Introduction
Colorectal cancer (CRC) is one of the major causes
of cancer-associated mortality and one of the most curable
gastrointestinal cancers (1). It is
therefore important to identify all the factors that may serve a
role in the diagnosis and prognosis estimation of CRC such that
timely diagnosis and treatment decisions may be made. A previous
study demonstrated that the number of CRC patients may be reduced
through routine screening (2), but
the rates of screening for CRC remain low (2–4).
Cancer causes under-nutrition and chronic
inflammation, and cancer-related inflammation has been reported to
be a crucial factor in cancer progression and cancer-associated
survival (5,6). In addition to the pathological
characteristics of cancer, other non-cancer factors, including
general health condition, may determine the outcomes of patients
with cancer (7). The
neutrophil-to-lymphocyte ratio (NLR) has been used as an indicator
of the inflammatory-related response (8). The NLR is equivalent to the number of
neutrophils divided by the number of lymphocytes. An elevated NLR
has been reported to be a valuable predictive indicator of various
cancer types, including epithelial ovarian, pancreatic, gastric and
breast cancer (9–12). The red cell distribution width (RDW)
is also a generally used laboratory indicator of inflammatory
response (13). The RDW reflects the
variation in erythrocyte size, and an increased RDW indicates
anisocytosis (14). Previous studies
have also demonstrated the role of an increased RDW, which predicts
a worse overall survival (OS) rate and an increased
disease-specific mortality rate in patients with chronic
inflammatory diseases and certain cancer types (15–19), in
addition to promoting the progression of cardiovascular and
cerebrovascular diseases (20–22).
However, few specific studies regarding the predictive value of NLR
and RDW in patients with CRC have been reported (23,24). The
present study systematically evaluated whether NLR and RDW
elevations may serve potential roles as biomarkers of CRC activity.
Any associations between the NLR or RDW and histopathological
parameters in patients with CRC were identified, any differences in
NLR and RDW prior to and following radical surgical resection were
determined, and the prognostic importance of NLR and RDW in CRC
patients was subsequently evaluated.
Materials and methods
Patients
Clinical data from 240 patients with CRC who had
undergone radical surgical resection at Shandong Provincial
Hospital Affiliated to Shandong University (Shandong, China)
between January 2011 and April 2015 were analyzed retrospectively.
Data from 110 patients with colon polyps and 48 healthy volunteers
were also collected to serve as controls for comparative analysis.
Following radical resection, patients were histopathologically
diagnosed by two pathologists. Patients with CRC were enrolled
according to the following inclusive criteria: CRC diagnosed by
histopathology (the invasion of the mucosal muscle layer by cancer
cells), radical resection under a microscope, blood parameters, and
clinicopathological and follow-up results. Patients with anemia,
hematological disorders, active infectious diseases, venous
thrombosis diagnosed within the last 6 months, a history of blood
transfusion within the last 3 months, a treatment history of
asiderosis, hypertension, cardiac failure, autoimmune disorders and
a history of other malignancies were excluded from the study. Blood
parameters were detected within 1 week prior to surgery and 3 weeks
after surgery. The complete blood count (including NLR and RDW) was
detected using a hematology analyzer XE-2100 (Sysmex Corporation,
Kobe, Japan). Tumor markers [carcinoembryonic antigen (CEA) and
carbohydrate antigen 19-9 (CA19-9)] were measured within 1 month
prior to surgery. CEA and CA19-9 were analyzed using a Cobas e601
analyzer (Roche Diagnostics GmbH, Mannheim, Germany). The high and
low values of NLR or RDW were compared in terms of the CRC
location, tumor diameter, Tumor-Node-Metastasis (TNM) stage
(25), degree of differentiation,
sex, age and tumor markers, as well as changes in the NLR and RDW
prior to and following radical surgical resection. A total of 128
patients, including 54 patients with metastatic CRC, were followed
up regularly through telephone interviews and patients received
their last follow-up in June 2016. The first research end-point was
OS time, which was defined as the time from the date of surgery to
mortality from any cause. The second study end-point was
disease-free survival (DFS) time, which was defined as the time
from the date of surgery to the date of identification of disease
recurrence, either radiological or histological. This study was
approved by the Ethics Committee of the Shandong Provincial
Hospital Affiliated to Shandong University. Written informed
consent was obtained from all participants.
Statistical analysis
SPSS statistical software version 19.0 (IBM Corp.,
Armonk, NY, USA) was used for statistical analysis. All parameters
are normally distributed and presented as the mean ± standard
deviation (SD). Categorical variables are presented as frequencies.
Receiver operating characteristic (ROC) curve analysis was used to
determine the cutoff values for the NLR and the RDW, and to
calculate the Youden index (YI), which was used to identify the
optimal cutoff values for the NLR and the RDW (2.06 and 13.45%,
respectively). All cases were divided into high and low NLR or RDW
groups in terms of these cutoff values. Comparison between groups
was evaluated using one-way analysis of variance and unpaired
Student's t-tests. Multiple comparison between the groups was
performed using Student-Newman-Keuls method. A χ2 test
or Fisher's exact test was performed with ≥1 variables (<5) to
analyze the differences between high and low NLR and RDW groups in
terms of each clinicopathological characteristic. Logistic
regression analysis was conducted to evaluate the
clinicopathological factors that likely caused the increased NLR
and RDW. Comparisons between NLR and RDW values prior to and
following surgery were made using the Wilcoxon matched-pairs signed
rank test. Kaplan-Meier analysis was used to calculate the OS and
DFS time and the log-rank test was used to compare the survival
rate curves. Significant indicators for survival determined in
univariate analysis were introduced into the multivariate Cox
proportional hazards model to establish independent prognostic
indicators. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison between groups in terms of
the RDW and NLR values
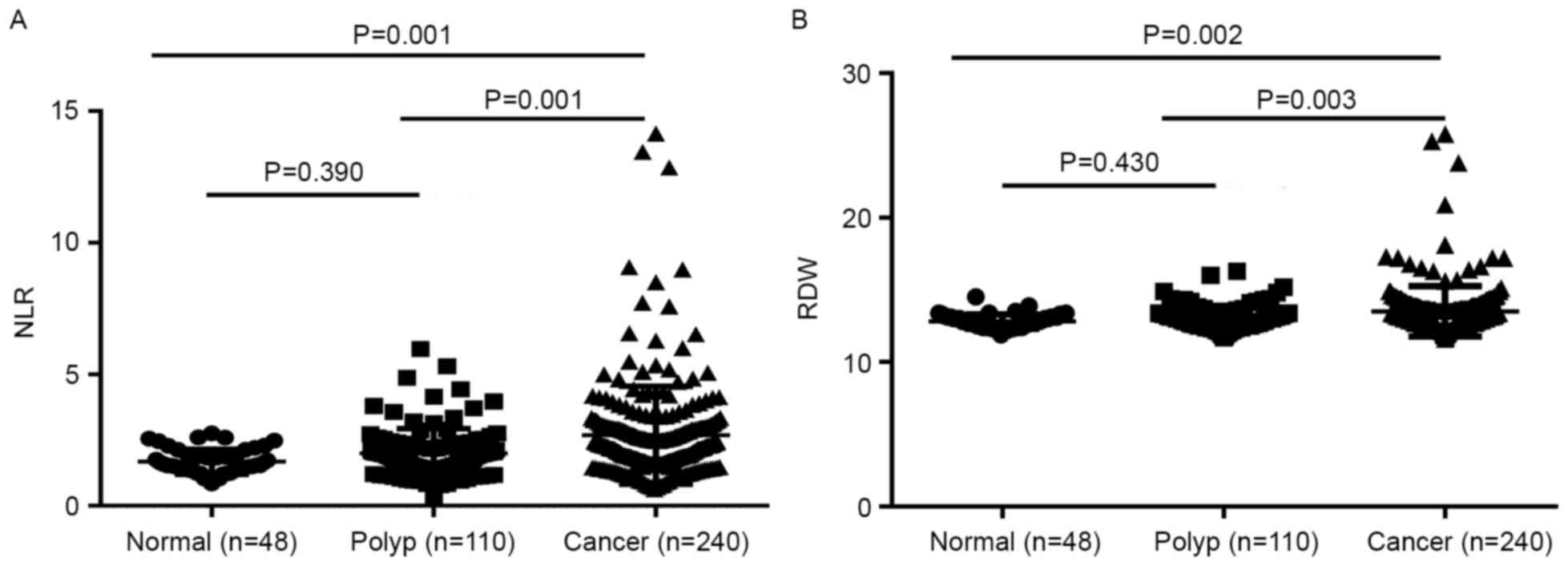
The mean ± SD of the NLR in the CRC, colon polyps
and healthy control groups was 2.81±2.60, 1.99±0.94 and 1.68±0.48,
respectively. The NLR in the CRC patients was significantly higher
compared with that in the colon polyps patients and the healthy
controls (P=0.001 and P=0.001, respectively). The colon polyps
group demonstrated no significant difference in NLR compared with
the healthy control group (P=0.390) (Fig.
