Introduction
Gastric cancer is the fourth most common malignancy
worldwide and has a high mortality rate (2008) (1). In 2012, there were 951,600 new cases of
stomach cancer and 723,100 mortalities (2). Due to the absence of typical symptoms at
an early stage of the disease, the majority of patients are
diagnosed at advanced stages and lose the opportunity for surgery.
Therefore, effective adjuvant chemotherapy is important in in the
treatment of gastric cancer, as well as early diagnosis. However,
multidrug resistance may lead to failure of chemotherapy, and the
mechanism underlying this is complex (3). Drug resistance affects the long-term
effectiveness of therapeutics for patients with cancer, and
therefore, identifying a way to solve this problem is of great
importance.
The phosphatidylinositol-3-kinase (PI3K)/protein
kinase B (Akt) signaling pathway has been frequently reported to be
involved in human cancer (4). Once
activated, PI3K, a lipid kinase, phosphorylates
phosphatidylinositol-4, 5-trisphosphate (PIP2) to form
phosphatidylinositol-3, 4, 5-trisphosphate (PIP3).
PIP3, a secondary messenger, transfers Akt to the plasma
membrane in order to be phosphorylated, activated and act on
downstream signal transduction pathways, which result in cell
proliferation and continuation of survival. Constitutive activation
of the PI3K/Akt signaling pathway leads to maintenance of cell
survival and uncontrolled proliferation, which then results in
cancer. On the other hand, the PI3K/Akt signaling pathway is also
activated when chemotherapy is administered, which leads to drug
resistance (5). In vitro and
in vivo studies have indicated that the inhibition of the
PI3K/Akt signaling pathway leads to increases in sensitivity to
drugs; for example, this increases the sensitivity of ovarian
cancer cells to cisplatin and gastric cancer to vincristine
(6,7).
The phosphatase and tensin homolog (PTEN)
gene is an anti-oncogene identified in 1997, and mutations in
PTEN have been identified in a number of types of cancer
(8). PTEN has protein and lipid
kinase activity, and dephosphorylates PIP3 through lipid
kinase activity, resulting in inhibition of the activation of Akt
and the promotion of anticancer effects (9). Like multiple other proteins, there are
various ways of regulating the activity of PTEN, among which
phosphatidylinositol 3,4,5-trisphosphate RAC exchanger 2a (P-REX2a)
is a novel downregulator. P-REX2a is a RAC-GTP guanine nucleotide
exchange factor with a structure similar to
phosphatidylinositol-3,4,5-trisphosphate dependent Rac exchange
factor 1, and inhibits the activation of PTEN by binding directly
with PTEN (10,11). The P-REX2a gene is located in
8q13.2, and P-REX2a amplification is frequently observed in
lung, prostatic, colon and ovarian cancer. Furthermore, P-REX2a
mRNA is often amplified in lung, prostatic, glioma and pancreatic
cancer, and may also participate in the tumorigenesis of breast
cancer (10).
As a first-line drug in cancer therapy, resistance
to doxorubicin has arisen, and this has drawn global attention. The
PI3K/Akt signaling pathway may be activated and promote resistance
to doxorubicin in gastric cancer cells (12). However, increased expression of PTEN
may reverse this resistance (12,13).
Considering that P-REX2a is able to bind with and
inactivate PTEN, the expression of P-REX2a was knocked down in
doxorubicin-treated SGC7901 gastric cancer cells, in order to
investigate the association between P-REX2a expression and
resistance to doxorubicin.
Materials and methods
Cancer tissues and cells
All 38 gastric cancer cases were obtained from the
People's Hospital of Three Gorges University (Yichang, China) from
February 2014 to February 2016, and included 26 males and 12
females (age range, 36–85 years; average age, 61.7). All patients
received surgery without preoperative chemotherapy or
immunotherapy, and diagnosis was by pathological examination for
gastric adenocarcinoma. All patients provided written informed
consent. Ethical approval was obtained from the Ethics Committee at
the People's Hospital of Three Gorges University (Yichang, China).
A total of two tissue blocks were obtained from each patient. In
addition to the cancer tissues, normal tissues from the edge of the
cancer tissues were obtained. Each tissue block of a thickness
about 1.5 mm was split into three parts, with one sample fixed in
4% formaldehyde at room temperature for 24 h and another two
samples stored at −80°C.
The human moderately-differentiated gastric
adenocarcinoma cell line SGC7901 was used for the present study. It
was purchased from the Cell Resource Center of iCell Bioscience,
Inc. (Shanghai, China). The cells were cultured in a humidified
incubator at 37°C supplemented with 5% CO2, and the
culture medium used was Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc. Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin.
The cells were subcultured every two days, and cells in the
logarithmic growth phase were used for subsequent experiments.
