Introduction
Hepatocellular carcinoma (HCC) is a highly malignant
type of cancer that is the 5th most commonly diagnosed cancer and
the second leading cause of cancer-associated mortality worldwide
(1). Hepatitis B virus (HBV) and
hepatitis C virus (HCV) infections are major risk factors for the
development of HCC (2). Due to the
high incidence of HBV and HCV infection, the incidence of HCC is
increasing, particularly in Southeast Asia and sub-Saharan Africa
(3). At present, surgical resection
and liver transplantation remain the most effective therapies for
HCC. Although improvements have been made regarding HCC diagnosis
and systematic therapies, the prognosis is not favorable
(three-year survival rate, 30–40%) (4,5). Cancer
metastasis and recurrence are the major causes for HCC-associated
mortality. The resistance of HCC to anticancer therapy results from
its highly heterogeneous character (6). Cancer metastasis and recurrence, and
embryonic development have many analogous properties, including
dynamic cell motility, cellular plasticity and integral interaction
with the microenvironment. Past research has identified that the
heterogeneity of HCC may result from the presence of hepatic cancer
cells with stem/progenitor features (7).
Cancer stem cells (CSCs) are a subpopulation of
cancer cells that can self-renew and differentiate to produce
heterogeneous tumors. CSCs may cause cancer relapse and metastases,
due to their resistance to drugs and radiation therapy. Past
research has indicated the existence of CSCs in various cancers by
isolation using cell surface stemness-associated markers, including
markers for leukemia (CD34), breast cancer (CD44) and colon cancer
(CD133) CSCs (8–10). Several stemness-associated markers of
HCC have been proposed for subtype classification, including CD133,
epithelial cell adhesion molecule (EpCAM), keratin 19, and
α-fetoprotein (AFP), which have been individually associated with a
poorer prognosis (11–13). However, subtype classification based
on only one stemness-associated marker may not be sufficient to
clearly define CSCs or decipher the heterogeneous nature of HCC.
The combination of multiple stemness-associated markers is required
to achieve a more precise subtype classification, which may enable
the prognostic stratification and effective assessment of patients
with HCC for personalized therapy.
Yamashita et al (7) identified novel HCC subtypes using EpCAM
expression and serum AFP levels to subclassify HCC into the four
groups: EpCAM+AFP+,
EpCAM−AFP−, EpCAM+AFP−,
and EpCAM−AFP+. These four subtypes exhibited
distinct gene expression patterns, with features resembling certain
developmental stages of hepatic lineages. The
EpCAM+AFP+ subtype exhibited the poorest
prognosis and displayed hepatic stem cell-like traits, whereas the
EpCAM−AFP− subtype was associated with a more
favorable outcome and exhibited mature hepatocyte-like features.
The Wnt/β-catenin signaling pathway, critical for maintaining
embryonic stem cells (14), was
activated in the EpCAM+AFP+ subtype, but not
in the EpCAM−AFP− subtype, indicating that
these four subtypes required different therapeutic interventions.
CD133 is one of the most commonly used stemness-associated markers
in HCC (15). To the best of our
knowledge, HCC subtype classification based on a combination of
CD133 expression and serum AFP levels has not been previously
reported.
In the present study, a CD133/AFP classification
system to subdivide HCC into four different subtypes is defined for
the prediction of the prognosis and clinicopathological
features.
Subjects and methods
Patients and clinicopathological
information
Tissue samples were obtained, with informed consent,
from 127 patients with HBV-associated HCC who underwent curative
hepatectomies at the Prince of Wales Hospital (Hong Kong, China)
from November 1995 to January 2013. The present study strictly
abided by the Reporting Recommendations for Tumor Marker Prognostic
Studies guidelines (16) and the
Transparent Reporting of a multivariable prediction model for
Individual Prognosis or Diagnosis Statement (17). The study was approved by the Joint
Chinese University of Hong Kong-New Territories East Cluster
Clinical Research Ethics Committee (Hong Kong, China). The patient
eligibility criteria were as follows: i) To have been diagnosed
with HCC for the first time, without distant metastasis and to have
not received any anticancer therapy before surgery; ii) to have
undergone a liver function test revealing Child-Pugh grade A
disease and tumor-negative resection margins following the curative
hepatectomy; iii) to have no other malignant tumors, autoimmune
diseases, or serious heart, lung, kidney or blood diseases; iv) to
be seropositive for hepatitis B surface antigen and seronegative
for HCV; and v) to have available follow-up information. All HCC
specimens were histologically documented by two independent
pathologists blinded to all patient identities and clinical
outcomes. Serum AFP levels were evaluated by an
electrochemiluminescence immunoassay (E170 Analytics; Roche
Diagnostics, Indianapolis, IN, USA) as previously described
(18) prior to hepatectomy. The
details of other biochemical markers, including albumin, alanine
aminotransferase and bilirubin, were acquired from the patients'
medical records. All patients' clinicopathological data are
summarized in Table I.