1A). The mean ± SD of RDW in the CRC, colon polyps and healthy
control groups were 13.51±1.74, 13.02±0.81 and 12.82±0.47%,
respectively. The RDW in the CRC group was increased significantly
compared with that of the colon polyps and the healthy control
groups (P=0.003 and P=0.002, respectively). No significant
difference in RDW was observed between the colon polyps patients
and the healthy controls (P=0.430) (Fig.
1B).
Association between NLR or RDW and
clinicopathological characteristics
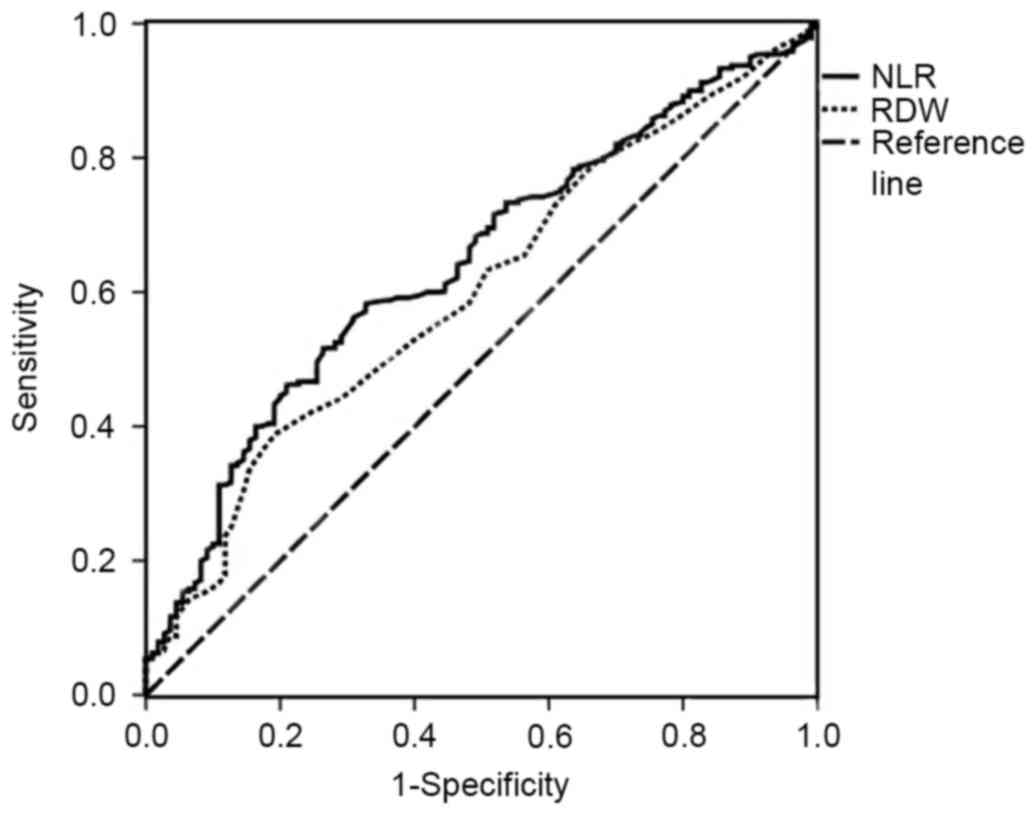
The area under the curve of the NLR was 0.642 [95%
confidence interval (CI), 0.582–0.703; P<0.001], and that of the
RDW was 0.601 (95% CI, 0.539–0.663; P<0.001) (Fig. 2). When the YI was at its maximum
(YI=0.256), the NLR was 2.06, the sensitivity was 58.3% and the
specificity was 67.3%, revealing that the optimal cutoff value for
NLR was 2.06. Thus, patients with CRC were divided into high NLR
(≥2.06) and low NLR (<2.06) groups. When the RDW was 13.45%, the
sensitivity was 38.8%, the specificity was 80.9% and the YI was at
its maximum (YI=0.197). The patients with CRC were then divided
into high RDW (≥13.45%) and low RDW (<13.45%) groups. The
clinicopathological characteristics of the 2 groups were compared
in terms of the NLR and the RDW (Table
I). The high NLR group was associated with tumor location
(colon), a larger tumor diameter, poor tumor differentiation,
deeper tumor infiltration, and high CEA and CA19-9 levels
(P<0.05). A high RDW was revealed to be associated with older
age and distant metastases (P<0.05). The clinicopathological
factors that likely caused the increased NLR and RDW were also
evaluated following evaluation of other factors using logistic
regression analysis. The results demonstrated that a larger tumor
diameter and poor tumor differentiation were independent risk
factors for increased NLR (P<0.05), while older age and distant
metastases were independent risk factors for increased RDW
(P<0.05) (Table II).
 | Table I.Association between NLR and RDW, and
clinicopathological characteristics. |
Table I.
Association between NLR and RDW, and
clinicopathological characteristics.