Drugs and antibodies
Doxorubicin (Adriamycin) was purchased from Selleck
Chemicals (Houston, TX, USA). Doxorubicin was dissolved in DMSO
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) to make a stock
solution (10 mmol/l) and stored at −20°C. The stock solution was
further diluted with culture media of DMEM containing 10% fetal
bovine serum to a final working solution of 0.3 µM in culture
plates. Primary antibodies against PTEN (1:1,000; cat. no. CST
9188P), Akt (1:1,000; cat. no. CST 4691P), phosphorylated (p-)Akt
(S473; 1:1,000; cat. no. CST 4060P), BCL2 associated X, apoptosis
protein (Bax; 1:1,000; cat. no. CST 2772S) and B cell lymphoma-2
(Bcl-2; 1:1,000; cat. no. CST 2870P) were purchased from Cell
Signaling Technology, Inc., (Danvers, MA, USA), and P-REX2a
(1:1,000; cat. no. ab169027) was purchased from Abcam (Cambridge,
UK). Primary antibodies against β-actin (1:200; cat. no. BM0627)
and secondary antibodies, goat anti-rabbit (1:5,000; cat. no.
BA1054) and goat-anti-mouse (1:5,000; cat. no. BA1051) were
purchased from Wuhan Boster Biological Technology, Ltd., (Wuhan,
China).
P-REX2a-small interfering (si)RNA
transfection and drug treatment
A total of three P-REX2a-siRNA sequences were
designed and synthesized by Suzhou GenePharma Co., Ltd. (Suzhou,
China), from which two sequences were selected for the interference
experiment. The sequences used were as follows: P-REX2a-Homo-883
(siRNA1) forward, 5′-GGCAUCUACAGAUGGACAUTT-3′ and reverse,
5′-AUGUCCAUCUGUAGAUGCCTT-3′; P-REX2a-Homo-262 (siRNA2) forward,
5′-CAUCCUUGCAGUACAUAAATT-3′ and reverse,
5′-UUUAUGUACUGCAAGGAUGTT-3′ and negative control forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse, 5′-ACGCACGUUCGGAGAATT-3′.
The cells (2×105) were seeded in each well of the 6-well
plate 24 h prior to transfection, and the original culture medium
was replaced with serum-free and antibiotic-free DMEM 2 h prior to
the transfection. The liposome-mediated method (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), was used according to
an optimized version of the supplier's (Thermo Fisher Scientific,
Inc.) protocol for transfection. P-REX2a-siRNA or negative control
(NC)-siRNA (4.0 µg) was diluted with serum-free and antibiotic-free
DMEM 250 µl, and Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) was also diluted with serum-free and
antibiotic-free DMEM in another two tubes, then the siRNA solutions
were mixed with Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) solutions respectively. The solution was
incubated at room temperature for 20 min and was then added to the
cells, and the swapped serum-free and antibiotic-free DMEM with
DMEM with 10% FBS 6 h later. The cells were divided into four
treatment groups as follows: Cells cultured under normal conditions
(NM, cells were untransfected), NC-siRNA-transfected cells, and
cells transfected with siRNA1 or siRNA2. The cells were treated
with 0.3 µM doxorubicin at 37°C for 24 h following transfection for
48 h.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR is a common method to detect mRNA (14), the present study repeated the
experiment in triplicate. Total RNA was extracted with TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and the
concentration was measured with the Nanodrop 2000 spectrophotometer
(NanoDrop Technologies; Thermo Fisher Scientific, Inc.). The
concentration of the RNA samples was adjusted to ~1 µg/µl with
RNase-free water. Total RNA (1 µg) was used in a 20-µl reaction
volume to perform reverse transcription with virus reverse
transcriptase (Takara Bio, Inc., Otsu, Japan) according to the
manufacturer's protocol. A total of 2 µl reverse transcription
product, 10 µl 2X SYBR mixture (Takara Bio, Inc.), 1.6 µl upstream
and downstream primers (10 µM) and 6.4 µl DNase-RNase-free water
was used for qPCR. The reaction was performed on the FCX96
Real-time PCR system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA), with 1 cycle of initial denaturation at 95°C for 30 sec,
followed by 40 cycles of denaturation at 95°C for 15 sec, annealing
and extension at 55°C for 30 sec. The fluorescence signals were
captured at the extension phase of each cycle. The primer sequences
were as follows: P-REX2a, forward 5′-TGGGAGGGGTCCAACATCA-3′ and
reverse, 5′-TCTTCAACCGTCTGTGTTTTCTT-3′; GADPH forward,
5′-GCCAAAAGGGTCATCATCTC-3′ and reverse,
5′-GTAGAGGCAGGGATGATGTTC-3′.
Western blot analysis
Every sample repeated twice. Clinical tissue samples
and cells collected were incubated with radioimmunoprecipitation
assay buffer (cat no. P0013B; Beyotime Institute of Biotechnology,
Haimen, China) and a protease inhibitor cocktail (1:100; cat no.
ST506; Beyotime Institute of Biotechnology) or phosphatase
inhibitors (cat no. S1873; Beyotime Institute of Biotechnology).
The protein samples were incubated on ice for 30 min, and then
centrifuged at 10,000 × g at 4°C for 8 min, supernatants were then
extracted and quantified using the bicinchoninic acid assay method.
Equal quantities of protein samples (~60 µg) were electrophoresed
on 10% polyacrylamide gels and electroblotted onto a polyvinylidene
difluoride membrane (EMD Millipore, Billerica, MA, USA) using a
semidry transfer system (Bio-Rad Laboratories, Inc.). The membrane
was blocked in TBST (containing 0.05% Tween-20) with 5% non-fat
milk for 1 h at room temperature. Primary antibodies were diluted
in TBST with 5% non-fat milk, according the manufacturer's
protocol, and the membrane was incubated with the primary
antibodies overnight at 4°C. The membranes were subsequently rinsed
four times with TBST (5 min per wash), and the appropriate
secondary antibodies were added for incubation at room temperature
for 2 h. Antibody staining was visualized using enhanced
chemiluminescence reagents (Thermo Fisher Scientific, Inc.) and
developed with exposure to X-ray films. β-actin served as an
internal reference. The absorbance values were analyzed using
BandScan software (version 5; Glyko Biomedical, Ltd., Toronto, ON,
Canada). The ratio to reference absorbance value for each protein
was measured for comparison.
