additionally investigate whether ursolic acid
affects the PTEN pathway of prostate cancer, the present study
detected the protein expression of phosphorylated PTEN (p-PTEN) in
all the experimental groups. In comparison with the control group
(0 µM ursolic acid), the p-PTEK protein expression of LNCaP cells
was significantly promoted by 20 or 40 µM of ursolic acid (Fig. 3).
Ursolic acid activates cofilin-1 of
prostate cancer
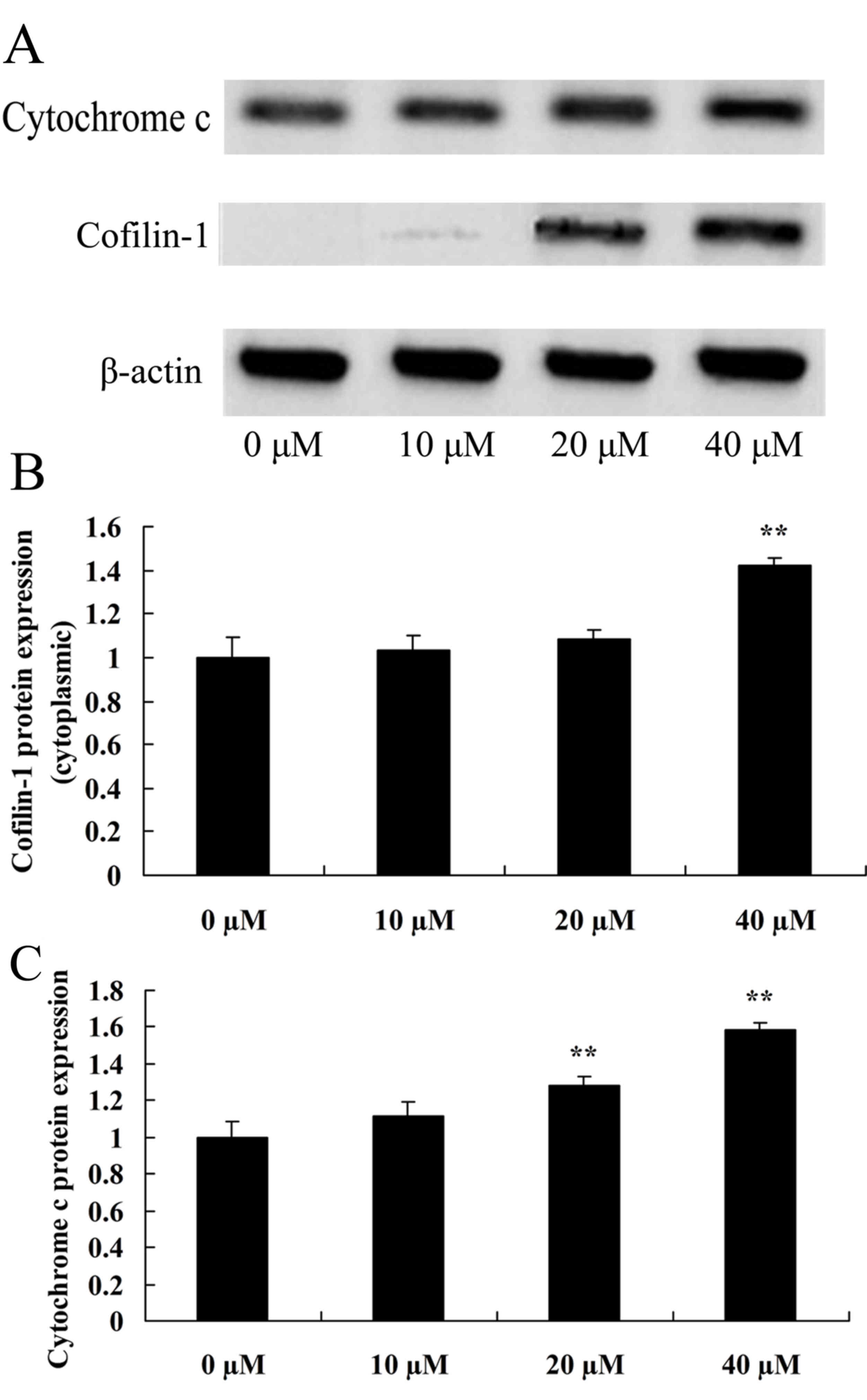
To improve the understanding of how ursolic acid
affects cofilin-1 of prostate cancer, the present study detected
cofilin-1 in LNCaP cells. Cofilin-1 protein expression in
cytoplasmic LNCaP cells was observed to be significantly enhanced
by treatment with 20 or 40 µM ursolic acid, compared with the
control group (Fig. 4).