|
Characteristics | Cases, n | NLR<2.06, n
(%) | NLR≥2.06, n
(%) | P-value | RDW<13.45, n
(%) | RDW≥13.45, n
(%) | P-value |
|---|
| Sex |
|
|
| 0.224 |
|
| 0.622 |
|
Male | 167 | 66 (65.3) | 101 (72.7) |
| 104 (70.7) | 63 (67.7) |
|
|
Female | 73 | 35 (34.7) | 38 (27.3) |
| 43 (29.3) | 30 (32.3) |
|
| Age, years |
|
|
| 0.676 |
|
| 0.022 |
|
<60 | 94 | 38 (37.6) | 56 (40.3) |
| 66 (44.9) | 28 (30.1) |
|
|
≥60 | 146 | 63 (62.4) | 83 (59.7) |
| 81 (55.1) | 65 (69.9) |
|
| Tumor location |
|
|
| 0.046 |
|
| 0.457 |
|
Colon | 199 | 78 (77.2) | 121 (87.1) |
| 124 (84.4) | 75 (80.6) |
|
|
Rectum | 41 | 23 (22.8) | 18 (12.9) |
| 23 (15.6) | 18 (19.4) |
|
| Tumor diameter,
cm |
|
|
| <0.001 |
|
| 0.442 |
| ≤4 | 95 | 55 (64.0) | 40 (33.9) |
| 65 (48.5) | 30 (42.9) |
|
|
>4 | 109 | 31 (36.0) | 78 (66.1) |
| 69 (51.5) | 40 (57.1) |
|
|
Differentiation |
|
|
| 0.004 |
|
| 0.799 |
|
Well |
7 | 2 (2.0) | 5 (3.6) |
| 5 (3.4) | 2 (2.2) |
|
|
Moderate | 167 | 82 (81.2) | 85 (61.1) |
| 103 (70.1) | 64 (68.8) |
|
|
Poor | 66 | 17 (16.8) | 49 (35.3) |
| 39 (26.5) | 27 (29.0) |
|
| Tumor depth |
|
|
| 0.012 |
|
| 0.634 |
| T1 |
5 | 5 (5.0) | 0 (0.0) |
| 4 (2.7) | 1 (1.1) |
|
| T2 | 25 | 7 (6.9) | 18 (12.9) |
| 13 (8.8) | 12 (12.9) |
|
| T3 | 51 | 26 (25.7) | 25 (18.0) |
| 31 (21.1) | 20 (21.5) |
|
| T4 | 159 | 63 (62.4) | 96 (69.1) |
| 99 (67.3) | 60 (64.5) |
|
| Lymph node
metastasis |
|
|
| 0.210 |
|
| 0.527 |
| N0 | 119 | 44 (43.6) | 75 (54.0) |
| 77 (52.4) | 42 (45.2) |
|
| N1 | 66 | 29 (28.7) | 37 (26.6) |
| 39 (26.5) | 27 (29.0) |
|
| N2 | 55 | 28 (27.7) | 27 (19.4) |
| 31 (21.1) | 24 (25.8) |
|
| Distant
metastasis |
|
|
| 0.395 |
|
| 0.023 |
| M0 | 219 | 94 (93.1) | 125 (89.9) |
| 139 (94.6) | 80 (86.0) |
|
| M1 | 21 | 7 (6.9) | 14 (10.1) |
| 8 (5.4) | 13 (14.0) |
|
| pStage |
|
|
| 0.117 |
|
| 0.109 |
| I | 22 | 10 (9.9) | 12 (8.6) |
| 15 (10.2) | 7 (7.5) |
|
| II | 91 | 31 (30.7) | 60 (43.2) |
| 60 (40.8) | 31 (33.3) |
|
|
III | 106 | 53 (52.5) | 53 (38.1) |
| 64 (43.5) | 42 (45.2) |
|
| IV | 21 | 7 (6.9) | 14 (10.1) |
| 8 (5.4) | 13 (14.0) |
|
| CEA, ng/ml |
|
|
| 0.045 |
|
| 0.881 |
| ≤5 | 100 | 49 (62.0) | 51 (47.2) |
| 61 (53.0) | 39 (54.2) |
|
|
>5 | 87 | 30 (38.0) | 57 (52.8) |
| 54 (47.0) | 33 (45.8) |
|
| CA19-9, U/ml |
|
|
| 0.030 |
|
| 0.142 |
|
≤39 | 155 | 71 (89.9) | 84 (77.8) |
| 99 (86.1) | 56 (77.8) |
|
|
>39 | 32 | 8 (10.1) | 24 (22.2) |
| 16 (13.9) | 16 (22.2) |
|
 | Table II.Logistic regression analysis of NLR-
and RDW-associated risk factors. |
Table II.
Logistic regression analysis of NLR-
and RDW-associated risk factors.
|
| NLR | RDW |
|---|
|
|
|
|
|---|
|
Characteristics | B | SE | Wald | HR (95% CI) | P-value | B | SE | Wald | HR (95% CI) | P-value |
|---|
| Sex
(male/female) | −0.182 | 0.453 | 0.160 | 0.834
(0.343–2.028) | 0.689 | 0.004 | 0.427 | 0.000 | 1.004
(0.435–2.318) | 0.992 |
| Age, years
(<60/≥60) | 0.460 | 0.419 | 1.205 | 1.584
(0.697–3.602) | 0.272 | 0.669 | 0.285 | 5.526 | 1.953
(1.118–3.412) | 0.019 |
| Tumor location
(colon/rectum) | −1.013 | 0.573 | 3.127 | 0.363
(0.118–1.116) | 0.077 | 0.022 | 0.526 | 0.002 | 1.022
(0.365–2.867) | 0.966 |
| Tumor diameter, cm
(≤4/>4) | 1.793 | 0.430 | 17.375 | 6.010
(2.586–13.966) | <0.001 | 0.289 | 0.388 | 0.553 | 1.335
(0.623–2.859) | 0.457 |
| Differentiation
(well+moderate/poor) | 0.501 | 0.237 | 4.465 | 1.651
(1.037–2.628) | 0.035 | 0.008 | 0.210 | 0.001 | 1.008
(0.667–1.522) | 0.971 |
| Depth of tumor
(T1+T2/T3+T4) | −0.157 | 0.438 | 0.128 | 0.855
(0.362–2.017) | 0.721 | 0.041 | 0.623 | 0.004 | 1.042
(0.307–3.537) | 0.947 |
| Lymph node
metastasis (N0/N1+N2) | 0.365 | 0.964 | 0.143 | 1.440
(0.218–9.522) | 0.705 | −1.026 | 1.511 | 0.461 | 0.358
(0.019–6.929) | 0.497 |
| Distant metastasis
(M0/M1) | 0.631 | 0.591 | 1.140 | 1.879
(0.590–5.981) | 0.286 | 1.093 | 0.478 | 5.222 | 2.983
(1.168–7.615) | 0.022 |
| pStage
(I+II/III+IV) | −1.041 | 0.999 | 1.085 | 0.353
(0.050–2.503) | 0.298 | 0.925 | 1.524 | 0.368 | 2.523
(0.127–50.060) | 0.544 |
| CEA, ng/ml
(≤5/>5) | 0.666 | 0.417 | 2.545 | 1.946
(0.859–4.410) | 0.111 | −0.172 | 0.382 | 0.201 | 0.842
(0.397–1.785) | 0.654 |
| CA19-9, U/ml
(≤39/>39) | 0.520 | 0.667 | 0.607 | 1.682
(0.455–6.223) | 0.436 | 0.415 | 0.557 | 0.555 | 1.514
(0.509–4.505) | 0.456 |
Differences in the NLR and RDW prior
to and following surgery
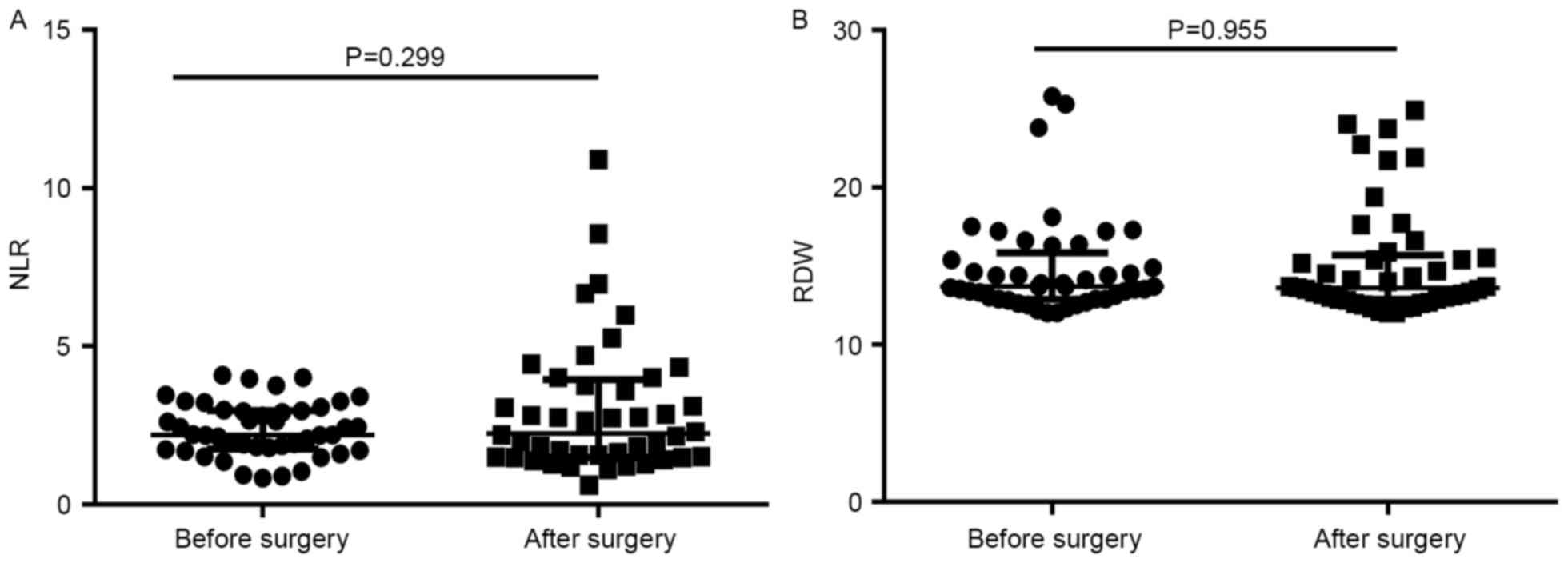
Differences in the NLR and RDW prior to and
following surgery were analyzed in 45 patients with CRC. The median
(interquartile range) values of the NLR and RDW prior to surgery
were 2.20 (1.765–3.025) and 13.7% (12.90–15.85%), respectively, and
following surgery were 2.30 (1.500–4.005) and 13.6% (12.85–15.70%),
respectively. No significant differences in the NLR or RDW were
observed prior to and following surgery (P=0.299 and P=0.955,
respectively) (Fig. 3). Furthermore,
the patients were divided into 2 groups according to the change in
NLR and RDW values (the value following surgery divided by the
value prior to surgery): NLR <1 and ≥1, and RDW <1 and ≥1.
