Introduction
Patients with pancreatic cancer increase in number
every year. Pancreatic cancer is typically associated with
extremely poor prognosis. Even with advanced imaging technology and
diagnosis, many patients are diagnosed at a stage when the cancer
is unresectable. Even if resected, most will eventually metastasize
to locations such as the liver or peritoneum (1–3).
Improvements in chemotherapy are necessary to improve the prognosis
of pancreatic cancer patients (4).
Gemcitabine was approved in the United States in 1996 and is a key
drug for patients with pancreatic cancer (5). In recent years, gemcitabine has been
administered with nab-paclitaxel. Although the survival rate is
improved, the prognosis of patients with pancreatic cancer remains
grim. In vivo animal models are important for facilitating
the rapid development of effective drugs. These models imitate
metastatic patterns and allow for close examination of the
therapeutic effects of new medications.
Therefore, several mouse models are used, including
orothotopically, heterotopically, syngenic, xenografted (6); patient-derived tumor xenografted or
genetically engineered cancer models (7,8). These
models have varied advantages pertaining to ease, cost,
reproducibility, etc. Generally, preclinical drug development
examines the effects of the subcutaneous implantation model on
tumor regression. Mia-pa-ca-2, Capan-1, and BX-PC-3 are used as
models for pancreatic cancer; however, only BX-PC-3 is used as a
model for gemcitabine resistance in pancreatic cancer. Our
laboratory has not confirmed distant metastasis in models involving
subcutaneous implantation of Capan-1 or SUIT-2 cell lines.
Therefore, mice that are subcutaneously transplanted with these
cells cannot be used to assess drug anti-tumor effects or
metastases. Tumor regression effects alone are insufficient for
evaluating survival extension and the curability of cancer. There
is a need to evaluate drugs in multiple ways, determining if the
drug acts on pancreatic tumors, if it suppresses metastases, and if
it can improve secondary pathologies.
Among these promising models, we expect orthotopic
xenografted pancreatic cancer models to be preferable because the
model can evaluate the drug effects on human pancreatic cancer
cells and imitate the natural metastatic cascades with high
reproducibility and convenience and without special gene
engineering technologies or ethical issues related to the use of
clinical pancreatic cancer specimens. Previous studies affirm the
usefulness of orthotopic xenografted pancreatic cancer model mice
in these studies (9–13). There are several pancreatic cancer
cell lines, and all cell lines do not metastasize from
orthotopically xenografted pancreas, as in the typical course of
human pancreatic cancer (14,15). In addition, few studies have examined
the cell lines suitable for evaluating drug efficacy when
xenografted cells spread from the primary pancreas to the
extra-pancreas and distant organs. We also do not know when target
drugs should be started in experimental animal models during
preclinical testing. Effective drug development requires that we
address these experimental problems and examine the systemic
pathological, and therapeutic, effects.
The purpose of this study was to establish an ideal
pancreatic cancer mouse model for reliable preclinical testing.
Such a model will accurately reflect human pancreatic cancer
phenotypes and estimate the results of future clinical trials. We
focused on the orthotopic pancreatic cancer mouse model using the
SUIT-2 cell line because this model has characteristics similar to
the progression phenotypes of human pancreatic cancer, with
extra-pancreatic invasion, intra-peritoneal dissemination, and
hematogenous metastases to other organs. First, we examined the
spread of inoculated SUIT-2 cells from primary pancreatic tumors by
monitoring the systemic and pathological findings. Subsequently,
using the model mice imitating Stage IV human pancreatic cancer, we
evaluated the prognostic metastatic inhibition effects of
gemcitabine treatment, which was started on day 7 and day 14 after
SUIT-2 inoculation. We sought to determine if our focused-SUIT-2
pancreatic cancer model could portray the effects of gemcitabine
treatment against typical human metastatic pancreatic cancer
cells.
Materials and methods
Cell cultures and animals
Human pancreatic cancer cell lines Capan-1 and
SUIT-2 were provided by American Type Culture Collection and the
Japanese Cancer Research Resources Bank (Tokyo, Japan). The cells
were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.,
Tokyo, Japan) and RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with streptomycin (100 mg/ml), penicillin (100 U/ml)
(Pen strep; Gibco; Thermo Fisher Scientific, Inc.), and 10% fetal
bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.) in a
humidified incubator with 5% CO2 at 37°C.
We purchased 5-week-old female BALBc nu/nu mice from
Japan SLC (Hamamatsu, Japan). These animals were transferred to a
temperature-(20–26°C) and humidity-controlled (40–60% relative
humidity) room with a 12-h light/12-h dark cycle during the
experimental period. All animal experiments were approved by the
FUJIFILM Animal Experimentation Committee.
Orthotopic implantation
The cells were treated with trypsin/EDTA (Gibco;
Thermo Fisher Scientific, Inc.) and washed with FBS-added and
serum-free media twice. Cell suspensions of 1×106
cells/0.01 ml were injected into the pancreatic tail of mice under
anesthesia (isoflurane) (Pfizer, Tokyo, Japan). A cotton swab was
held over the injection site for 1 min to prevent leakage of
intrapancreatic tumor cells. We measured survival times until death
or moribundity (e.g., marked decrease in body weight, hypothermia,
or other conditions requiring euthanasia).
We orthotopically transplanted Capan-1 and SUIT-2
lines (1×106 cells) into mice (Capan-1 n=8, SUIT-2 n=8)
and plotted the Kaplan-Meier survival curve 72 days after
transplantation. The mice were sacrificed and examined for tumor
spreading through the use of macroscopic and microscopic
observations by hematoxylin and eosin (H&E) staining.
To evaluate the time course of metastatic cascades
in the orthotopic SUIT-2 model, we made 23 SUIT-2 (1×106
cells) model mice and pathologically examined tumor spreading in
each pancreas, spleen surface, peritoneum, liver, and lung on days
3, 7, and 14 after inoculation. H&E sections of the heart,
lung, trachea, submandibular gland, aorta, liver, kidney, spleen,
pancreas, adrenal gland, peritoneal cavity, bladder, stomach,
duodenum, jejunum, ileum, colon, rectum, female genital organs,
sternum, lymphatic tissues, and abdominal wall were examined.