MTT assay
The cells were cultured in 24-well plates at 60%
confluence. The cells were divided into four groups and transfected
as aforementioned. The cells were cultured for 24, 48 or 72 h,
respectively. A total of 5 wells were used for each treatment group
and 100 µl MTT solution was added to each well, followed by
incubation for an additional 4 h at 37°C. The culture medium was
subsequently removed, and 1,500 µl DMSO was added. The cells were
suspended with trypsin and 200 µl of the suspension was transferred
to a 96-well plate. The optical density (OD) was measured at 570 nm
with a spectrophotometer.
Annexin V-fluorescein isothiocyanate
(FITC) apoptosis assay
SGC7901 cells were cultured in 6-well plates at 60%
confluence. The cells in the four treatment groups were transfected
as aforementioned. The cells were treated with 0.3 µM doxorubicin
for 24 h at 37°C after following transfection for 48 h. The
apoptosis assay was performed according to the manufacturer's
protocol, using an Annexin V-FITC and propidium iodide (PI) double
staining apoptosis detection kit with flow cytometry (BD
Biosciences, Franklin Lakes, USA). The protocol was as follows:
Cells were collected and washed twice with PBS, centrifuged at 900
× g at 4°C for 5 min and resuspended with 500 µl Annexin binding
buffer. Cells were incubated with 5 µl Annexin V-FITC solution for
5 min at room temperature. PI (5 µg/ml, 5 µl) was added and
incubated for a further 5 min at room temperature. Cell suspensions
were analyzed with flow cytometry (FACSCalibur; CellQuest Pro
software, version 5.1; BD Biosciences).
Detection of enzymatic activity of
PTEN
SGC7901 cells were cultured in 6-well plates at 60%
confluence. The cells in the four treatment groups were transfected
as aforementioned. The cells were treated with 0.3 µM doxorubicin
for 24 h following transfection for 48 h. The detection of PTEN
enzymatic activity was performed by cell PTEN activity colorimetric
assay kit (cat. no. GMS50064.1; Shanghai Genmeds Scientific, Inc.,
Wilmington, DE, USA) and a spectrophotometer. According to the
manufacturer's protocol, the cells were collected using
phosphate-buffered saline (reagent A in the aforementioned kit) and