Ursolic acid activates cytochrome c of
prostate cancer
The present study also examined the expression of
cytochrome c in LNCaP cells in order to understand how ursolic acid
affects cytochrome c in prostate cancer. As demonstrated in
Fig. 4, there was a significant
increase in cytochrome c protein expression of LNCaP cells in the
20 and 40 µM ursolic acid group in comparison with the control
group.
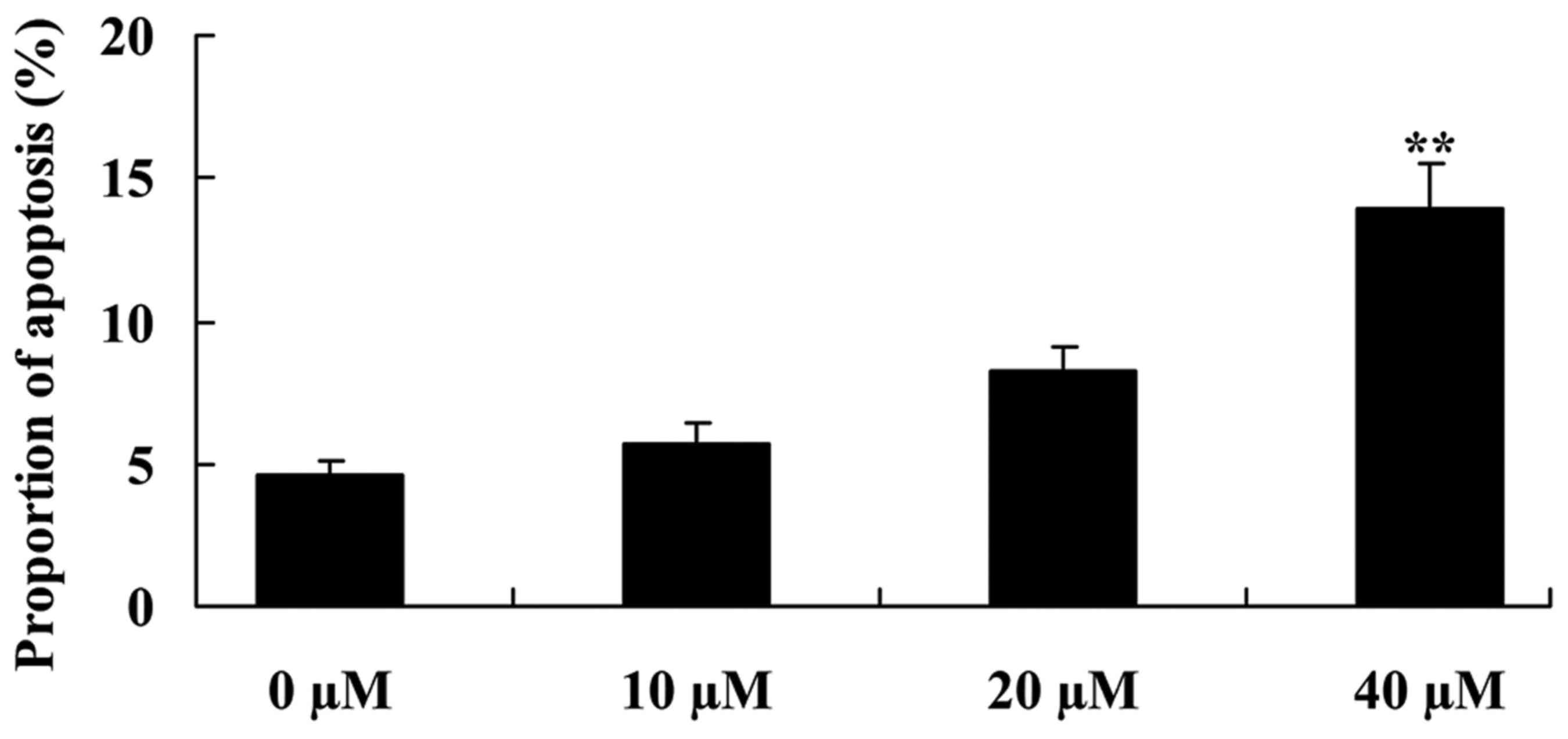
Ursolic acid activates apoptosis of
prostate cancer
The apoptosis of LNCaP cells was also investigated
in order to investigate the effect of ursolic acid on prostate
cancer cells. Fig. 5 demonstrates
that treatments with 20 and 40 µM ursolic acid significantly
induced apoptosis of LNCaP cells in comparison with the 0 µM
ursolic acid group (control).