Evaluation of gemcitabine effects
using the orthotopic SUIT-2 pancreatic cancer model
Overall, 40 BALBc nu/nu mice were orthotopically
inoculated with 1×106 SUIT-2 cells (day 0). The mice
were randomly divided into three groups: the vehicle group (n=20),
gemcitabine day 7 group (n=10, weekly intravenous injection started
7 days after inoculation; 240 mg/kg/week, weekly) (Teva
Pharmaceutical Industries Netanya, Israel), and gemcitabine day 14
group (n=10, weekly intravenous injection was started 14 days after
inoculation; 240 mg/kg/week, weekly).
For weekly treatment, 240 mg/kg is reported to be
the maximal tolerated dose of gemcitabine in mice (16). We plotted Kaplan-Meier survival curves
100 days after transplantation for each group. This treatment was
performed until the final observation week excluding death or
moribundity. Tumor spreading was evaluated as mentioned above.
H&E stains
Organs and tissues were fixed in 10% neutral
buffered formalin (Wako Pure Chemical Industries, Ltd., Osaka,
Japan) and embedded in paraffin (Sakura Finetek Japan Co., Ltd.,
Tokyo, Japan), and 2-µm sections were prepared. The sections were
stained with H&E (Hematoxylin 3G, Sakura Finetek Japan Co.,
Ltd.; Eosin, Wako Pure Chemical Industries, Ltd.) using standard
procedures (Hematoxylin, 1 min and Eosin, 1 min). Snapshots of
histology were taken using Olympus BX51 microscope. Images were
generated using an attached Olympus DP70 camera and the cellSens
software (Olympus Corporation, Tokyo, Japan).
Statistical analysis
Overall survival was measured from the day of SUIT-2
injection and plotted according to the Kaplan-Meier method; a
log-rank test was used for comparison. P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using the GraphPad Prism5 software package
(GraphPad Software, Inc., La Jolla, CA, USA).
Results
The orthotopic SUIT-2 xenografted
mouse model resembled the spread of typical human pancreatic cancer
compared to the Capan-1 mouse model
Among several human pancreatic cell lines, we
selected Capan-1 and SUIT-2 cells for our orthotopic pancreatic
cancer mouse model because these cells are transplantable into the
mice, and transplanted tumor pathology was relatively similar to
that of human pancreatic cancer tissues, with ductal components and
cancer stromal components or poor differentiation. Capan-1 and
SUIT-2 cells were transplanted orthotopically and observed.
Capan-1-transplanted mice (n=8) had no remarkable abnormal
findings, including body weight loss, jaundice, or abdominal
ascites during the observation period.
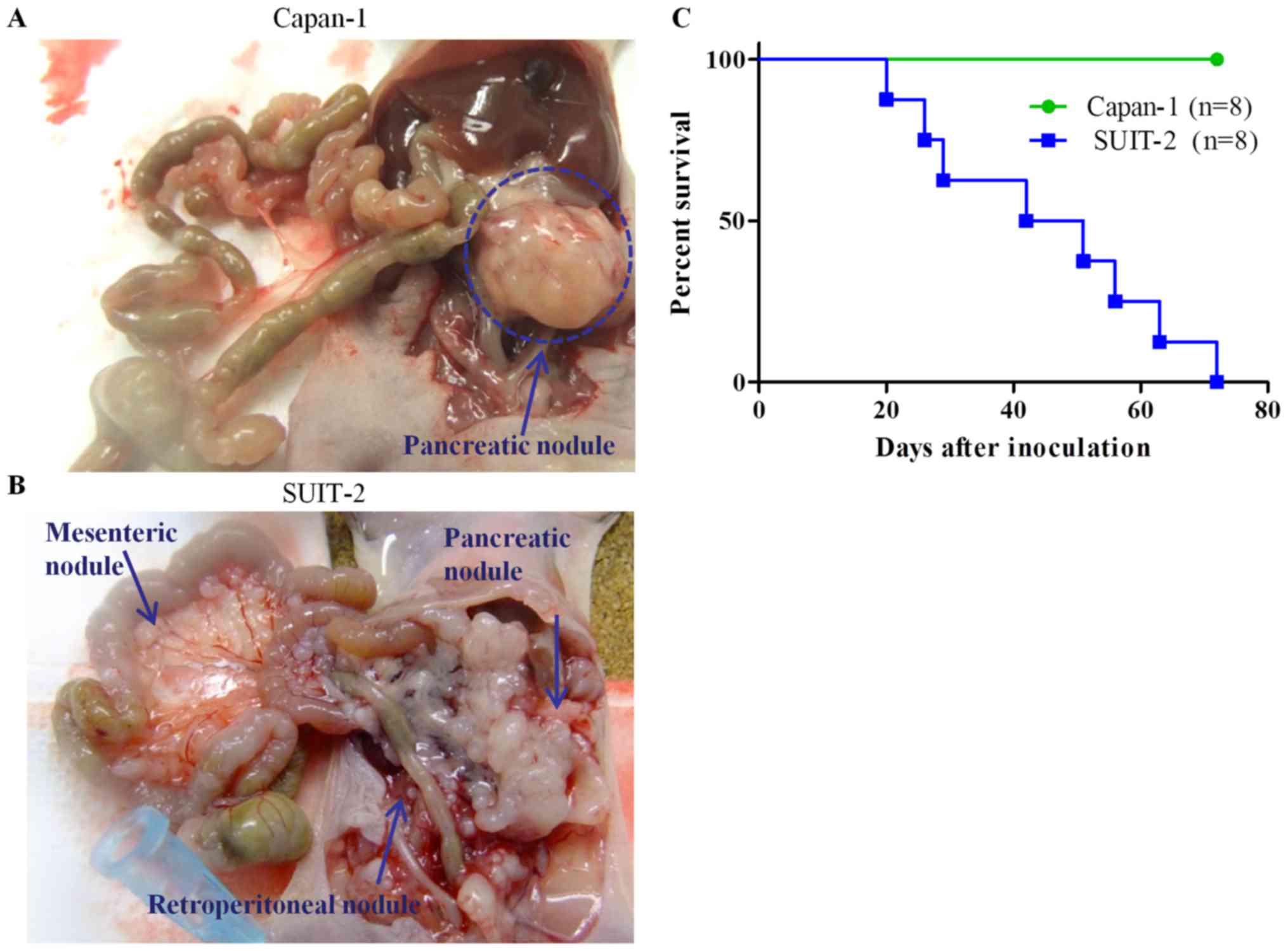
Tumor progression in two orthotopic transplantation
mouse models of pancreatic cancer were evaluated (Fig. 1). On dissection on day 72 after
inoculation, pancreatic cancer nodules were observed in all mice
(Fig. 1A). Transplanted Capan-1 cells
were observed in all pancreatic tumors (Fig. 2); however, the rate of metastasis was
low (50%). SUIT-2-transplanted mice (n=8) began to die or became
moribund from day 20 after transplantation. Tumor nodules were
scattered over the pancreas to the spleen, mesenterium, and
retroperitoneum on necropsy (Fig.