cell scraper, and protein was obtained using a protein extraction
lysate (reagent B in the kit). A standard curve was constructed,
and the samples were prepared following the manufacturer's
protocol. The OD was measured at 660 nm using a spectrophotometer
and the concentration of phosphorus was calculated.
Statistical analysis
All the data are presented as the mean ± standard
deviation of at least three independent experiments. Statistical
analysis was performed with SPSS 22.0 statistical software (IBM
Corp., Armonk, NY, USA) using independent Student's t-tests or
one-way analysis of variance followed by Fisher's least significant
difference tests. Graphs were constructed using GraphPad Prism 5.0
software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
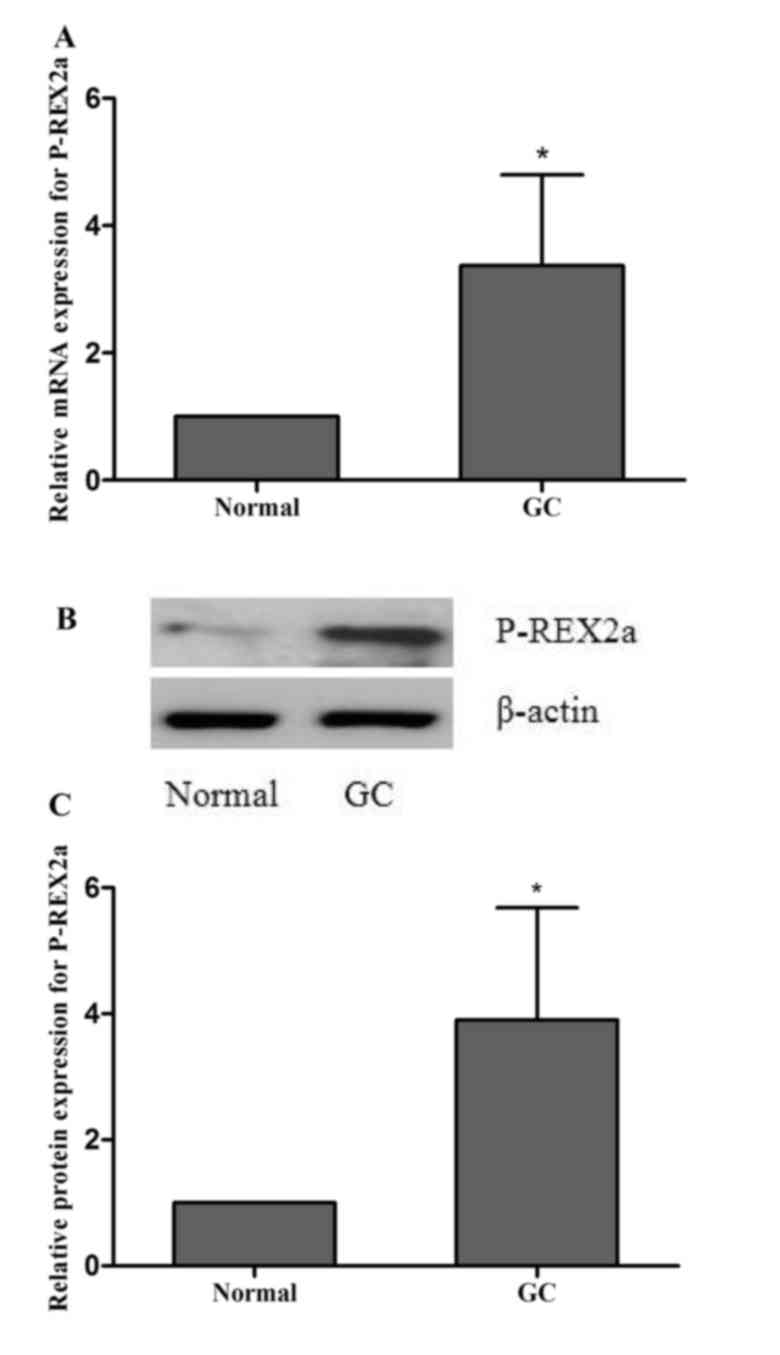
Expression of P-REX2a in clinical
gastric cancer cases
Normal and cancer tissues were separated from each
case. P-REX2a mRNA expression was detected by RT-qPCR. The results
revealed that the P-REX2a mRNA expression was significantly higher
in the cancer tissue group compared with the normal tissue group
(P<0.05; Fig. 1A). Western
blotting was performed to detect the protein expression of P-REX2a,
and the results indicated that P-REX2a protein expression
significantly increased in the cancer tissue group compared with
the normal tissue group (P<0.05; Fig.
1B), which was consistent with the results from RT-qPCR.
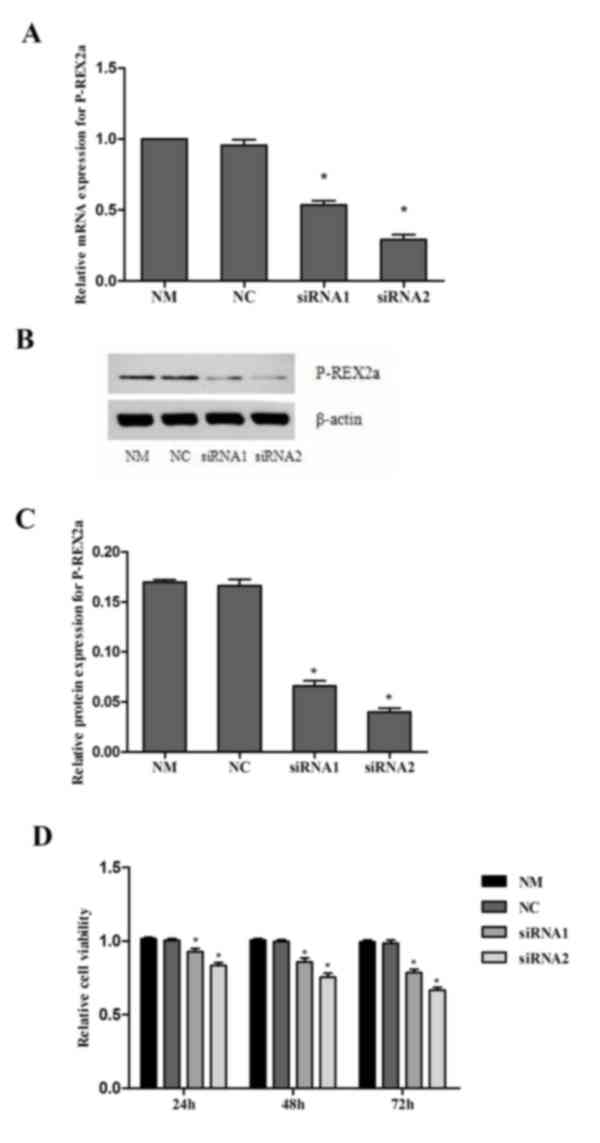
Knock down of P-REX2a inhibits the
proliferation of SGC7901 cells
The expression of P-REX2a was knocked down in
SGC7901 cells by RNA interference. RT-qPCR and western blotting
were performed to confirm the efficiency of P-REX2a siRNA knockdown
(Fig. 2A-C). The analysis revealed
that transfection with siRNA1 and siRNA2 was able to markedly
inhibit P-REX2a expression in SGC7901 cells compared with the NM
group, particularly transfection with siRNA2. However, no marked
changes were observed in the NC group, where the cells were
transfected with NC-siRNA. These results indicated that the siRNA1
and siRNA2 used in the present study were able to efficiently
silence the P-REX2a expression of SGC7901 cells.