 | Table I.Main demographic, biochemical and
clinical characteristics of the 127 patients with HCC. |
Table I.
Main demographic, biochemical and
clinical characteristics of the 127 patients with HCC.
| Variable | Unit | Value |
|---|
| Age, median
(range) | years | 57.3 (13–84) |
| Sex (n, %) |
|
|
|
Male |
| 103 (81.1) |
|
Female |
| 14 (18.9) |
| Albumin, median
(range) | g/l | 38.2 (29–46) |
| ALT, median
(range) | U/l | 49.8 (11–227) |
| Total bilirubin,
median (range) | g/l | 10.5 (3–20) |
| HCC diameter,
median (range) | cm | 5.3 (1.1–15) |
| AFP, median
(range) | ng/ml | 92 (2–699,800) |
Follow-up information
Patients visited the outpatient clinic every 3
months in the first year after surgery, every 4 months in the
second year after surgery, and every 6 months thereafter; tumor
recurrence was diagnosed by contrast-enhanced computed tomography
or magnetic resonance images, which were obtained at least every
three months during the postoperative follow-up. All patients were
followed-up until 8th November 2014 or the event of mortality.
Information regarding patient mortality was obtained from the
Social Security Death Index, medical records or notification from
the family of the deceased.
Immunohistochemical analysis
Immunohistochemical staining was performed as
described by Kang et al (19).
The primary antibody was anti-human CD133 mouse monoclonal antibody
(cat. no. MAB4399; Millipore, Billerica, MA, USA). CD133 expression
levels were semi-quantitatively scored from 0–3, according to the
proportion of tumor cells with positive cytoplasmic/membranous
staining, as follows: 0 (negative), staining in <1% of tumor
cells; 1, weak staining in ≥1%; 2, moderate staining in ≥1%; and 3,
strong staining in ≥1% of tumor cells. Staining scores of 2 and 3
were defined as positive staining, whereas 0 and 1 were regarded as
negative staining (11).
Statistical analysis
Continuous data were expressed as the median
(range). The association of CD133 expression or AFP level with
clinicopathological characteristics was examined using
χ2 tests. Overall survival (OS) was defined as the time
from curative hepatectomy until 8th November 2014 or the event of
mortality. Recurrence-free survival (RFS) was defined as the time
from curative hepatectomy to the first recurrence. The Kaplan-Meier
method was used to calculate OS and RFS, and the log rank-test was
applied to determine the differences between survival curves. Our
previous study (13) demonstrated
that an AFP cut-off value of 400 ng/ml was optimal to assess the
link between AFP levels and OS/RFS, rather than 300 ng/ml as
recommended by the existing literature (20). Therefore, the AFP cut-off value was
defined as 400 ng/ml in the present study. All statistical analyses
were performed using the SPSS software package (version 16.0; SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics and
clinicopathological features
A total of 127 eligible patients with HBV-associated
HCC were recruited to the present study. Their characteristics are
summarized in Tables I and II. The mean follow-up time was 106.3
(range, 24–215) months.
 | Table II.Associations of serum AFP levels and
CD133 protein expression in surgical specimens of HCC with
clinicopathological characteristics. |
Table II.