1B). We plotted Kaplan-Meier survival curves for Capan-1- and
SUIT-2-inoculated mice. The median survival of SUIT-2-transplanted
mice was 46.5 days. This model led to premature death (Fig. 1C). Capan-1-transplanted mice
experienced superior prognoses than the SUIT-2-transplanted
mice.
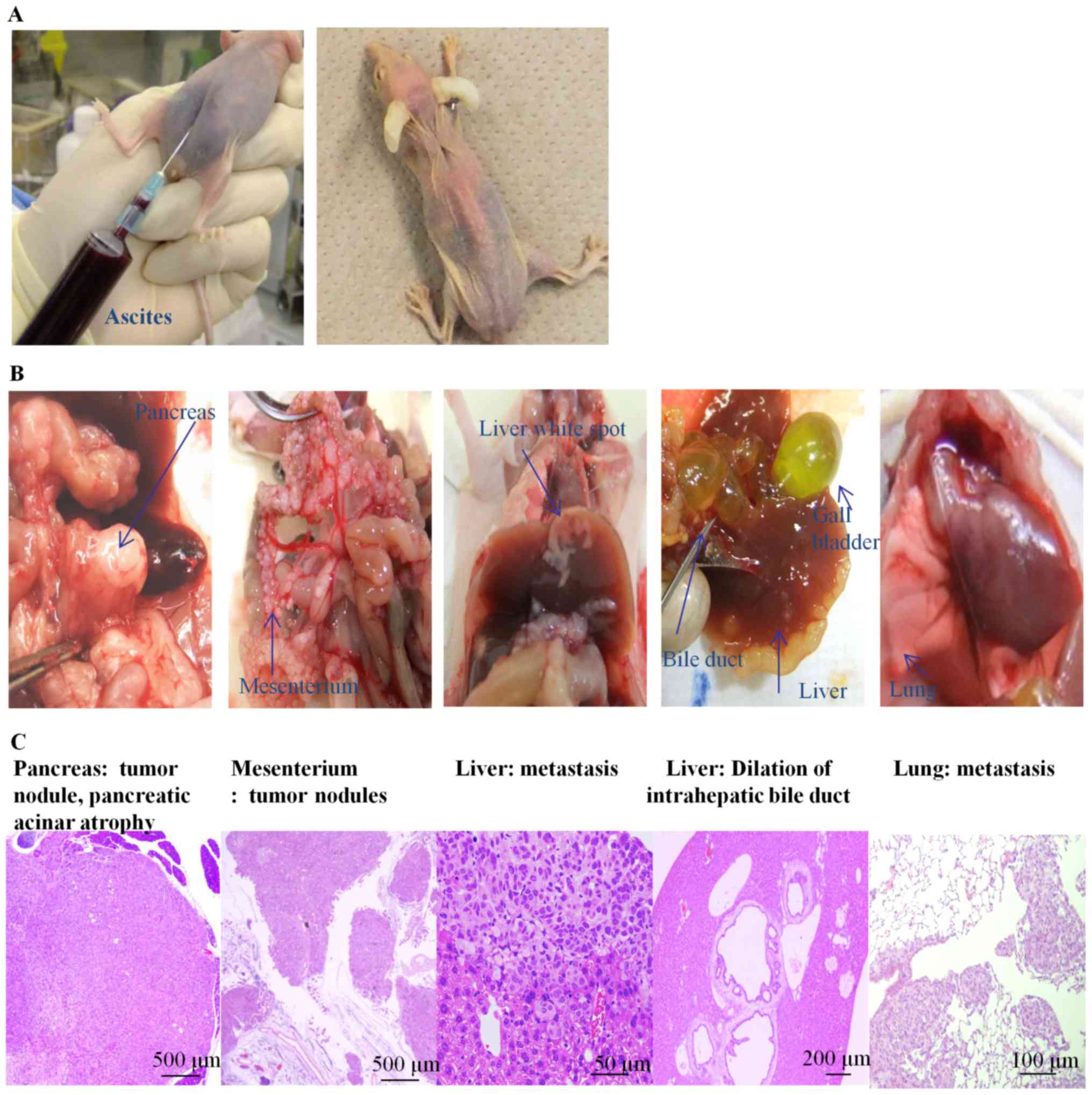
To clarify the SUIT-2 spread and the causes of
death, dead or moribund mice were subjected to macroscopic and
pathological observation (Fig. 3).
Many mice had carcinomatous ascites with bleeding. In some mice,
the ascites were more than 10 ml (Fig.
3A, left). Jaundiced mice appeared to have yellow-colored skin,
noses, and limbs (Fig. 3A, right).