The association between the two groups and TNM stage was analyzed.
These results demonstrated that no apparent association existed
between changes in the NLR or RDW and TNM stage (Table III).
 | Table III.Association between the change in NLR
and RDW values following surgery compared with those prior to
surgery and TNM stage. |
Table III.
Association between the change in NLR
and RDW values following surgery compared with those prior to
surgery and TNM stage.
|
| Change in NLR | Change in RDW |
|---|
|
|
|
|
|---|
|
Characteristics | <1 (n=23) | ≥1 (n=22) | P-value | <1 (n=24) | ≥1 (n=21) | P-value |
|---|
| Depth of tumor |
|
| 0.699 |
|
| 0.443 |
|
T1+T2 | 5
(21.7) | 3
(13.6) |
| 3
(12.5) | 5
(23.8) |
|
|
T3+T4 | 18 (78.3) | 19 (86.4) |
| 21 (87.5) | 16 (76.2) |
|
| Lymph node
metastasis |
|
| 0.772 |
|
| 0.469 |
| N0 | 4
(17.4) | 5
(22.7) |
| 6
(25.0) | 3
(14.3) |
|
|
N1+N2 | 19 (82.6) | 17 (77.3) |
| 18 (75.0) | 18 (85.7) |
|
| Distance
metastasis |
|
| 0.092 |
|
| 1.000 |
| M0 | 18 (78.3) | 12 (54.5) |
| 16 (66.7) | 14 (66.7) |
|
| M1 | 5
(21.7) | 10 (54.5) |
| 8
(33.3) | 7
(33.3) |
|
| pStage |
|
| 1.000 |
|
| 1.000 |
|
I+II | 4
(17.4) | 3
(13.6) |
| 4
(16.7) | 3
(14.3) |
|
|
III+IV | 19 (82.6) | 19 (86.4) |
| 20 (83.3) | 18 (85.7) |
|
Survival analysis of prognostic
factors
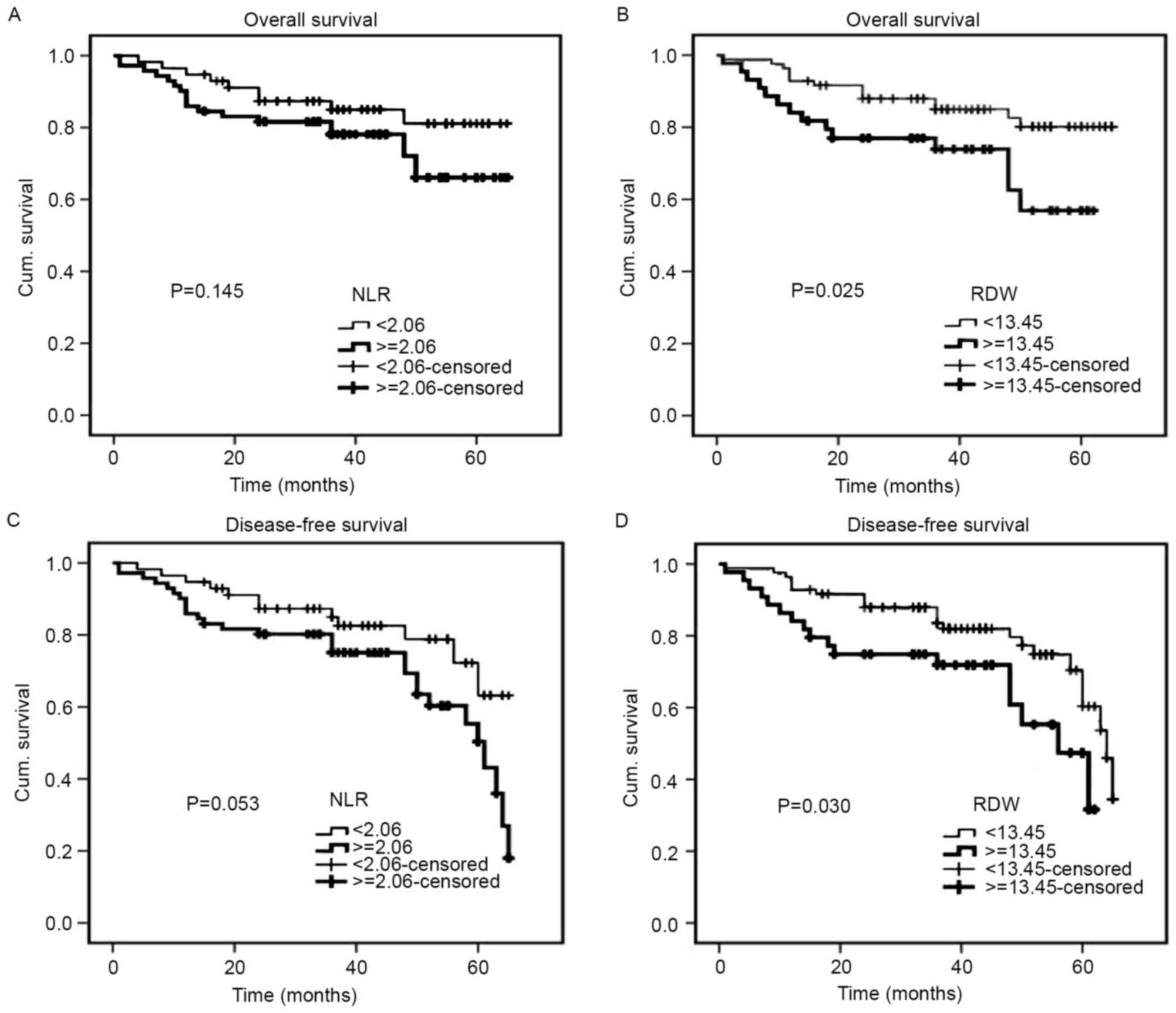
The median follow-up duration was 40 months (range,
1–65 months). The Kaplan-Meier cumulative survival curves for 128
patients with CRC are presented (Fig.