The MTT results revealed that, compared with the NM
and NC group, the proliferation of SGC7901 cells in the siRNA1 and
siRNA2-transfceted groups was significantly reduced (P<0.05),
and lower activity was observed in the siRNA2-transfected group,
which suggested that the inhibition of P-REX2a expression may
inhibit the proliferation of SGC7901 cells. When comparing the
inhibition of proliferation in the NC group with that of the NM
group, there were no significant differences (Fig. 2D).
Knock down of P-REX2a increases the
sensitivity of SGC7901 cells to doxorubicin in vitro
The cells were treated with 0.3 µM doxorubicin for
another 24 h following transfection for 48 h. The rate of apoptosis
was determined with an Annexin V/PI double-dye apoptosis kit. The
expression of Bax and Bcl-2 was detected using western blotting.
Compared with the NM group, there were no significant changes in
the NC group. By contrast, the rate of apoptosis was significantly
increased in the siRNA1 and siRNA2-transfected groups compared with
the NM group (P<0.05). However, there were no significant
changes between the siRNA1 and siRNA2-transfected groups (Fig. 3A and B). Furthermore, the expression
of Bax significantly increased in the siRNA1 and siRNA2-transfected
groups compared with the NM and NC groups, while the expression of
Bcl-2 was reduced (P<0.05; Fig. 3C and
D).
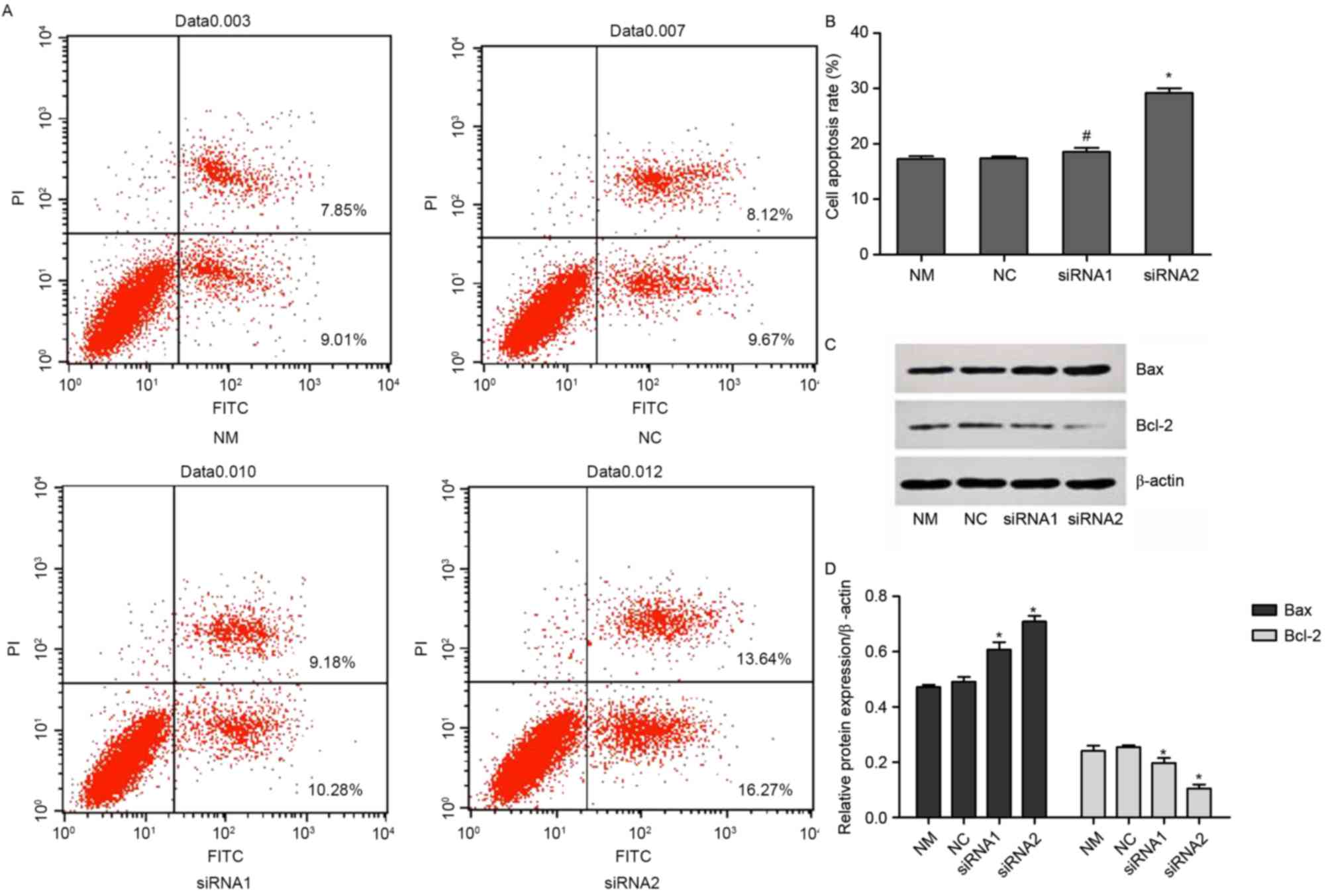 | Figure 3.Rate of apoptosis of P-REX2a knocked
down cells. The cells were treated with 0.3 µM doxorubicin for 24 h
and then transfected for 48 h. (A) Apoptosis was analyzed using an
Annexin V/propidium iodide double-dye apoptosis kit with (B)
quantification. (C). The levels of Bax and Bcl-2 expressions were
analyzed by western blotting with (D) quantification. Values are
represented as the mean ± standard deviation (n=3). *P<0.05 vs.