Most mice had single SUIT-2 nodules in the pancreas, with adhesion
to surrounding organs such as the spleen and the stomach. SUIT-2
cells appeared as white spots and were spread throughout the whole
body, including the mesenterium, liver, and lung (Fig. 3B). Some mice had intestinal dilation
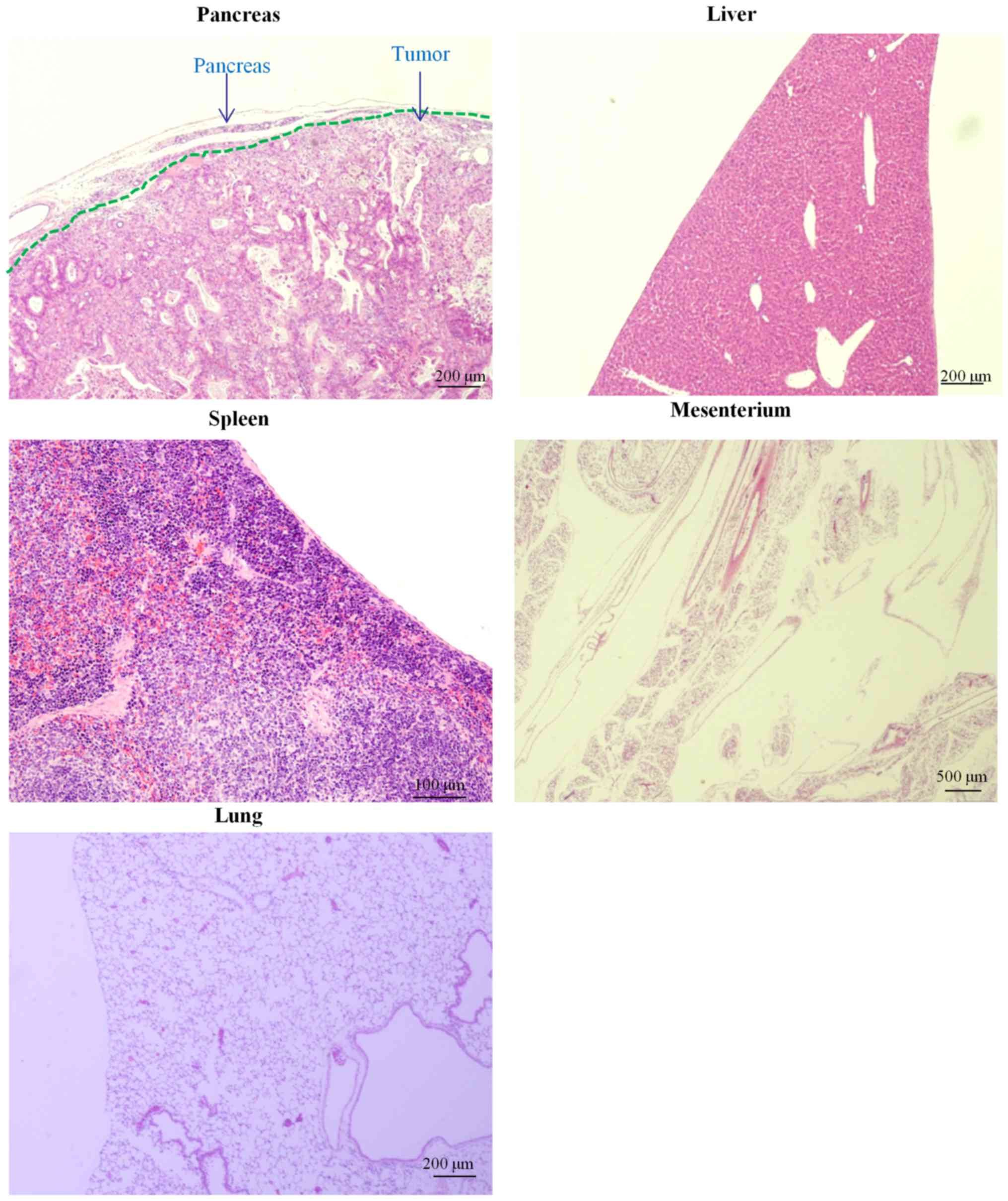
due to peritoneal dissemination, lymph node metastasis, and pleural
effusion (data not shown). SUIT-2 tumors in the pancreas (moderate
to poorly differentiated), atrophy of pancreatic acinar cells,
mesenteric tumors, hepatic metastases, and lung metastases were
observed using microscopic observations of the SUIT-2 model mice
(Fig. 3C). Jaundiced mice displayed
bile duct dilation (Fig. 3C). Tumor
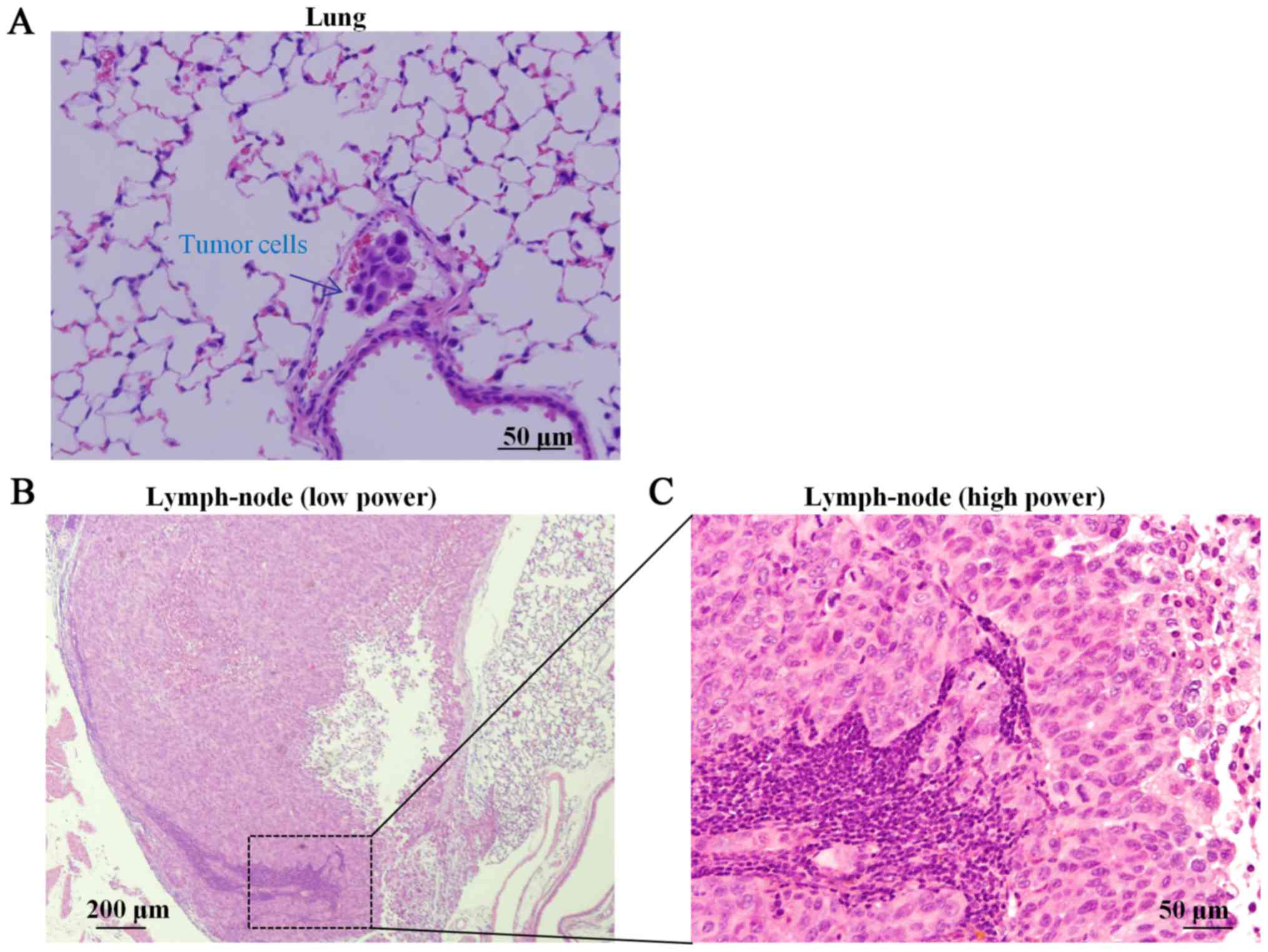
cells (Fig. 4A) were observed in the
blood vessels of the lung, and lymph node metastases to the
mediastinum were also observed by pathological examination of the
whole body (Fig. 4B and C).