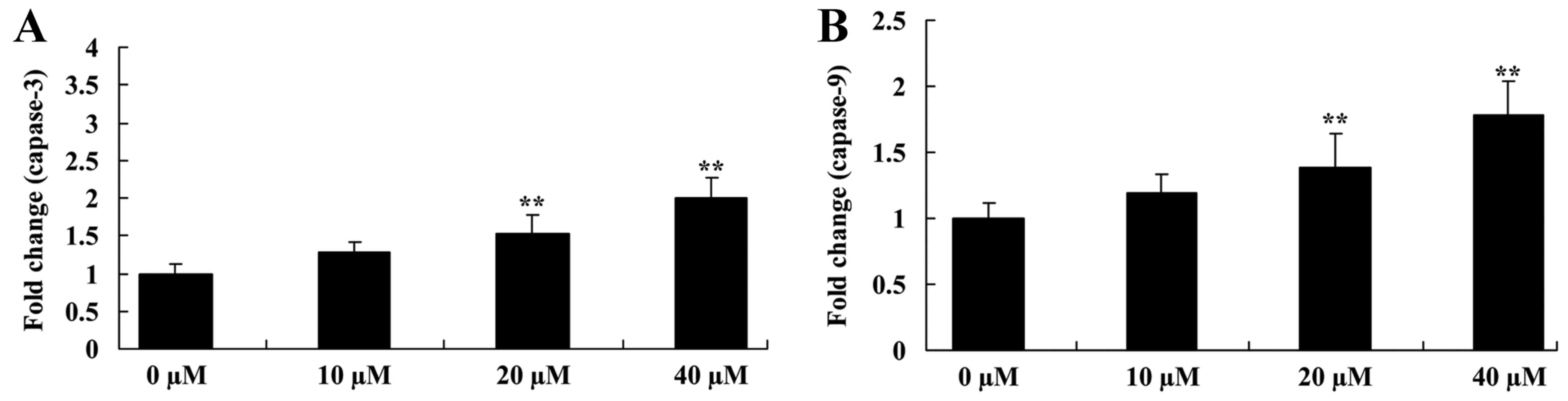
Ursolic acid activates caspase-3 and
caspase-9 activities of prostate cancer
The present study also investigated the mechanism of
apoptosis, by examining caspase-3 and caspase-9 activities of LNCaP
cells. Compared with the 0 µM ursolic acid group, caspase-3 and
caspase-9 activities of LNCaP cells were significantly increased by
the 20 and 40 µM ursolic acid treatment groups (Fig. 6).