another siRNA group and NM group. NM, normal cultured cells; NC,
negative control; Bcl-2, B-cell lymphoma 2; Bax, BCL2 associated X,
apoptosis regulator; FITC, fluorescein isothiocyanate; P-REX2a,
phosphatidylinositol 3,4,5-trisphosphate RAC exchanger 2a; siRNA,
small-interfering RNA; NM, normal cultured cells. |
Changes in the PTEN/PI3K/Akt signaling
pathway
The cells were treated with 0.3 µM doxorubicin for
another 24 h following transfection for 48 h. Western blotting was
performed to detect the protein expression of PTEN, Akt and p-Akt
(S473). PTEN enzymatic activity was determined using a kit with
PIP3 substrate. Independent experiments were performed
at least three times. The results revealed that there were no
significant changes in the expression of total PTEN and total AKT
between all the treatment groups, but p-Akt expression was reduced
significantly in the siRNA1 and siRNA2-trasnfected groups compared
with the NM and NC groups (P<0.05; Fig. 4A and B). PTEN enzymatic activity
increased significantly in the siRNA1 and siRNA2-transfected groups
compared with the NM and NC groups (P<0.05; Fig. 4C).
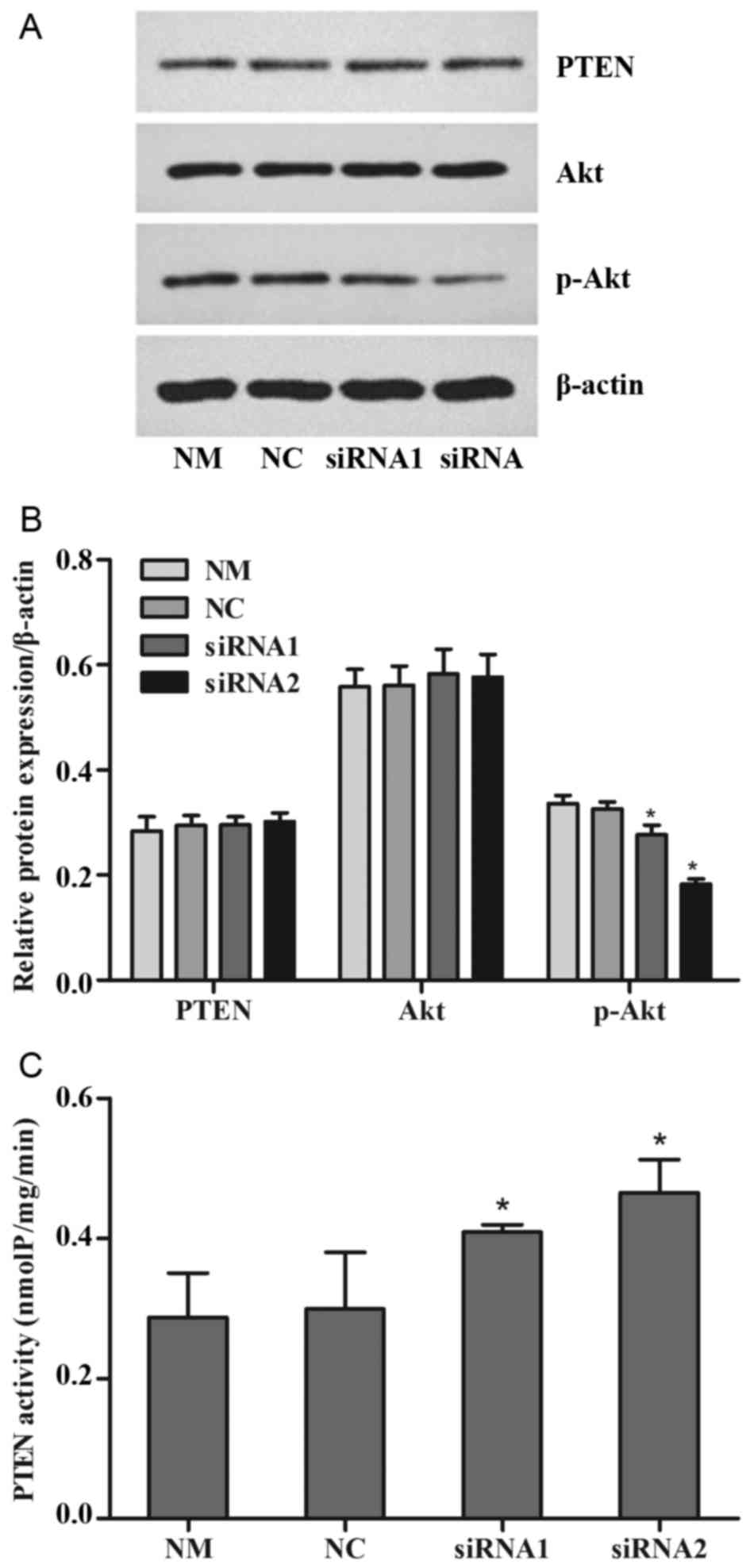 | Figure 4.Levels of PTEN expression and
activity. The cells were treated with 0.3 µM doxorubicin for 24 h
following transfection for 48 h. (A) The expression of PTEN, Akt
and p-Akt were detected by western blotting, with (B)
quantification. (C) The enzymatic activity of PTEN was analyzed
with a PIP3 substrate-based kit. Values are represented as the mean
± standard deviation (n=3). *P<0.05 vs. another siRNA group and
NM group. p-, phosphorylated; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; PTEN, phosphatase and
tensin homolog; P-REX2a, RAC exchanger 2a; siRNA, small-interfering
RNA; NM, normal cultured cells; NC, negative control cells. |
Discussion
Gastric cancer is one of the most common malignant
tumors worldwide. A lack of typical symptoms present at the early
stage of the disease leads to patients being frequently diagnosed
at advanced stages of the disease, resulting in a poor prognosis
(14). Surgery is sufficient to cure
a patient at the early stage of the disease. However, prognosis is
dependent on the efficacy of chemotherapy in conjunction with
surgery at advanced stages (15).