4). The mean OS time was 57.3 months in the low RDW group (95%
CI, 53.5–61.1) and 46.9 months in the high RDW group (95% CI,
40.3–53.6). In addition, the mean DFS time was 55.1 months in the
low RDW group (95% CI, 51.2–59.0) and 45.4 months in the high RDW
group (95% CI, 38.8–52.0). The high RDW group exhibited an
unfavorable OS time (P=0.025) and a shorter DFS time (P=0.030)
compared with the low RDW group (Fig. 4B
and D). With regards to the NLR, the mean OS and DFS times were
57.4 months and 55.7 months, respectively, in the low NLR group
(95% CI, 52.8–62.0 and 50.9–60.4, respectively), and 52.0 months
and 49.4 months, respectively, in the high NLR group (95% CI,
46.9–57.1 and 44.3–54.5, respectively). Therefore, the NLR was not
significantly associated with OS or DFS (P=0.145 and P=0.053,
respectively) (Fig. 4A and C). For 54
patients with metastatic CRC, an increased RDW was associated with
a significantly shorter OS (P=0.014) and DFS (P=0.005), while an
increased NLR was only associated with a significantly shorter DFS
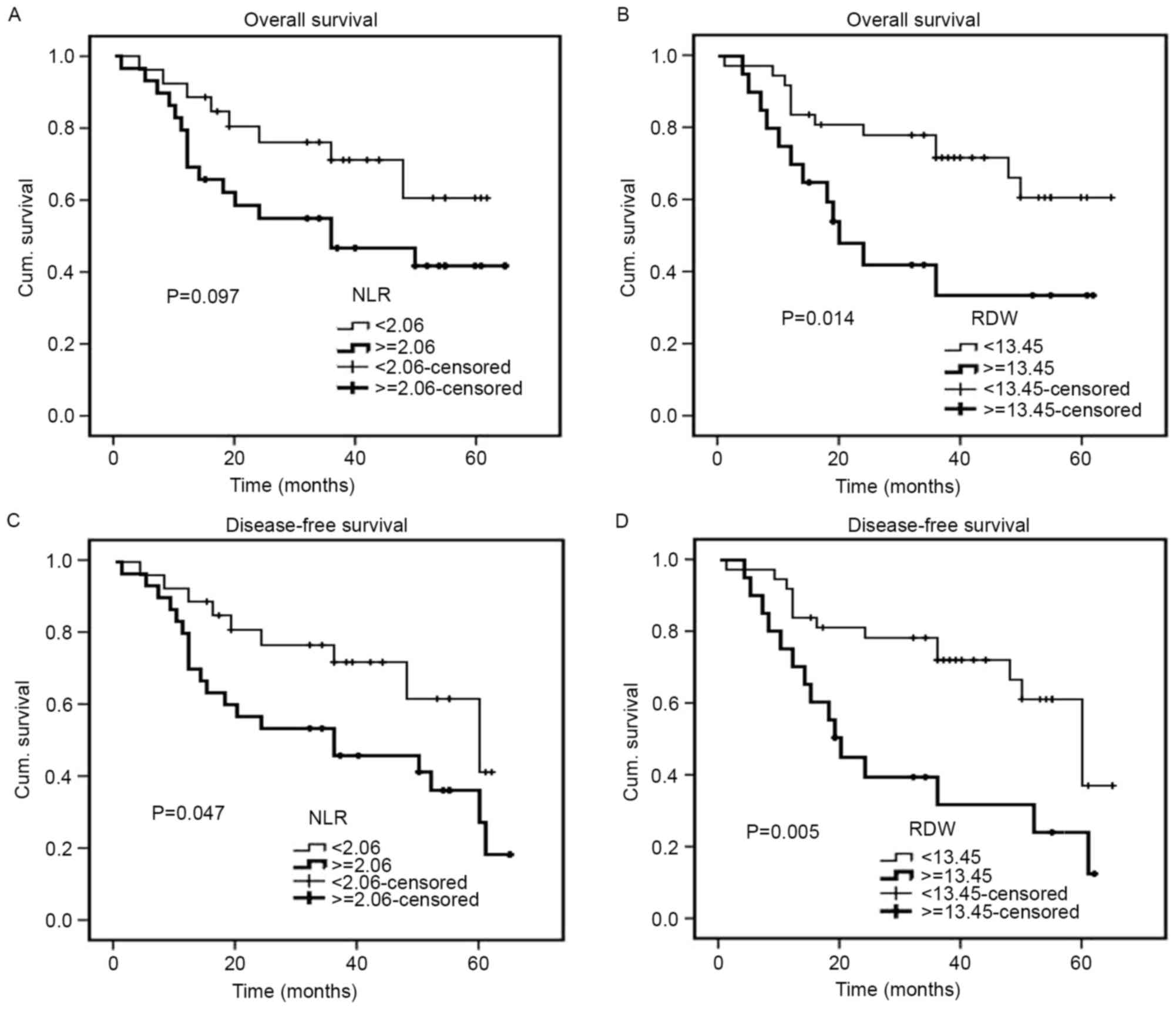
(P=0.047) (Fig. 5). For 128 patients
with CRC, univariate analysis revealed that OS was significantly
associated with RDW (P=0.030), degree of differentiation
(P<0.001), pStage (P<0.001) (25), depth of tumor (P=0.028), lymph node
metastasis (P<0.001), distant metastasis (P<0.001), CEA
(P=0.031) and CA19-9 (P=0.008), while DFS demonstrated a similar
association with RDW (P=0.035), tumor diameter (P=0.042), degree of
differentiation (P<0.001), pStage (P<0.001), depth of tumor
(P=0.009), lymph node metastasis (P=0.001), distant metastasis
(P<0.001) and CEA (P=0.011). The multivariate analyses revealed
that the degree of differentiation (P=0.003) and lymph node
metastasis (P=0.001) may act as independent prognostic indicators
of OS and DFS (Table IV). For 54
patients with metastatic CRC, univariate analysis revealed that OS
was significantly associated with RDW (P=0.019), degree of
differentiation (P=0.008), pStage (P=0.012), distant metastasis
(P=0.012) and CA19-9 (P=0.046), while DFS exhibited a similar
association with RDW (P=0.007), degree of differentiation
(P=0.005), pStage (P=0.014), distant metastasis (P=0.014), CEA
(P=0.041) and CA19-9 (P=0.037). The multivariate analyses revealed
that RDW, the degree of differentiation and CA19-9 may act as
independent prognostic indicators of OS and DFS in patients with
metastatic CRC (Table V).
 | Table IV.Univariate and multivariate Cox
regression analyses for OS and DFS in 128 patients with colorectal
cancer. |
Table IV.