Associations of serum AFP levels and
CD133 protein expression in surgical specimens of HCC with
clinicopathological characteristics.
|
| AFP (ng/ml), n |
| CD133, n |
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
parameter | ≤400 | >400 | P-value | Negative | Positive | P-value |
|---|
| Total | 80 | 47 |
| 75 | 52 |
|
| Age, years |
|
| 0.275 |
|
| 0.614 |
|
≥50 | 60 | 31 |
| 55 | 36 |
|
|
<50 | 20 | 16 |
| 20 | 16 |
|
| Sex |
|
| 0.377 |
|
| 0.316 |
|
Male | 63 | 40 |
| 63 | 40 |
|
|
Female | 17 | 7 |
| 12 | 12 |
|
| No. of tumor
lesions |
|
| 0.280 |
|
|
<0.001a |
|
Solitary | 63 | 33 |
| 65 | 31 |
|
|
Multiple | 17 | 14 |
| 10 | 21 |
|
| Cirrhosis |
|
| 0.738 |
|
| 0.396 |
|
Absent | 35 | 22 |
| 36 | 21 |
|
|
Present | 45 | 25 |
| 39 | 31 |
|
| Tumor size |
|
| 0.094 |
|
| 0.650 |
| ≥5
cm | 32 | 26 |
| 33 | 25 |
|
| <5
cm | 48 | 21 |
| 42 | 27 |
|
| AFP |
|
|
|
|
| 0.927 |
| >400
µg/l | – | – |
| 28 | 19 |
|
| ≤400
µg/l | – | – |
| 47 | 33 |
|
|
Differentiation |
|
| 0.022a |
|
| 0.487 |
|
Well/moderate | 73 | 36 |
| 63 | 46 |
|
|
Poor | 7 | 11 |
| 12 | 6 |
|
| Vascular
invasion |
|
| 0.016a |
|
| 0.054 |
|
Absent | 70 | 33 |
| 65 | 38 |
|
|
Present | 10 | 14 |
| 10 | 14 |
|
The distribution of AFP levels was skewed, with a
range of 2–699,800 (median, 92) ng/ml. A serum AFP cut-off value of
(400 ng/ml) was used to classify HCC as AFP+ or
AFP−. AFP+ HCC was associated with poorer
histological differentiation (P=0.022) and vascular invasion
(P=0.016). No associations were identified between AFP and patient
age, sex, number of tumor lesions (solitary or multiple), cirrhosis
(absence or presence) or tumor size (≤5 vs. >5 cm; Table II).