SUIT-2 spread from the pancreas to the
extra-pancreatic tissues, including distant organs, in sacrificed
mice
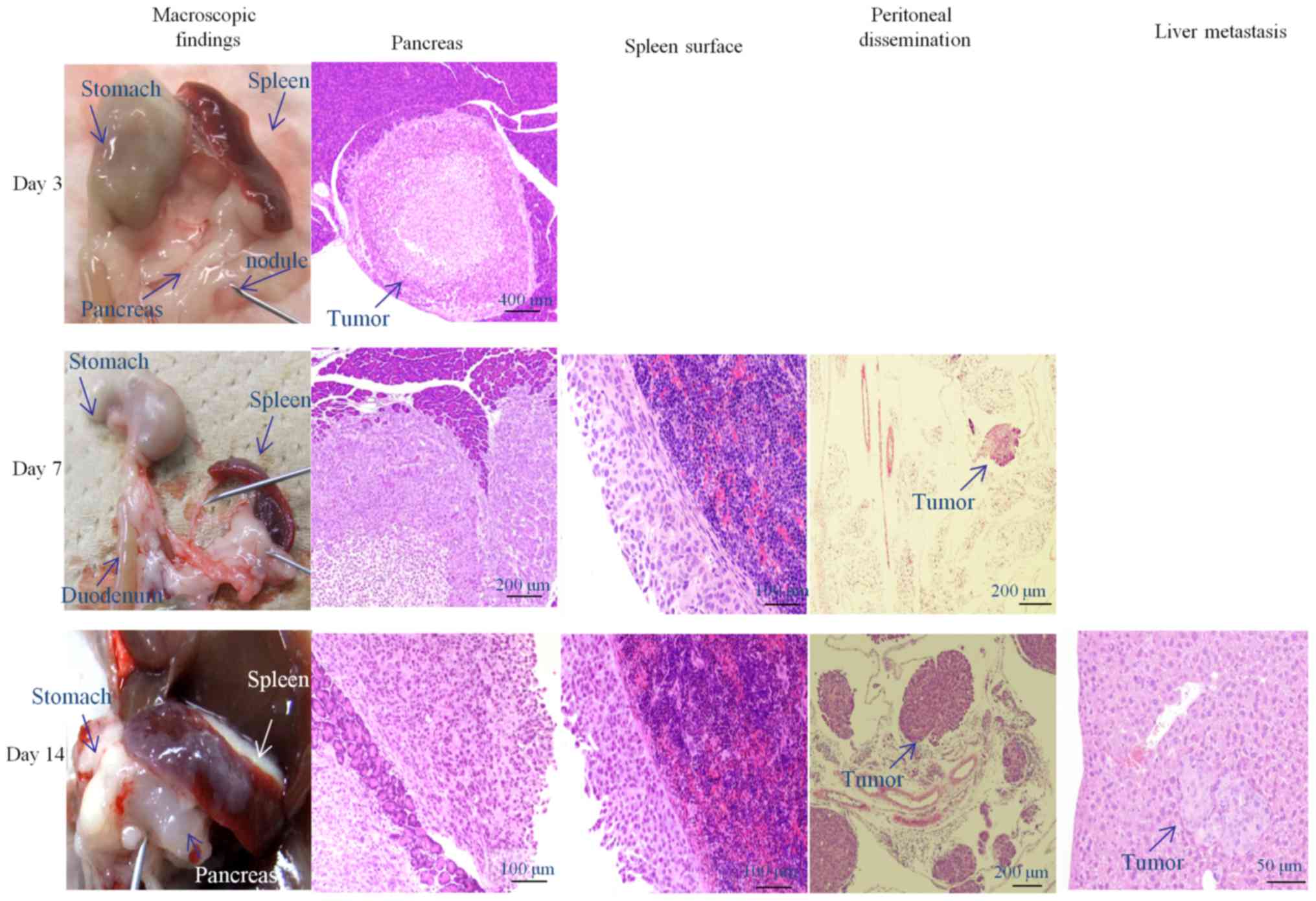
To investigate cancer spread following
transplantation, 23 mice were sacrificed on days 3, 7 and 14 after
transplantation. Representative examples are shown in Fig. 5. The microscopic findings and
metastatic rates are summarized in Table
I. At sacrifice 3 days after inoculation, we observed
pancreatic nodules, covered with a transparent membrane.
Microscopic examination showed nodule tumor cell formation, covered
by a membrane. Tumor cells were not observed in other organs.
 | Table I.Establishment of microscopic SUIT-2
tumors in the orthotopic pancreatic cancer mouse model according to
the observation time. |
Table I.