Univariate and multivariate Cox
regression analyses for OS and DFS in 128 patients with colorectal
cancer.
|
| Univariate
(OS) | Multivariate
(OS) | Univariate
(DFS) | Multivariate
(DFS) |
|---|
|
|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| NLR
(<2.06/≥2.06) | 1.781 | 0.806–3.938 | 0.154 |
|
|
| 1.923 | 0.976–3.788 | 0.059 |
|
|
|
| RDW
(<13.45/≥13.45) | 2.273 | 1.082–4.774 | 0.030 |
|
|
| 2.011 | 1.052–3.846 | 0.035 |
|
|
|
| Sex
(male/female) | 1.672 | 0.781–3.579 | 0.186 |
|
|
| 1.561 | 0.811–3.007 | 0.183 |
|
|
|
| Age, years
(<60/≥60) | 1.974 | 0.839–4.647 | 0.119 |
|
|
| 1.312 | 0.676–2.548 | 0.422 |
|
|
|
| Tumor location
(colon/rectum) | 1.529 | 0.619–3.776 | 0.357 |
|
|
| 1.439 | 0.629–3.292 | 0.388 |
|
|
|
| Tumor diameter, cm
(≤4/>4) | 2.313 | 0.302–17.683 | 0.419 |
|
|
| 4.686 | 1.057–20.777 | 0.042 |
|
|
|
| Differentiation
(well/moderate/poor) | 4.534 | 2.132–9.641 | <0.001 | 4.398 | 1.631–11.860 | 0.003 | 3.544 | 1.907–6.588 | <0.001 | 3.887 | 1.649–9.165 | 0.002 |
| Depth of tumor
(T1/T2/T3/T4) | 3.991 | 1.161–13.715 | 0.028 |
|
|
| 3.067 | 1.320–7.128 | 0.009 |
|
|
|
| Lymph node
metastasis (N0/N1/N2) | 2.364 | 1.530–3.654 | <0.001 | 2.734 | 1.521–4.914 | 0.001 | 1.850 | 1.299–2.634 | 0.001 | 1.817 | 1.112–2.968 | 0.017 |
| Distant metastasis
(M0/M1) | 8.282 | 2.823–24.302 | <0.001 |
|
|
| 6.633 | 2.527–17.407 | <0.001 |
|
|
|
| pStage
(I/II/III/IV) | 3.861 | 2.118–7.041 | <0.001 |
|
|
| 2.377 | 1.712–4.364 | <0.001 |
|
|
|
| CEA, ng/ml
(≤5/>5) | 2.900 | 1.101–7.637 | 0.031 |
|
|
| 2.849 | 1.268–6.402 | 0.011 |
|
|
|
| CA19-9, U/ml
(≤39/>39) | 4.041 | 1.452–11.250 | 0.008 |
|
|
| 2.205 | 0.823–5.911 | 0.116 |
|
|
|
 | Table V.Univariate and multivariable Cox
regression analyses for OS and DFS in 54 patients with metastatic
colorectal cancer. |
Table V.
Univariate and multivariable Cox
regression analyses for OS and DFS in 54 patients with metastatic
colorectal cancer.
|
| Univariate
(OS) | Multivariate
(OS) | Univariate
(DFS) | Multivariate
(DFS) |
|---|
|
|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| NLR
(<2.06/≥2.06) | 2.012 | 0.859–4.712 | 0.107 |
|
|
| 2.158 | 0.980–4.752 | 0.056 |
|
|
|
| RDW
(<13.45/≥13.45) | 2.628 | 1.170–5.901 | 0.019 | 2.755 | 1.194–6.361 | 0.018 | 2.742 | 1.313–5.724 | 0.007 | 2.684 | 1.258–5.725 | 0.011 |
| Sex
(male/female) | 1.419 | 0.629–3.199 | 0.399 |
|
|
| 1.606 | 0.771–3.346 | 0.206 |
|
|
|
| Age, years
(<60/≥60) | 1.781 | 0.705–4.499 | 0.222 |
|
|
| 1.993 | 0.847–4.691 | 0.114 |
|
|
|
| Tumor location
(colon/rectum) | 1.692 | 0.619–4.623 | 0.305 |
|
|
| 1.603 | 0.591–4.350 | 0.354 |
|
|
|
| Tumor diameter, cm
(≤4/>4) | 1.383 | 0.180–10.644 | 0.755 |
|
|
| 2.811 | 0.629–12.573 | 0.176 |
|
|
|
| Differentiation
(well/moderate/poor) | 3.187 | 1.357–7.481 | 0.008 | 2.933 | 1.239–6.947 | 0.014 | 3.017 | 1.397–6.514 | 0.005 | 2.605 | 1.193–5.686 | 0.016 |
| Depth of tumor
(T1/T2/T3/T4) | 1.263 | 0.370–4.307 | 0.709 |
|
|
| 0.958 | 0.355–2.585 | 0.933 |
|
|
|
| Lymph node
metastasis (N0/N1/N2) | 1.265 | 0.574–2.785 | 0.560 |
|
|
| 1.196 | 0.576–2.483 | 0.631 |
|
|
|
| Distant metastasis
(M0/M1) | 4.065 | 1.365–12.111 | 0.012 |
|
|
| 3.472 | 1.281–9.415 | 0.014 |
|
|
|
| pStage
(I/II/III/IV) | 4.065 | 1.365–12.111 | 0.012 |
|
|
| 3.472 | 1.281–9.415 | 0.014 |
|
|
|
| CEA, ng/ml
(≤5/>5) | 1.578 | 0.950–2.621 | 0.078 |
|
|
| 1.600 | 1.020–2.509 | 0.041 |
|
|
|
| CA19-9, U/ml
(≤39/>39) | 1.906 | 1.011–3.595 | 0.046 | 2.240 | 1.180–4.252 | 0.014 | 1.883 | 1.039–3.411 | 0.037 | 2.061 | 1.139–3.731 | 0.017 |
Discussion
CRC is one of the most common cancers and the fourth
highest cause of cancer-associated mortality in the world (26). In China, with the increasing number of
risk factors, including an aging population and changes in dietary
habits (e.g., reduced fiber intake), CRC has become the fifth most
common cancer in the country (27).
Tumor-associated inflammatory cytokines and
mediators may mediate inflammatory responses, which lead to tumor
growth, infiltration and metastasis (28). Previous studies have demonstrated that
blood parameters, including C-reactive protein, albumin,
hemoglobin, mean corpuscular volume, NLR, white blood cell count
and RDW, are significantly associated with the host inflammatory
response and the poorer nutritional status induced by numerous
types of cancer, partially through acting as predictors of disease
progression and prognosis (29–31).
Firstly, elevated neutrophils facilitate tumor proliferation,
migration and vasculogenesis. Secondly, lymphocytes can promote
cytotoxic cell activation and cytokine production, which inhibit
tumor proliferation and migration. Thus, low lymphocyte levels
destroy the antitumor immune response and result in a poorer
prognosis. Therefore, NLR reflects the balance between the
pro-tumor inflammatory response and the antitumor immune response
(32). Furthermore, several studies
have reported that an increased preoperative NLR is associated with
the activated inflammatory response, advanced stage and poorer
survival in patients with CRC, non-small cell lung cancer and
hepatocellular carcinoma (23,33–35).
RDW has been used as an early indicator of increased oxidative
stress and disorders in iron deficiency anemia and iron
mobilization, and its increase is associated with inflammation
markers, including C-reactive protein and interleukin-6 (36,37).
Despite previous studies that have evaluated the clinical value of
RDW as a prognostic indicator in patients with impaired
cardiometabolic function and active inflammation, there is limited
data available concerning the potential use of RDW as a biomarker
of cancer growth and metastatic activity in solid cancer types
(20,36). A previous study indicated that a high
preoperative RDW could be used to predict the long-term survival
rate of patients with lung cancer (38). Another study reported that for
patients with symptomatic multiple myeloma, a preoperative increase
in RDW may reflect worse progression-free survival (39). Albayrak et al (19) demonstrated that an increased RDW was
significantly associated with an elevated risk of progressing into
advanced prostate cancer. RDW has been gradually used to predict
inflammatory status and tumor stress.