Discussion
In western countries, prostate cancer is the most
common type of cancer for males and also the leading cause of
cancer associated mortality (3). This
is also now observed in China. Castration is an effective treatment
option for early prostate cancer patients (15). In the present study, it was observed
that ursolic acid suppressed cell proliferation and induced the
apoptosis of LNCaP cells. Park et al (16) suggested that ursolic acid induced
apoptosis in prostate cancer PC-3 cells via caspase-9 and −3. Zhang
et al (17) suggested that
ursolic acid inhibits the proliferation and promotes apoptosis in
human ovarian cancer.
By inducing actin to connect with proteins and
myosin, ROCK regulates protein phosphorylation through the
contraction of actomyosin (18). The
contraction of actomyosin is important for cell movement, and the
inhibition of ROCK activity may negatively affect the contraction
of actomyosin (19). Microtubules
perform an essential role in maintaining cell polarity and
extra-cellular transportation. The interaction between ROCK and
Diaphanous-related formin (Dia) contribute to the regulation of
cell polarity and canaliculus (20).
The present study identified that ursolic acid significantly
inhibited ROCK protein expression and elevated cleaved ROCK protein
expression in LNCaP cells. Li et al (21) suggested that ursolic acid promotes the
apoptosis of gastric cancer cells via the ROCK/PTEN pathway.
The importance of PTEN can be demonstrated by its
frequent destruction to cancer cells (21). PTEN is the first known phosphatase
which can inhibit tumor activity. In tumor cells, mechanisms which
regulate the expression and functional changes of PTEN include the
regulation of PTEN transcription, post-transcriptional regulation
of encoding RNA, modification following interpretation and protein
interactions (22). Slight changes to
the expression levels of PTEN may influence the occurrence and
progression of tumors (23). The
present study demonstrated that ursolic acid significantly promoted
p-PTEK protein expression in prostate cancer LNCaP cells. Wu et
al (24) also reported that
ursolic acid induced apoptosis in K562 cells by upregulating PTEN
gene expression and cytochrome c. Li et al (21) suggested that ursolic acid promotes the
apoptosis of gastric cancer cells through the ROCK/PTEN
pathway.
Cofilin-1 is a fundamental regulatory factor in the
invasion and metastasis of cancer cells (25). The overexpression of cofilin-1
increases the speed of tumor migration, and the inhibition of its
expression can therefore significantly reduce the invasion of tumor
cells (6,9). The present study revealed that ursolic
acid significantly enhanced cofilin-1 protein expression in
cytoplasmic LNCaP cells. Li et al (21) reported that ursolic acid promotes the
apoptosis of gastric cancer cells via ROCK/PTEN mediated cofilin-1
expression in the SGC-7901 cell line.
Cytochrome c is the control center for cell
movement, and is not only the center of cellular respiratory chains
and oxidative phosphorylation, but is also the regulatory center of
cell apoptosis (26). The release of
cytochrome c is a key step in cellular apoptosis. Under the
conditions of deoxyadenosine triphosphate (dATP), cytochrome can
combine with apoptotic protease activating factor 1 (APaf-1), which
in turn promotes the formation of polymers and also enhances the
formation of apoptosome (27).
Activated caspase-9 results in the activation of other caspases
including caspase-3. Caspase-3 triggers cascade reactions of
Caspases, which subsequently results in apoptosis (28). A new study demonstrated that ursolic
acid significantly increased the protein expression of cytochrome c
and augmented the activities of caspase-3 and caspase-9 in LNCaP
cells (28). In addition, Shyu et
al (12) suggested that ursolic
acid can induce the apoptosis of human hepatocellular carcinoma
cells via the activation of caspase-9 and caspase-3. Similarly,
Park et al (16) suggested
that ursolic acid induced apoptosis in prostate cancer PC-3 cells
through caspase-9 and caspase-3. Wu et al (24) also reported that ursolic acid induced
apoptosis following the upregulation of PTEN and cytochrome c in
K562 cells.
In conclusion, the present study demonstrates that
ursolic acid activates the apoptosis of prostate cancer, at least
in part by directly targeting ROCK/PTEN mediated mitochondrial
translocation of cofilin-1. In future studies, the authors aim to
focus on the drug development of ursolic acid for the treatment of
human prostate cancer.