Establishment of microscopic SUIT-2
tumors in the orthotopic pancreatic cancer mouse model according to
the observation time.
| Microscopic
findings | Day 3 (n=3), (%) | Day 7 (n=10),
(%) | Day 14 (n=10),
(%) |
|---|
| Xenografted SUIT-2
(Pancreas) | 3/3 (100) | 10/10 (100) | 10/10 (100) |
| Extracapsular
invasion (Pancreas) | 0/3 (0) | 7/10 (70) | 10/10 (100) |
| Peritonal
dissemination | 0/3 (0) | 4/10 (40) | 6/10 (60) |
| Liver metastasis | 0/3 (0) | 0/10 (0) | 4/10 (40) |
| Lung metastasis | 0/3 (0) | 0/10 (0) | 0/10 (0) |
At sacrifice, 7 days after inoculation, white
nodular areas were observed on the surfaces of the stomach, spleen,
and peritoneum, surrounding the transplanted pancreas. Pancreatic
tumors invaded the pancreatic acinar cells (70%), with spleen
capsule surface thickening and cancer cell seeding. Cancer cells
were observed in the mesenterium but not in other organs. At this
time, we observed 40% peritoneal dissemination. At sacrifice on day
14 after inoculation, a large nodule from the inoculated pancreas
invaded the surrounding tissues, and we observed white tumor spots
on the surfaces of the spleen and stomach. Microscopically, tumor
cells disseminated throughout the abdominal cavity over the
pancreatic serosa. Tumor cells were seeded in the capsule of the
spleen, as on day 7. Peritoneal dissemination was 60% at this time.
Many tumor nodules were observed in the mesenterium, with 40% liver
metastases. Lung metastases were not observed on any specimens;
however, tumor cells in the blood vessels of the lung and
mesenteric lymph vessels (30%) were noted (data not shown).
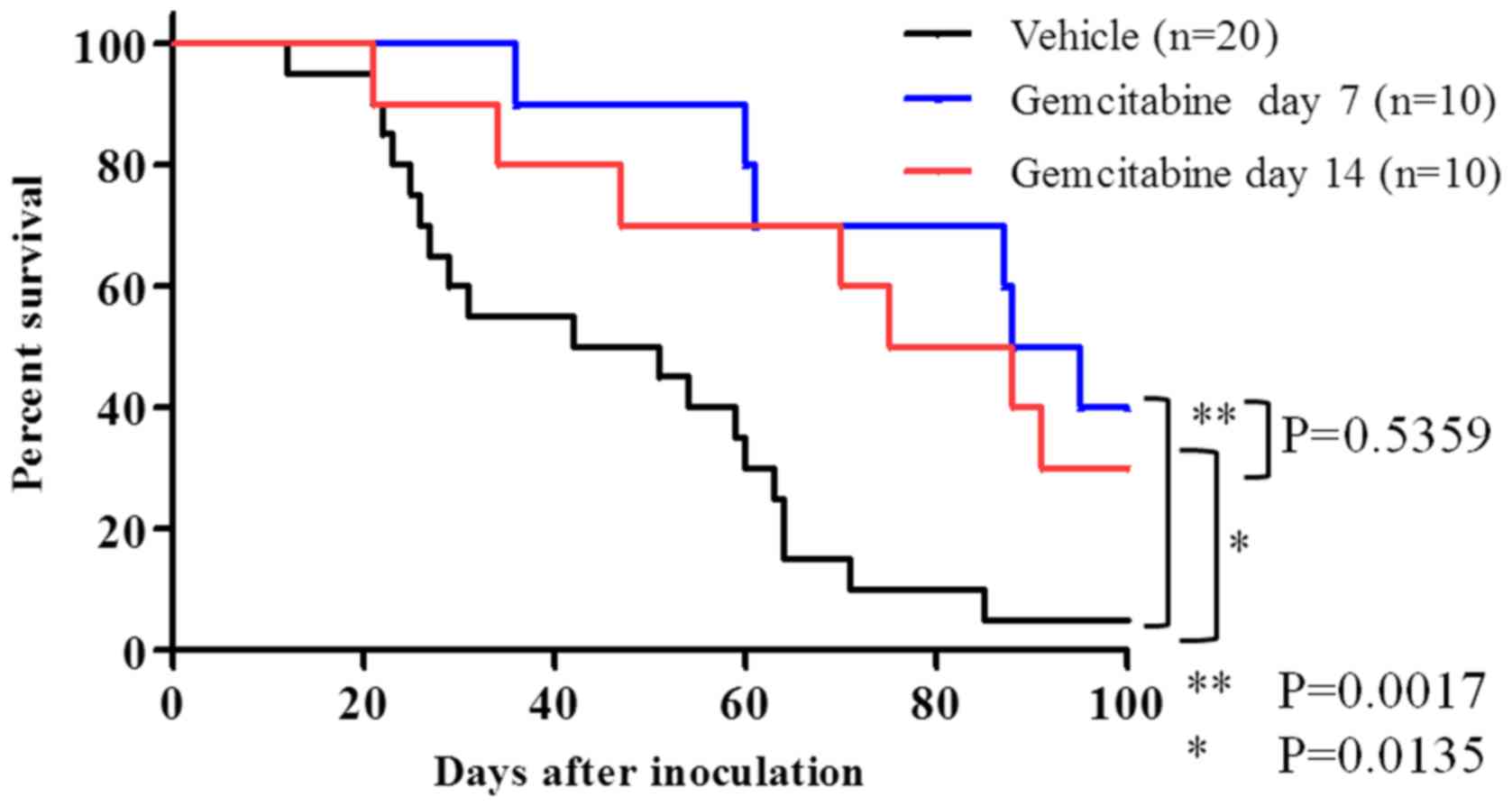
Efficacy of gemcitabine using an
orthotopic pancreatic cancer model
Survival tests were performed on gemcitabine, a
standard treatment for pancreatic cancer. Weekly gemcitabine
treatment occurred on day 7 and day 14 after cell transplantation.
A survival-prolonging effect was observed in the groups receiving
gemcitabine as compared with that in the group receiving the
vehicle (Gemcitabine day 7; P=0.0017) (Gemcitabine day 14:
P=0.0135; Fig. 6). Median survivals
were 46 days for the vehicle group, 91.5 days for the gemcitabine
day 7 group, and 81.5 days for the gemcitabine day 14 group. Mice
who died during the test in both the vehicle group and
gemcitabine-administered groups were dissected and examined.