Therefore, in the present study, the clinical value
of NLR and RDW in patients with CRC was detected. Karaman et
al (40) reported that NLR may be
used to distinguish neoplastic from non-neoplastic colon polyps, as
the NLR was revealed to be elevated in neoplastic polyps. Ay et
al (24) observed that a
significantly higher RDW was detected in patients with CRC compared
with that in individuals with colon polyps. In the present study,
the NLR and RDW values were higher in patients with CRC compared
with those in patients with colon polyps and healthy controls,
which is consistent with the results of the aforementioned study.
At present, the mechanism underlying this effect has not been
confirmed. It is generally believed that the onset of CRC begins
with an infection or an inflammatory response. NLR and RDW are
sensitive indicators that reflect the activation of the
inflammatory system and are involved in the inflammatory response
(41). Neutrophils remodel the
extracellular matrix to promote tumor growth and invasion, and
inhibit lymphocytes from killing the malignant tumor cells
(42). RDW is a sensitive and
specific indicator of early iron deficiency and malnutrition in CRC
(43). Therefore, when the NLR and
the RDW are elevated in CRC, the body's defense mechanism is
weakened and the barrier against malignant cells is destroyed,
ultimately leading to a poor survival prognosis. This concept is
consistent with the results of the present study.
In the present study, the cutoff values for NLR and
RDW were determined to be 2.06 and 13.45%, respectively, using the
ROC curve. CRC patients with an elevated NLR (NLR ≥2.06) appeared
to exhibit more clinicopathological characteristics associated with
advanced conditions, including a larger tumor diameter, poor tumor
differentiation, deeper tumor infiltration, and high CEA and CA19-9
levels (P<0.05). A higher RDW was also detected in patients with
clinicopathological features associated with advanced conditions,
including older age and distant metastasis (P<0.05).
Furthermore, the logistic regression analysis revealed that a
larger tumor diameter and poor tumor differentiation were
independent risk factors associated with an increased NLR
(P<0.05), while older age and distant metastases were
independent risk factors associated with an increased RDW
(P<0.05).
Few studies have compared the changes in the NLR and
RDW prior to and following surgery; however, no significant
difference was observed and there was no apparent association
between the changes in the NLR and RDW and TNM stage in the present
study.
The Kaplan-Meier cumulative survival rates for OS
and DFS demonstrated that a high RDW value indicated significantly
shorter OS and DFS times in the 128 CRC patients and in the 54
patients with metastases. The high NLR group had no association
with OS or DFS in the 128 patients with CRC. Furthermore, an
increased NLR was indicative of a significantly shorter DFS time
for the 54 patients with metastatic CRC. These results revealed
that RDW serves an important role in predicting the survival of
patients with CRC, particularly those with metastatic CRC.
Furthermore, univariate and multivariate analyses
indicated that, for CRC, only lymph node metastasis and the degree
of differentiation were independent prognostic indicators for OS
and DFS. The potential use of NLR and RDW as independent prognostic
indicators for CRC was not demonstrated in the present study.
Patients with metastasis were analyzed separately in order to
reduce bias and it was revealed that for metastatic CRC, RDW may
act as an independent prognostic indicator for OS and DFS, which
has also been confirmed in previous studies. Zou et al
(41) reported that the NLR acted as
an independent prognostic indicator in patients with CRC.
Furthermore, Malietzis et al (44) demonstrated that the preoperative NLR
could be an independent prognostic indicator for patients with CRC.
Shibutani et al (45) reported
that the preoperative NLR was a simple biomarker and indicator of
poor prognosis for CRC following surgery. Zhao et al
(46) detected that patients with
hepatocellular carcinoma exhibiting high preoperative RDW values
had significantly poorer survival compared with those with low
levels of RDW.
There were a number of limitations to the present
study. As is the case for the majority of retrospective studies,
there may have been unavoidable errors in the data collection. In
addition, the number of subjects in the present study was
relatively small and the follow-up duration was not that long.
Thus, the findings of this study should be validated in further
investigations with larger subject sizes and longer follow-up
durations.
Pre-operative NLR and RDW values are simple and
conveniently measured biomarkers of clinical diagnosis and
prognostic assessment in patients with CRC. NLR and RDW may
function as novel indicators that precisely predict the prognosis
in patients with CRC, particularly in patients with metastatic
CRC.
Acknowledgements
This study was supported by grants obtained from the
National Natural Science Foundation of China (grant nos. 81000731
and 81202307) and the Promoted Research Fund for Excellent Young
and Middle-aged Scientists of Shandong Province (grant no.
BS2010YY045).
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Winawer S, Fletcher R, Rex D, Bond J, Burt
R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D, et al:
Colorectal cancer screening and surveillance: Clinical guidelines
and rationale - Update based on new evidence. Gastroenterology.
124:544–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Byers T, Levin B, Rothenberger D, Dodd GD
and Smith RA: American Cancer Society guidelines for screening and
surveillance for early detection of colorectal polyps and cancer:
Update 1997. American Cancer Society Detection and Treatment
Advisory Group on Colorectal Cancer. CA Cancer J Clin. 47:154–160.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rex DK, Johnson DA, Lieberman DA, Burt RW
and Sonnenberg A: Colorectal cancer prevention 2000: Screening
recommendations of the American College of Gastroenterology.
American College of Gastroenterology. Am J Gastroenterol.
95:868–877. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guthrie GJ, Charles KA, Roxburgh CS,
Horgan PG, McMillan DC and Clarke SJ: The systemic
inflammation-based neutrophil-lymphocyte ratio: Experience in
patients with cancer. Crit Rev Oncol Hematol. 88:218–230. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zahorec R: Ratio of neutrophil to
lymphocyte counts-rapid and simple parameter of systemic
inflammation and stress in critically ill. Bratisl Lek Listy.
102:5–14. 2001.(In English). PubMed/NCBI
|
|
9
|
Yang JJ, Hu ZG, Shi WX, Deng T, He SQ and
Yuan SG: Prognostic significance of neutrophil to lymphocyte ratio
in pancreatic cancer: A meta-analysis. World J Gastroenterol.
21:2807–2815. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim
YT and Lee K: Pre-treatment neutrophil to lymphocyte ratio is
elevated in epithelial ovarian cancer and predicts survival after
treatment. Cancer Immunol Immunother. 58:15–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Azab B, Bhatt VR, Phookan J, Murukutla S,
Kohn N, Terjanian T and Widmann WD: Usefulness of the
neutrophil-to-lymphocyte ratio in predicting short- and long-term
mortality in breast cancer patients. Ann Surg Oncol. 19:217–224.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aliustaoglu M, Bilici A, Ustaalioglu BB,
Konya V, Gucun M, Seker M and Gumus M: The effect of peripheral
blood values on prognosis of patients with locally advanced gastric
cancer before treatment. Med Oncol. 27:1060–1065. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yesil A, Senates E, Bayoğlu IV, Erdem ED,
Demirtunc R and Kurdas OA: Red cell distribution width: A novel
marker of activity in inflammatory bowel disease. Gut Liver.