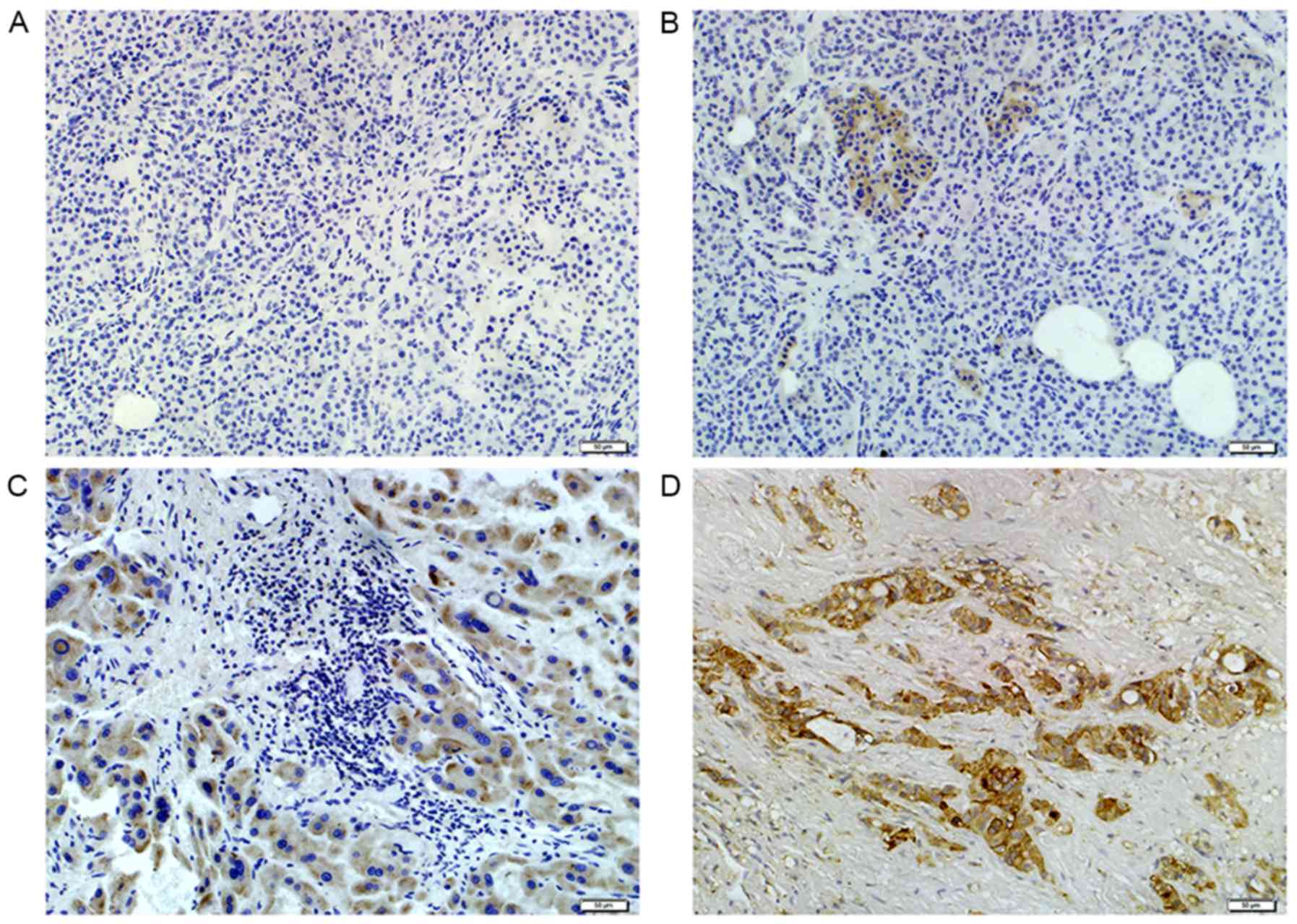
The CD133 cytoplasmic/membranous expression was
observed as negative, weak, moderate or strong staining (Fig. 1). Positive CD133 expression was
detected in 52 out of 127 of the patients with HCC. Positive CD133
staining was detected more often in patients with multiple tumor
lesions (P<0.001), and was trending towards an association with
vascular invasion status (P=0.054). No association between CD133
expression and other clinicopathological features was identified
(Table II).
According to CD133 expression and serum AFP levels,
all 127 HBV-associated HCC cases were subclassified into four
groups: i) CD133+AFP+, 19 cases (15.0%); ii)
CD133−AFP+, 28 cases (22.0%); iii)
CD133+AFP−, 33 cases (26.0%) and iv)
CD133−AFP−, 47 cases (37.0%; Table III). Among these four HCC subtypes,
the number of tumor lesions, the histological grade and vascular
invasion were significantly different (P=0.002, P=0.018 and
P=0.022, respectively). Patients classified with
CD133+AFP+ HCC were more liable to developing
multiple tumor lesions and vascular invasion compared with patients
classified with CD133−AFP− HCC, which
typically presented with single tumor lesions without vascular
invasion. CD133+AFP− HCC was also associated
with multiple tumor lesions, and CD133−AFP+
HCC was associated with the patients demonstrating ‘poor’
histological differentiation, with the highest frequency of all
types (32.1%; 9/28).
 | Table III.Clinicopathological characteristics
of HCC subtypes defined by AFP and CD133 expression. |
Table III.
Clinicopathological characteristics
of HCC subtypes defined by AFP and CD133 expression.