References
|
1
|
Hoare D, Skinner TA, Black A and Robert
Siemens D: Serum follicle-stimulating hormone levels predict time
to development of castration-resistant prostate cancer. Can Urol
Assoc J. 9:122–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoon BI, Shin TS, Cho HJ, Hong SH, Lee JY,
Hwang TK and Kim SW: Is it effective to perform two more prostate
biopsies according to prostate-specific antigen level and prostate
volume in detecting prostate cancer? Prospective study of 10-core
and 12-core prostate biopsy. Urol J. 9:491–497. 2012.PubMed/NCBI
|
|
3
|
Ankerst DP, Till C, Boeck A, Goodman PJ,
Tangen CM and Thompson IM: Predicting risk of prostate cancer in
men receiving finasteride: Effect of prostate volume, number of
biopsy cores and american urological association symptom score.
Urology. 82:1076–1081. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yli-Hemminki TH, Laurila M, Auvinen A,
Määttänen L, Huhtala H, Tammela TL and Kujala PM: Histological
inflammation and risk of subsequent prostate cancer among men with
initially elevated serum prostate-specific antigen (PSA)
concentration in the Finnish prostate cancer screening trial. BJU
Int. 112:735–741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu B, Fukada K, Zhu H and Kyprianou N:
Prohibitin and cofilin are intracellular effectors of transforming
growth factor beta signaling in human prostate cancer cells. Cancer
Res. 66:8640–8647. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Kuramitsu Y, Ueno T, Suzuki N,
Yoshino S, Iizuka N, Zhang X, Oka M and Nakamura K: Differential
expression of up-regulated cofilin-1 and down-regulated cofilin-2
characteristic of pancreatic cancer tissues. Oncol Rep.
26:1595–1599. 2011.PubMed/NCBI
|
|
7
|
Tang Q, Ji Q, Tang Y, Chen T, Pan G, Hu S,
Bao Y, Peng W and Yin P: Mitochondrial translocation of cofilin-1
promotes apoptosis of gastric cancer BGC-823 cells induced by
ursolic acid. Tumour Biol. 35:2451–2459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li M, Yin J, Mao N and Pan L: Upregulation
of phosphorylated cofilin 1 correlates with taxol resistance in
human ovarian cancer in vitro and in vivo. Oncol Rep. 29:58–66.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu LI, Fu NI, Luo XU, Li XY and Li XP:
Overexpression of cofilin 1 in prostate cancer and the
corresponding clinical implications. Oncol Lett. 9:2757–2761. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zang LL, Wu BN, Lin Y, Wang J, Fu L and
Tang ZY: Research progress of ursolic acid's anti-tumor actions.
Chin J Integr Med. 20:72–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mazumder K, Tanaka K and Fukase K:
Cytotoxic activity of ursolic acid derivatives obtained by
isolation and oxidative derivatization. Molecules. 18:8929–8944.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shyu MH, Kao TC and Yen GC: Oleanolic acid
and ursolic acid induce apoptosis in HuH7 human hepatocellular
carcinoma cells through a mitochondrial-dependent pathway and
downregulation of XIAP. J Agric Food Chem. 58:6110–6118. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu HY, Chang CI, Lin BW, Yu FL, Lin PY,
Hsu JL, Yen CH, Liao MH and Shih WL: Suppression of hepatitis B
virus × protein-mediated tumorigenic effects by ursolic Acid. J
Agric Food Chem. 59:1713–1722. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma JQ, Ding J, Xiao ZH and Liu CM: Ursolic
acid ameliorates carbon tetrachloride-induced oxidative DNA damage
and inflammation in mouse kidney by inhibiting the STAT3 and NF-κB
activities. Int Immunopharmacol. 21:389–395. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gontero P, Marra G, Soria F, Oderda M,
Zitella A, Baratta F, Chiorino G, Gregnanin I, Daniele L, Cattel L,
et al: A randomized double-blind placebo controlled phase I–II
study on clinical and molecular effects of dietary supplements in
men with precancerous prostatic lesions. Chemoprevention or
‘chemopromotion’? Prostate. 75:1177–1186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park JH, Kwon HY, Sohn EJ, Kim KA, Kim B,
Jeong SJ, Song JH, Koo JS and Kim SH: Inhibition of
Wnt/beta-catenin signaling mediates ursolic acid-induced apoptosis
in PC-3 prostate cancer cells. Pharmacol Rep. 65:1366–1374. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Wang W, Qian L, Zhang Q, Lai D
and Qi C: Ursolic acid inhibits the proliferation of human ovarian
cancer stem-like cells through epithelial-mesenchymal transition.