Chemotherapy is vital for improving the prognosis of
gastric carcinoma in general. However, resistance to
chemotherapeutics in gastric cancer has become more common in the
clinic, lowering the efficacy of chemotherapy (16). Doxorubicin is one of the classical
drugs with reported cases of resistance against it. Furthermore,
the PI3K/Akt signaling pathway has been implicated in the majority
of cases of resistance (17–20).
P-REX2a is a guanine-nucleotide exchange factor
(GEF) for Rac (21), which binds to
PTEN directly and inhibits its phosphatase activity, resulting in
the accumulation of PIP3 in vitro (10). The activation of PI3K results in the
conversion of PIP2 into PIP3, which as a
second messenger activates Akt, resulting in cell proliferation and
differentiation. However, the lipid phosphatase activity of PTEN
dephosphorylates PIP3 to PIP2 (9). High expression of P-REX2a has been
reported in a number of types of cancer. However, to the best of
our knowledge, only one report to date has investigated P-REX2a
expression in gastric cancer (22).
Therefore, high expression of P-REX2a may promote cell
proliferation and differentiation. In the present study, high
expression of P-REX2a was confirmed in gastric cancer tissues. As
>95% cases of gastric cancer are adenocarcinomas, the SGC7901
cell line was selected for the present study. The results of the
MTT assay, which assessed cell viability following the knockdown of
P-REX2a, indicated that P-REX2a expression was able to promote
proliferation in SGC7901 gastric cancer cells.
The PI3K/Akt signaling pathway has often been
reported to be activated in chemotherapy, and inhibiting the
pathway may reverse drug resistance to a certain degree, including
resistance to doxorubicin in gastric cancer (12,23,24). A
previous study by our group on the association between doxorubicin
and the PI3K/Akt signaling pathway in gastric cancer has indicated
that, following treatment of SGC7901 cells with 0.3 µM doxorubicin
and a PI3K/Akt pathway inhibitor for 24 h, a marked increase in
apoptosis and alterations in protein levels were detected (17).
Activation of the PI3K/Akt signaling pathway
inhibits apoptosis, which leads to a low chemotherapy efficiency
(12). P-REX2a participates in the
regulation of PTEN/PI3K/Akt, which may be associated with drug
resistance. In the present study, SGC7901 cells were treated with
doxorubicin, and the rate of apoptosis increased when P-REX2a was
knocked down. Bcl-2 and Bax are anti-apoptotic and pro-apoptotic
effectors, respectively, and are important regulators in the
PI3K/Akt signaling pathway (25). The
expression of Bcl-2 was decreased and the expression of Bax was
increased in cells transfected with P-REX2a-siRNA compared with the
negative control. These results indicated that P-REX2a may promote
resistance to doxorubicin in gastric cancer cells.
A previous study has reported that overexpression of
PTEN in originally PTEN deficient cells may reduce phosphorylation
of Akt. Phosphorylation did not markedly change if only P-REX2a was
overexpressed, but Akt was activated at both S473 and T308 sites if
PTEN and P-REX2a were overexpressed at the same time (10). This result indicated that P-REX2a
regulates the PI3K/Akt signaling pathway, which is dependent on
PTEN. In the present study, SGC7901 cells were treated with
doxorubicin following the knockdown of P-REX2a, and the expression
of PTEN, Akt, p-Akt (S473) and the lipid phosphatase activity of
PTEN were detected. The results in the present study indicated that
knockdown of P-REX2a was able to increase the activity of PTEN,
leading to the downregulation of p-Akt during chemotherapy.
In conclusion, P-REX2a promotes resistance to
doxorubicin in gastric cancer cells and may inactivate PTEN via the
PTEN/PI3K/Akt signaling pathway. Therefore, it may be possible to
increase the sensitivity of gastric cancer to doxorubicin by
inhibiting P-REX2a in the future. At present, the research is
limited, and further studies on the expression of P-REX2a in
gastric cancer and confirmatory experiments in different cell lines
are required.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun X, Bao J and Shao Y: Mathematical
modeling of therapy-induced cancer drug resistance: Connecting
cancer mechanisms to population survival rates. Sci Rep.