|
| HCC subtype |
|
|---|
|
|
|
|
|---|
| Clinicopathological
parameter |
AFP−CD133− |
AFP−CD133+ |
AFP+CD133− |
AFP+CD133+ | P-value |
|---|
| Age, years | 47 | 33 | 28 | 19 | 0.526 |
|
≥50 | 35 | 25 | 20 | 11 |
|
|
<50 | 12 | 8 | 8 | 8 |
|
| Sex |
|
|
|
| 0.594 |
|
Male | 38 | 25 | 25 | 15 |
|
|
Female | 9 | 8 | 3 | 4 |
|
| No. of tumor
lesions |
|
|
|
| 0.002a |
|
Solitary | 41 | 22 | 24 | 9 |
|
|
Multiple | 6 | 11 | 4 | 10 |
|
| Cirrhosis |
|
|
|
| 0.843 |
|
Absent | 22 | 13 | 14 | 8 |
|
|
Present | 25 | 20 | 14 | 11 |
|
| Tumor size, cm |
|
|
|
| 0.312 |
| ≥5 | 17 | 15 | 16 | 10 |
|
|
<5 | 30 | 18 | 12 | 9 |
|
|
Differentiation |
|
|
|
| 0.018a |
|
Well/moderate | 44 | 29 | 19 | 17 |
|
|
Poor | 3 | 4 | 9 | 2 |
|
| Vascular
invasion |
|
|
|
| 0.022a |
|
Absent | 44 | 26 | 21 | 12 |
|
|
Present | 3 | 7 | 7 | 7 |
|
Prognosis of HCC subtypes defined by
AFP or CD133
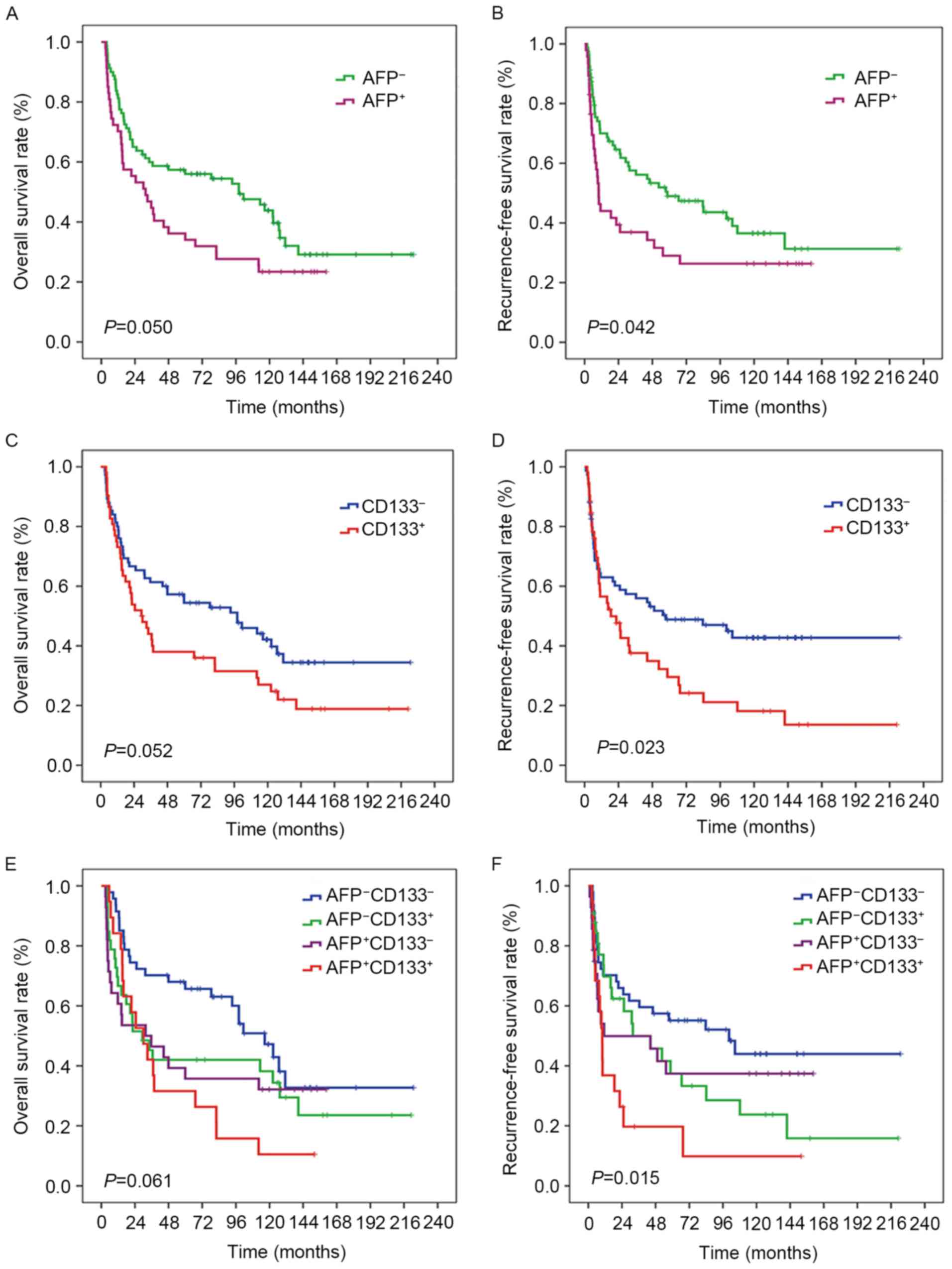
OS and RFS curves according to AFP levels (cut-off
value, 400 ng/ml) are illustrated in Fig.