All mice displayed a wide range of metastatic cancer
cells (data not shown). Surviving mice treated with gemcitabine
were dissected on day 100 (Table
II). Comparing the day 7 treatment group with the day 14
treatment group, earlier treatment was slightly inhibitory to both
liver and lung metastases (day 7: 67%, and day 14: 100%). In both
groups, pancreatic tumor invasion, peritoneal dissemination, liver
metastases, and lung metastases increased on dissection (day 100),
in spite of surviving mice.
 | Table II.Macroscopic and microscopic SUIT-2
tumors in survival mice on the last observation day according to
the starting date of gemcitabine administration. |
Table II.
Macroscopic and microscopic SUIT-2
tumors in survival mice on the last observation day according to
the starting date of gemcitabine administration.
| Pathological
Characteristics | Day 7 (n=3), (%) | Day 14 (n=3),
(%) |
|---|
| Macroscopic findings
(tumorous lesion) |
|
|
| Pancreas,
nodule | 3/3 (100) | 3/3 (100) |
| Spleen
surface, white spot | 2/3 (67) | 2/3 (67) |
|
Mesenterium, white nodule | 3/3 (100) | 3/3 (100) |
| Kidney
surface, white nodule | 3/3 (100) | 3/3 (100) |
| Liver
white spot | 2/3 (67) | 3/3 (100) |
| Microscopic
findings |
|
|
|
Xenografted SUIT-2
(Pancreas) | 3/3 (100) | 3/3 (100) |
|
Extracapsular invasion
(Pancreas) | 3/3 (100) | 3/3 (100) |
| Peritonal
dissemination | 3/3 (100) | 3/3 (100) |
| Liver
metastasis | 2/3 (67) | 3/3 (100) |
| Lung
metastasis | 2/3 (67) | 3/3 (100) |
Discussion
The SUIT-2 orthotopic pancreatic cancer model was
similar to the phenotypic progression of human pancreatic cancer,
with extra-pancreatic invasion, intra-peritoneal dissemination, and
other hematogenous organ metastases, compared to the Capan-1
model.
Pathology results clarified when these metastatic
lesions spread from primary pancreatic tumors. The prognostic
efficacy of gemcitabine treatment against metastatic pancreatic
cancer lesions, similar to Stage IV human pancreatic cancer, was
also evaluable using this model. Moreover, necropsy on the final
observation day showed the suppression of distant metastases in the
day 7 treatment group compared to that in the day 14 group.
However, all mice displayed xenografted tumors in spite of the
continuous gemcitabine treatment, similar to typical human
pancreatic cancer.
The SUIT-2 orthotopic pancreatic cancer mouse model
has been used in other studies for evaluating promising new
anticancer agents (14,17–20).
Cherubini et al (17) used the
model to evaluate target drug survival effects. Saimura et
al (19)evaluated the tumor
reduction effects by measuring tumor weight in a mouse model.
SUIT-2 is reported not only in an orthotopic but also in a
subcutaneous model, a peritoneal dissemination model, and a lung
metastasis model. Previous studies evaluated the anti-tumor effects
in a subcutaneous implantation model, drug effects on peritoneal
dissemination in a dissemination model, and anti-tumor effects in a
lung metastasis model (21,22). In this study, we used 30-G thin
needles and 10 µl of cell suspension for orthotopical injection
into the pancreas to prevent these cells from leaking into the
abdominal cavity after inoculation. As shown in Table I, spreading of the macroscopic tumors
into the abdominal cavity was not observed on day 3, which is
similar to early human pancreatic cancer. We closed the pancreatic
puncture holes by pressing the punctured tissue using cotton swabs
for 1 min. This process may be effective for reproducing early
pancreatic cancer in an orthotopic mouse model. On the other hand,
some model mice had the SUIT-2 spreading in the extra-pancreatic
area, including pancreatic surface and abdominal cavity, on day 7
despite our procedures using cotton swab. It is suggested that
small number of SUIT-2 cells are leaked into the extra-pancreatic
areas in a part of mice. To exclude this possibility, it may be
effective to use Matrigel for cell suspension and to press the
puncture holes using swab for more long interval.
The orthotopic mouse model can be used to mimic the
natural course of human pancreatic cancer metastases. However, few
researchers have reported on the spreading patterns of systemic
pancreatic cancer, including invasion, peritoneal dissemination
around the pancreas, and distant metastases. Our model indicated
that orthotopically-injected SUIT-2 cells sequentially spread from
the pancreas to the peritoneum, diaphragm, liver, and lungs,
similar to human pancreatic cancer. We validated this model's
ability to mimic the terminal stage of pancreatic cancer with
hemorrhagic ascites, tumor cell metastases, liver failure with
jaundice, intussusception, and pleural effusion. This experimental
mouse model was similar to human pancreatic cancers.
We sought to identify the best mouse model for
predicting the effects of anticancer drugs in humans. Many mouse
models may not reflect human clinical trial results. For instance,
ganitumab, aflibercept, and exatecan reportedly induce strong
anticancer effects without severe side effects in preclinical mouse
models. However, the agents could not show the similar effect in
human cancer patients during clinical trials (11,23,24). Our
model accurately mimics the action of gemcitabine in human
pancreatic cancers. This mouse model can reproduce the anticancer
effects from a single gemcitabine treatment, including survival
prolongation, because all mice that survived to day 100 had cancer
and displayed limited anticancer effects, similar to the
gemcitabine effects in human patients with pancreatic cancer. If
new investigational agents can cure pancreatic SUIT-2 cells in this
mouse model, the agent may be useful for treating human pancreatic
cancer. In other words, using this model, it may be possible to
predict drug effects within clinical trial contexts, contributing
to efficient drug development.
We believe that our mouse model correctly reflects
the drug efficacy of gemcitabine alone therapy in the clinic
because the monotherapy could not eradicate SUIT-2 cells spreading
to the whole body. In future, we want to examine the drug efficacy
of gemcitabine combined therapy using our model because the
combined therapy with gemcitabine and nab-paclitaxel has often been
used as a standard chemo-regimen for advanced pancreatic
patients.