6:224982016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Obenauf AC, Zou Y, Ji AL, Vanharanta S,
Shu W, Shi H, Kong X, Bosenberg MC, Wiesner T, Rosen N, et al:
Therapy-induced tumour secretomes promote resistance and tumour
progression. Nature. 520:368–372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai Y, Tan X, Liu J, Shen Y, Wu D, Ren M,
Huang P and Yu D: Inhibition of PI3K/Akt/mTOR signaling pathway
enhances the sensitivity of the SKOV3/DDP ovarian cancer cell line
to cisplatin in vitro. Chin J Cancer Res. 26:564–572.
2014.PubMed/NCBI
|
|
7
|
Xie X, Tang B, Zhou J, Gao Q and Zhang P:
Inhibition of the PI3K/Akt pathway increases the chemosensitivity
of gastric cancer to vincristine. Oncol Rep. 30:773–782. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eng C: PTEN: One gene, many syndromes. Hum
Mutat. 22:183–198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chalhoub N and Baker SJ: PTEN and the
PI3-kinase pathway in cancer. Annu Rev Pathol. 4:127–150. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fine B, Hodakoski C, Koujak S, Su T, Saal
LH, Maurer M, Hopkins B, Keniry M, Sulis ML, Mense S, et al:
Activation of the PI3K pathway in cancer through inhibition of PTEN
by exchange factor P-REX2a. Science. 325:1261–1265. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leslie NR: P-REX2a driving tumorigenesis
by PTEN inhibition. Sci Signal. 2:pe682009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu HG, Ai YW, Yu LL, Zhou XD, Liu J, Li
JH, Xu XM, Liu S, Chen J, Liu F, et al: Phosphoinositide
3-kinase/Akt pathway plays an important role in chemoresistance of
gastric cancer cells against etoposide and doxorubicin induced cell
death. Int J Cancer. 122:433–443. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu XQ, Yu JP, Luo HS and Yu HG: The
expression and role of PTEN in doxorubicin induced gastric cancer
cell apoptosis. Zhonghua Nei Ke Za Zhi. 49:422–425. 2010.(In
Chinese). PubMed/NCBI
|
|
14
|
Schmidt N, Peitz U, Lippert H and
Malfertheiner P: Missing gastric cancer in dyspepsia. Aliment
Pharmacol Ther. 21:813–820. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Noh SH, Park SR, Yang HK, Chung HC, Chung
IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W, et al: Adjuvant
capecitabine plus oxaliplatin for gastric cancer after D2
gastrectomy (CLASSIC): 5-year follow-up of an open-label,
randomised phase 3 trial. Lancet Oncol. 15:1389–1396. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu BQ and Xie JW: Identifying therapeutic
targets in gastric cancer: The current status and future direction.
Acta Biochim Biophys Sin (Shanghai). 48:90–96. 2016.PubMed/NCBI
|
|
17
|
Ai YW, Yu HG, Yu JP, Yang Y, Li H, Hu XW
and Luo HS: Impact of PI3K/Akt/mdm2 signaling pathway on the
sensitivity of gastric cancer cell line SGC7901 to doxorubicin.
Zhonghua Zhong Liu Za Zhi. 30:494–497. 2008.(In Chinese).
PubMed/NCBI
|
|
18
|
Hu Y, Guo R, Wei J, Zhou Y, Ji W, Liu J,
Zhi X and Zhang J: Effects of PI3K inhibitor NVP-BKM120 on
overcoming drug resistance and eliminating cancer stem cells in
human breast cancer cells. Cell Death Dis. 6:e20202015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smolensky D, Rathore K and Cekanova M:
Phosphatidylinositol-3-kinase inhibitor induces chemosensitivity to
a novel derivative of doxorubicin, AD198 chemotherapy in human
bladder cancer cells in vitro. BMC Cancer. 15:9272015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Z, Yang L, Xia Y, Guo C and Kong L:
Icariin enhances cytotoxicity of doxorubicin in human
multidrug-resistant osteosarcoma cells by inhibition of ABCB1 and
down-regulation of the PI3K/Akt pathway. Biol Pharm Bull.
38:277–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Donald S, Hill K, Lecureuil C, Barnouin R,
Krugmann S, John Coadwell W, Andrews SR, Walker SA, Hawkins PT,
Stephens LR and Welch HC: P-Rex2, a new guanine-nucleotide exchange
factor for Rac. FEBS Lett. 572:172–176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo B, Liu L, Yao J, Ma R, Chang D, Li Z,
Song T and Huang C: miR-338-3p suppresses gastric cancer
progression through a PTEN-AKT axis by targeting P-REX2a. Mol
Cancer Res. 12:313–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goler-Baron V, Sladkevich I and Assaraf
YG: Inhibition of the PI3K-Akt signaling pathway disrupts
ABCG2-rich extracellular vesicles and overcomes multidrug
resistance in breast cancer cells. Biochem Pharmacol. 83:1340–1348.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fan QW and Weiss WA: Targeting the
RTK-PI3K-mTOR axis in malignant glioma: overcoming resistance. Curr
Top Microbiol Immunol. 347:279–296. 2010.PubMed/NCBI
|
|
25
|
Rasul A, Khan M, Yu B, Ali M, Bo YJ, Yang
H and Ma T: Isoalantolactone, a sesquiterpene lactone, induces
apoptosis in SGC-7901 cells via mitochondrial and
phosphatidylinositol kinase/Akt signaling pathways. Arch Pharm Res.
36:1262–1269. 2013. View Article : Google Scholar : PubMed/NCBI
|