5:460–467. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Forhecz Z, Gombos T, Borgulya G, Pozsonyi
Z, Prohaszka Z and Jánoskuti L: Red cell distribution width in
heart failure: Prediction of clinical events and relationship with
markers of ineffective erythropoiesis, inflammation, renal function
and nutritional state. Am Heart J. 158:659–666. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lorente L, Martin MM, Abreu-Gonzalez P,
Solé-Violán J, Ferreres J, Labarta L, Díaz C, González O, García D,
Jiménez A and Borreguero-León JM: Red blood cell distribution width
during the first week is associated with severity and mortality in
septic patients. PLoS One. 9:e1054362014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang R, Yang C, Wu K, Cao S, Liu Y, Su R,
Xiong Y, Huang A and Wu C: Red cell distribution width as a
potential index to assess the severity of hepatitis B virus-related
liver diseases. Hepatol Res. 44:E464–E470. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koma Y, Onishi A, Matsuoka H, Oda N,
Yokota N, Matsumoto Y, Koyama M, Okada N, Nakashima N, Masuya D, et
al: Increased red blood cell distribution width associates with
cancer stage and prognosis in patients with lung cancer. PLoS One.
8:e802402013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smirne C, Grossi G, Pinato DJ, Burlone ME,
Mauri FA, Januszewski A, Oldani A, Minisini R, Sharma R and Pirisi
M: Evaluation of the red cell distribution width as a biomarker of
early mortality in hepatocellular carcinoma. Dig Liver Dis.
47:488–494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Albayrak S, Zengin K, Tanik S, Bakirtas H,
Imamoglu A and Gurdal M: Red cell distribution width as a predictor
of prostate cancer progression. Asian Pac J Cancer Prev.
15:7781–7784. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Söderholm M, Borné Y, Hedblad B, Persson M
and Engström G: Red cell distribution width in relation to
incidence of stroke and carotid atherosclerosis: A population-based
cohort study. PLoS One. 10:e1249572015. View Article : Google Scholar
|
|
21
|
Sahin I, Karabulut A, Kaya A, Güngör B,
Avcı İİ, Okuyan E, Can MM, Sığırcı S, Ayça B and Dinçkal MH:
Increased level of red cell distribution width is associated with
poor coronary collateral circulation in patients with stable
coronary artery disease. Turk Kardiyol Dern Ars. 43:123–130.
2015.PubMed/NCBI
|
|
22
|
Huang YL, Hu ZD, Liu SJ, Sun Y, Qin Q, Qin
BD, Zhang WW, Zhang JR, Zhong RQ and Deng AM: Prognostic value of
red blood cell distribution width for patients with heart failure:
a systematic review and meta-analysis of cohort studies. PLoS One.
9:e1048612014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen ZY, Raghav K, Lieu CH, Jiang ZQ, Eng
C, Vauthey JN, Chang GJ, Qiao W, Morris J, Hong D, et al: Cytokine
profile and prognostic significance of high neutrophil-lymphocyte
ratio in colorectal cancer. Br J Cancer. 112:1088–1097. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ay S, Eryilmaz MA, Aksoy N, Okus A, Unlu Y
and Sevinc B: Is early detection of colon cancer possible with red
blood cell distribution width? Asian Pac J Cancer Prev. 16:753–756.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wittekind C: 2010 TNM system: On the 7th
edition of TNM classification of malignant tumors. Pathologe.
31:331–332. 2010.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in globocan. Int J Cancer. 136:E359–E386. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao P, Dai M, Chen W and Li N: Cancer
trends in China. Jpn J Clin Oncol. 40:281–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song ZB, Lin BC, Li B, He CX, Zhang BB,
Shao L and Zhang YP: Preoperative elevation of serum C-reactive
protein as an indicator of poor prognosis for early-stage
esophageal squamous cell carcinoma. Kaohsiung J Med Sci.
29:662–666. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng YZ, Dai SQ, Li W, Cao X, Li Y, Zhang
LJ, Fu JH and Wang JY: Prognostic value of preoperative mean
corpuscular volume in esophageal squamous cell carcinoma. World J
Gastroenterol. 19:2811–2817. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yuan D, Zhu K, Li K, Yan R, Jia Y and Dang
C: The preoperative neutrophil-lymphocyte ratio predicts recurrence
and survival among patients undergoing R0 resections of
adenocarcinomas of the esophagogastric junction. J Surg Oncol.
110:333–340. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
An X, Ding PR, Li YH, Wang FH, Shi YX,
Wang ZQ, He YJ, Xu RH and Jiang WQ: Elevated neutrophil to
lymphocyte ratio predicts survival in advanced pancreatic cancer.
Biomarkers. 15:516–522. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kayadibi H, Sertoglu E, Uyanik M and Tapan
S: Neutrophil-lymphocyte ratio is useful for the prognosis of
patients with hepatocellular carcinoma. World J Gastroenterol.
20:9631–9632. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang H, Zhang L, Zhu K, Shi B, Yin Y, Zhu
J, Yue D, Zhang B and Wang C: Prognostic significance of
combination of preoperative platelet count and
neutrophil-lymphocyte ratio (COP-NLR) in patients with non-small
cell lung cancer: Based on a large cohort study. PLoS One.
10:e01264962015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shau HY and Kim A: Suppression of
lymphokine-activated killer induction by neutrophils. J Immunol.
141:4395–4402. 1988.PubMed/NCBI
|
|
36
|
Agarwal S: Red cell distribution width,
inflammatory markers and cardiorespiratory fitness: Results from
the national health and nutrition examination survey. Indian Heart
J. 64:380–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Karabulut A and Uzunlar B: Correlation
between red cell distribution width and coronary ectasia in the
acute myocardial infarction. Clin Appl Thromb Hemost. 18:551–552.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Warwick R, Mediratta N, Shackcloth M, Shaw
M, McShane J and Poullis M: Preoperative red cell distribution
width in patients undergoing pulmonary resections for
non-small-cell lung cancer. Eur J Cardiothorac Surg. 45:108–113.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee H, Kong SY, Sohn JY, Shim H, Youn HS,
Lee S, Kim HJ and Eom HS: Elevated red blood cell distribution
width as a simple prognostic factor in patients with symptomatic
multiple myeloma. Biomed Res Int. 2014:1456192014.PubMed/NCBI
|
|
40
|
Karaman H, Karaman A, Erden A, Poyrazoglu
OK, Karakukcu C and Tasdemir A: Relationship between colonic polyp
type and the neutrophil/lymphocyte ratio as a biomarker. Asian Pac
J Cancer Prev. 14:3159–3161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zou ZY, Liu HL, Ning N, Li SY, DU XH and
Li R: Clinical significance of pre-operative neutrophil lymphocyte
ratio and platelet lymphocyte ratio as prognostic factors for
patients with colorectal cancer. Oncol Lett. 11:2241–2248. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Emir S, Aydin M, Can G, Bali I, Yildirim
O, Öznur M, Yildiz ZD, Sözen S and Gürel A: Comparison of
colorectal neoplastic polyps and adenocarcinoma with regard to NLR
and PLR. Eur Rev Med Pharmacol Sci. 19:3613–3618. 2015.PubMed/NCBI
|
|
43
|
Spell DW, Jones DV jr, Harper WF and David
Bessman J: The value of a complete blood count in predicting cancer
of the colon. Cancer Detect Prev. 28:37–42. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Malietzis G, Giacometti M, Askari A,
Nachiappan S, Kennedy RH, Faiz OD, Aziz O and Jenkins JT: A
preoperative neutrophil to lymphocyte ratio of 3 predicts
disease-free survival after curative elective colorectal cancer
surgery. Ann Surg. 260:287–292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shibutani M, Maeda K, Nagahara H, Noda E,
Ohtani H, Nishiguchi Y and Hirakawa K: A high preoperative
neutrophil-to-lymphocyte ratio is associated with poor survival in
patients with colorectal cancer. Anticancer Res. 33:3291–3294.
2013.PubMed/NCBI
|
|
46
|
Zhao T, Cui L and Li A: The significance
of RDW in patients with hepatocellular carcinoma after radical
resection. Cancer Biomark. 16:507–512. 2016. View Article : Google Scholar : PubMed/NCBI
|