2A and B. A significantly increased risk of mortality and
recurrence was identified in AFP-positive patients compared with
AFP-negative patients (P=0.050 and P=0.042, respectively). Fig. 2C and D includes OS and RFS curves
based on the expression of CD133; patients with CD133-positive
tumors had a higher risk of mortality that was trending towards
significance (P=0.052) and a significantly higher risk of disease
recurrence (P=0.023) than CD133-negative patients.
Prognosis of HCC subtypes defined by
AFP and CD133
Kaplan-Meier survival and recurrence analysis were
performed to assess the prognosis of the four HCC subtypes. The
result of Kaplan-Meier OS and RFS analyses (Fig. 2E and F) revealed that
CD133+AFP+ HCC was associated with a
relatively poor prognosis, CD133−AFP− HCC was
associated with a relatively good prognosis, and CD133+
AFP− HCC and CD133−AFP+ HCC were
associated with intermediate prognoses. Statistical analysis
demonstrated a borderline result for the association with OS
(P=0.061) and statistical significance for RFS (P=0.015).
Discussion
HCC is a highly heterogeneous type of tumor. Several
studies have demonstrated that the presence of HCC cells with
stem/progenitor features may lead to tumor heterogeneity (12,21).
Experimental evidence has indicated that liver stem cells can
develop into hepatoblasts, differentiate into hepatocytic or
biliary lineage progenitor cells, and mature into hepatocytes
(22,23). CD133 or AFP have been used to identify
hepatic CSCs (24,25). CD133 is a five-pass transmembrane cell
surface glycoprotein that is expressed in normal and malignant stem
cells of hematopoietic, neuronal/glial, renal/prostatic and
hepatic/endothelial lineages (26–30). Other
studies have indicated that CD133-positive cells separated from HCC
cell lines are able to self-renew and differentiate (25,31),
suggesting that CD133 is a common and robust HCC CSC marker. AFP is
a biomarker for HCC, expressed in fetal livers and HCC tumors, but
not in the adult liver, analogous to the expression of CD133
(32). A previous study has indicated
that AFP-producing cells in primary liver cancers may exhibit
CSC-like properties (24). Therefore,
the combination approach of using CD133 and AFP to define CSCs in
HCC is reasonable.
The present study revealed that patients with
AFP+ HCC exhibited poorer histological differentiation,
positive vascular invasion and an increased risk of mortality and
recurrence. CD133-immunopositivity was more frequently observed in
patients with multiple tumor lesions, vascular invasion and higher
risks of mortality and recurrence. Therefore, AFP and CD133 were
identified as independent poor prognosis factors for patients with
HCC, which corroborates the results of previous studies (13,33). A
poor prognosis may be associated with the existence of CSCs
(AFP+ or CD133+), which facilitate cancer
therapy resistance and accelerate cancer progression. The
combination of CD133 and AFP may be necessary for a more precise
classification of prognosis-associated subtypes.
HCC was classified into four subtypes in the present
study based on CD133 expression and serum AFP levels. The four HCC
subtypes corresponded to different clinicopathological features and
prognosis. Among the four subtypes,
CD133+AFP+ HCC was associated with the
poorest prognosis and CD133−AFP− HCC with the
most favorable prognosis.