Oncol Rep. 34:2375–2384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Li X, Liu KR, Zhang JN, Liu Y and
Zhu Y: Visfatin derived from ascites promotes ovarian cancer cell
migration through Rho/ROCK signaling-mediated actin polymerization.
Eur J Cancer Prev. 24:231–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sturge J, Wienke D and Isacke CM:
Endosomes generate localized Rho-ROCK-MLC2-based contractile
signals via Endo180 to promote adhesion disassembly. J Cell Biol.
175:337–347. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Richerioux N, Blondeau C, Wiedemann A,
Rémy S, Vautherot JF and Denesvre C: Rho-ROCK and Rac-PAK signaling
pathways have opposing effects on the cell-to-cell spread of
Marek's disease virus. PLoS One. 7:e440722012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li R, Wang X, Zhang XH, Chen HH and Liu
YD: Ursolic acid promotes apoptosis of SGC-7901 gastric cancer
cells through ROCK/PTEN mediated mitochondrial translocation of
cofilin-1. Asian Pac J Cancer Prev. 15:9593–9597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pabona JM, Dave B, Su Y, Montales MT, de
Lumen BO, de Mejia EG, Rahal OM and Simmen RC: The soybean peptide
lunasin promotes apoptosis of mammary epithelial cells via
induction of tumor suppressor PTEN: Similarities and distinct
actions from soy isoflavone genistein. Genes Nutr. 8:79–90. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sfakianos JP, Lin Gellert L, Maschino A,
Gotto GT, Kim PH, Al-Ahmadie H and Bochner BH: The role of PTEN
tumor suppressor pathway staining in carcinoma in situ of the
bladder. Urol Oncol. 32:657–662. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu B, Wang X, Chi ZF, Hu R, Zhang R, Yang
W and Liu ZG: Ursolic acid-induced apoptosis in K562 cells
involving upregulation of PTEN gene expression and inactivation of
the PI3K/Akt pathway. Arch Pharm Res. 35:543–548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Atefi M, Avramis E, Lassen A, Wong DJ,
Robert L, Foulad D, Cerniglia M, Titz B, Chodon T, Graeber T, et
al: Effects of MAPK and PI3K pathways on PD-L1 expression in
melanoma. Clin Cancer Res. 20:3446–3457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Banafa AM, Roshan S, Liu YY, Chen HJ, Chen
MJ, Yang GX and He GY: Fucoidan induces G1 phase arrest and
apoptosis through caspases-dependent pathway and ROS induction in
human breast cancer MCF-7 cells. J Huazhong Univ Sci Technolog Med
Sci. 33:717–724. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Balachandran C, Sangeetha B, Duraipandiyan
V, Raj MK, Ignacimuthu S, Al-Dhabi NA, Balakrishna K, Parthasarathy
K, Arulmozhi NM and Arasu MV: A flavonoid isolated from
Streptomyces sp. (ERINLG-4) induces apoptosis in human lung cancer
A549 cells through p53 and cytochrome c release caspase dependant
pathway. Chem Biol Interact. 224:24–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li HH, Su JH, Chiu CC, Lin JJ, Yang ZY,
Hwang WI, Chen YK, Lo YH and Wu YJ: Proteomic investigation of the
sinulariolide-treated melanoma cells A375: Effects on the cell
apoptosis through mitochondrial-related pathway and activation of
caspase cascade. Mar Drugs. 11:2625–2642. 2013. View Article : Google Scholar : PubMed/NCBI
|