This classification system is similar to the model
of Yamashita et al (7), which
demonstrated that EpCAM+AFP+ HCC, associated
with the poorest prognosis, displayed hepatic stem cell-like
traits, whereas EpCAM−AFP− HCC, associated
with a more favorable prognosis, exhibited the features of mature
hepatocytes. We hypothesize that the four subtypes classified based
on AFP and CD133 may also resemble different hepatocyte lineages,
and that this classification system may reflect the cellular origin
of the heterogeneous nature of HCC. The present study indicates
that the expression of various stem cell markers in CSCs may be
different in each HCC subtype, possibly resulting from the
heterogeneity of the activated CSC signaling pathways. Yamashita
et al (21) indicated that
EpCAM+AFP+ HCC cells displayed hepatic
CSC-like traits, including the abilities to self-renew and
differentiate by the activation of Wnt/β-catenin signaling.
Therefore, experimental design to test this hypothesis should
consider the molecular pathways potentially involved in the
maintenance of CD133+AFP+ HCC cells.
The present study is constrained by a number of
limitations. The sample size of the study is relatively small due
to the rigorous eligibility criteria for the selection of patients
with HBV-associated HCC; however, including such a homogeneous
group of patients provides internal validity to the results.
Furthermore, study regarding why CD133+AFP+
HCC is associated with a poor prognosis is required. Future studies
by this group will aim to explore whether
CD133+AFP+ HCC cells exhibit hepatic stem
cell-like traits and determine the associated molecular pathways
that are activated to sustain the CSC features of
CD133+AFP+ HCC cells.
In conclusion, the present study defined a novel and
simple classification system for subclassifying HCC into four
different prognostic subtypes based on CD133 expression and AFP
serum levels. This classification system may allow the provision of
personalized therapy to patients with HCC.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Altekruse SF, McGlynn KA, Dickie LA and
Kleiner DE: Hepatocellular carcinoma confirmation, treatment, and
survival in surveillance, epidemiology, and end results registries,
1992–2008. Hepatology. 55:476–482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bosch FX, Ribes J, Cléries R and Diaz M:
Epidemiology of hepatocellular carcinoma. Clin Liver Dis.
9:191–211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tanaka S and Arii S: Molecularly targeted
therapy for hepatocellular carcinoma. Cancer Sci. 100:1–8. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lau WY and Lai EC: The current role of
radiofrequency ablation in the management of hepatocellular
carcinoma: A systematic review. Ann Surg. 249:20–25. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thorgeirsson SS and Grisham JW: Molecular
pathogenesis of human hepatocellular carcinoma. Nat Genet.
31:339–346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamashita T, Forgues M, Wang W, Kim JW, Ye
Q, Jia H, Budhu A, Zanetti KA, Chen Y, Qin LX, et al: EpCAM and
alpha-fetoprotein expression defines novel prognostic subtypes of
hepatocellular carcinoma. Cancer Res. 68:1451–1461. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 100:pp.
3983–3988. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim H, Choi GH, Na DC, Ahn EY, Kim GI, Lee
JE, Cho JY, Yoo JE, Choi JS and Park YN: Human hepatocellular
carcinomas with ‘Stemness’-related marker expression: Keratin 19
expression and a poor prognosis. Hepatology. 54:1707–1717. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Terris B, Cavard C and Perret C: EpCAM, a
new marker for cancer stem cells in hepatocellular carcinoma. J
Hepatol. 52:280–281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang SL, Liu LP, Yang S, Liu L, Ren JW,
Fang X, Chen GG and Lai PB: Preoperative serum α-fetoprotein and
prognosis after hepatectomy for hepatocellular carcinoma. Br J
Surg. 103:716–724. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma S, Tang KH, Chan YP, Lee TK, Kwan PS,
Castilho A, Ng I, Man K, Wong N, To KF, et al: miR-130b promotes
CD133(+) liver tumor-initiating cell growth and self-renewal via
tumor protein 53-induced nuclear protein 1. Cell Stem Cell.
7:694–707. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Altman DG, McShane LM, Sauerbrei W and
Taube SE: Reporting recommendations for tumor marker prognostic
studies (REMARK): Explanation and elaboration. PLoS Med.
9:e10012162012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moons KG, Altman DG, Reitsma JB and
Collins GS: Transparent Reporting of a Multivariate PredictionModel
for Individual Prognosis or Development Initiative: New guideline
for the reporting of studies developing, validating, or updating a
multivariable clinical prediction model: The TRIPOD statement. Adv
Anat Pathol. 22:303–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chan SL, Mo F, Johnson PJ, Siu DY, Chan
MH, Lau WY, Lai PB, Lam CW, Yeo W and Yu SC: Performance of serum
α-fetoprotein levels in the diagnosis of hepatocellular carcinoma
in patients with a hepatic mass. HPB (Oxford). 16:366–372. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang YK, Hong SW, Lee H and Kim WH:
Prognostic implications of ezrin expression in human hepatocellular
carcinoma. Mol Carcinog. 49:798–804. 2010.PubMed/NCBI
|
|
20
|
Lee JS, Chu IS, Heo J, Calvisi DF, Sun Z,
Roskams T, Durnez A, Demetris AJ and Thorgeirsson SS:
Classification and prediction of survival in hepatocellular
carcinoma by gene expression profiling. Hepatology. 40:667–676.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamashita T, Ji J, Budhu A, Forgues M,
Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, et al:
EpCAM-positive hepatocellular carcinoma cells are tumor-initiating
cells with stem/progenitor cell features. Gastroenterology.
136:1012–1024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmelzer E, Wauthier E and Reid LM: The
phenotypes of pluripotent human hepatic progenitors. Stem Cells.
24:1852–1858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roskams T: Liver stem cells and their
implication in hepatocellular and cholangiocarcinoma. Oncogene.
25:3818–3822. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ishii T, Yasuchika K, Suemori H, Nakatsuji
N, Ikai I and Uemoto S: Alpha-fetoprotein producing cells act as
cancer progenitor cells in human cholangiocarcinoma. Cancer Lett.
294:25–34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO,
Zheng BJ and Guan XY: Identification and characterization of
tumorigenic liver cancer stem/progenitor cells. Gastroenterology.
132:2542–2556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lindgren D, Boström AK, Nilsson K, Hansson
J, Sjölund J, Möller C, Jirström K, Nilsson E, Landberg G, Axelson
H and Johansson ME: Isolation and characterization of
Progenitor-like cells from human renal proximal tubules. Am J
Pathol. 178:828–837. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmelzer E, Zhang L, Bruce A, Wauthier E,
Ludlow J, Yao HL, Moss N, Melhem A, McClelland R, Turner W, et al:
Human hepatic stem cells from fetal and postnatal donors. J Exp
Med. 204:1973–1987. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Richardson GD, Robson CN, Lang SH, Neal
DE, Maitland NJ and Collins AT: CD133, a novel marker for human
prostatic epithelial stem cells. J Cell Sci. 117:3539–3545. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Uchida N, Buck DW, He DP, Reitsma MJ,
Masek M, Phan TV, Tsukamoto AS, Gage FH and Weissman IL: Direct
isolation of human central nervous system stem cells. Proc Natl
Acad Sci USA. 97:pp. 14720–14725. 2000; View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yin AH, Miraglia S, Zanjani ED,
Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J and Buck
DW: AC133, a novel marker for human hematopoietic stem and
progenitor cells. Blood. 90:5002–5012. 1997.PubMed/NCBI
|
|
31
|
Yin S, Li J, Hu C, Chen X, Yao M, Yan M,
Jiang G, Ge C, Xie H, Wan D, et al: CD133 positive hepatocellular
carcinoma cells possess high capacity for tumorigenicity. Int J
Cancer. 120:1444–1450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parpart S, Roessler S, Dong F, Rao V,
Takai A, Ji J, Qin LX, Ye QH, Jia HL, Tang ZY and Wang XW:
Modulation of miR-29 expression by α-fetoprotein is linked to the
hepatocellular carcinoma epigenome. Hepatology. 60:872–883. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sasaki A, Kamiyama T, Yokoo H, Nakanishi
K, Kubota K, Haga H, Matsushita M, Ozaki M, Matsuno Y and Todo S:
Cytoplasmic expression of CD133 is an important risk factor for
overall survival in hepatocellular carcinoma. Oncol Rep.
24:537–546. 2010. View Article : Google Scholar : PubMed/NCBI
|