In this model, metastases form throughout the whole
body, and cancer death results from causes such as liver failure
and peritoneal dissemination. This model may not be suitable for
evaluating drug effects against conditions such as liver
metastases. Previous studies describe the peritoneal dissemination
model by intra-peritoneal injection, the liver metastasis model by
splenic injection or direct liver injection, and the lung
metastasis model by tail vein injection for preclinical studies of
pancreatic cancer (25). Such models
can evaluate drug effects on peritoneal dissemination, liver
metastases, and lung metastases. After evaluating the effects of
survival prolongation and organ-specific anti-tumor effects, using
our orthotopic mouse model, it might be better to use the
abovementioned specific metastasis mouse model to understand the
therapeutic characteristics of the target drugs against organ
metastases. Moreover, our model has another limitation: our nude
mice have activity of macrophases and natural killer cells to
prevent the cancer spreading. In future, we hope to establish the
orthotopic pancreatic cancer model using specific mice with
humanized immunoreactivity (26,27) and to
investigate the metastatic cascades and the therapeutic efficacy of
new drug candidates using our model.
In conclusion, there are prior reports of the SUIT-2
orthotopic pancreatic cancer mouse model for evaluating tumor
volume or survival time. We clarified the process and timing of
pancreatic cancer progression in this mouse model, similar to that
observed in typical human pancreatic cancer patients. Through this
model, researchers can validate the prognostic and therapeutic
efficacy of gemcitabine as a key drug for treating human pancreatic
cancer. Future investigations may further affirm the use of this
model as a standard model for drug development.
Acknowledgements
The authors thank Hiroyuki Iwamura, Akira Inomata,
Takeshi Yamaura, Shinji Mima, Hiroki Nishikawa, Hiroko Fujisaki and
Hiroko Nemoto for helpful discussion and technical assistance. We
also thank the staff at Biotechnical center Japan SLC for providing
technical help with the animal experiments.
References
|
1
|
Okusaka T, Matsumura Y and Aoki K: New
approaches for pancreatic cancer in Japan. Cancer Chemother
Pharmacol. 54 Suppl 1:S78–S82. 2004.PubMed/NCBI
|
|
2
|
Takahashi H, Ohigashi H, Gotoh K,
Marubashi S, Yamada T, Murata M, Ioka T, Uehara H, Yano M and
Ishikawa O: Preoperative gemcitabine-based chemoradiation therapy
for resectable and borderline resectable pancreatic cancer. Ann
Surg. 258:1040–1050. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oberstein PE and Olive KP: Pancreatic
cancer: Why is it so hard to treat? Therap Adv Gastroenterol.
6:321–337. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gnanamony M and Gondi CS: Chemoresistance
in pancreatic cancer: Emerging concepts. Oncol lett. 13:2507–2513.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kapischke M and Pries A: Animal models of
pancreatic cancer for drug research. Expert Opin Drug Discov.
3:1177–1188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aguirre AJ, Bardeesy N, Sinha M, Lopez L,
Tuveson DA, Horner J, Redston MS and DePinho RA: Activated Kras and
Ink4a/Arf deficiency cooperate to produce metastatic pancreatic
ductal adenocarcinoma. Genes Dev. 17:3112–3126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bardeesy N, Aguirre AJ, Chu GC, Cheng KH,
Lopez LV, Hezel AF, Feng B, Brennan C, Weissleder R, Mahmood U, et
al: Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain
progression of pancreatic adenocarcinoma in the mouse. Proc Natl
Acad Sci USA. 103:pp. 5947–5952. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma L and Saiyin H: LSL-KrasG12D;
LSL-Trp53R172H/+; Ink4flox/+; Ptf1/p48-Cre mice are an applicable
model for locally invasive and metastatic pancreatic cancer. PLoS
One. 12:e01768442017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hoang NT, Kadonosono T, Kuchimaru T and
Kizaka-Kondoh S: Hypoxia-inducible factor-targeting prodrug TOP3
combined with gemcitabine or TS-1 improves pancreatic cancer
survival in an orthotopic model. Cancer Sci. 107:1151–1158. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun FX, Tohgo A, Bouvet M, Yagi S,
Nassirpour R, Moossa AR and Hoffman RM: Efficacy of camptothecin
analog DX-8951f (Exatecan Mesylate) on human pancreatic cancer in
an orthotopic metastatic model. Cancer Res. 63:80–85.
2003.PubMed/NCBI
|
|
12
|
Bouvet M, Wang J, Nardin SR, Nassirpour R,
Yang M, Baranov E, Jiang P, Moossa AR and Hoffman RM: Real-time
optical imaging of primary tumor growth and multiple metastatic
events in a pancreatic cancer orthotopic model. Cancer Res.
62:1534–1540. 2002.PubMed/NCBI
|
|
13
|
Ammons WS, Wang JW, Yang Z, Tidmarsh GF
and Hoffman RM: A novel alkylating agent, glufosfamide, enhances
the activity of gemcitabine in vitro and in vivo. Neoplasia.
9:625–633. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tomioka D, Maehara N, Kuba K, Mizumoto K,
Tanaka M, Matsumoto K and Nakamura T: Inhibition of growth,
invasion, and metastasis of human pancreatic carcinoma cells by NK4
in an orthotopic mouse model. Cancer Res. 61:7518–7524.
2001.PubMed/NCBI
|
|
15
|
Metildi CA, Kaushal S, Hoffman RM and
Bouvet M: In vivo serial selection of human pancreatic cancer cells
in orthotopic mouse models produces high metastatic variants
irrespective of Kras status. J Surg Res. 184:290–298. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Veerman G, Ruiz van Haperen VW, Vermorken
JB, Noordhuis P, Braakhuis BJ, Pinedo HM and Peters GJ: Antitumor
activity of prolonged as compared with bolus administration of
2′,2′-difluorodeoxycytidine in vivo against murine colon tumors.
Cancer Chemother Pharmacol. 38:335–342. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cherubini G, Kallin C, Mozetic A,
Hammaren-Busch K, Müller H, Lemoine NR and Halldén G: The oncolytic
adenovirus AdΔΔ enhances selective cancer cell killing in
combination with DNA-damaging drugs in pancreatic cancer models.
Gene Ther. 18:1157–1165. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kizaka-Kondoh S, Itasaka S, Zeng L, Tanaka
S, Zhao T, Takahashi Y, Shibuya K, Hirota K, Semenza GL and Hiraoka
M: Selective killing of hypoxia-inducible factor-1-active cells
improves survival in a mouse model of invasive and metastatic
pancreatic cancer. Clin Cancer Res. 15:3433–3441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saimura M, Nagai E, Mizumoto K, Maehara N,
Okino H, Katano M, Matsumoto K, Nakamura T, Narumi K, Nukiwa T and
Tanaka M: Intraperitoneal injection of adenovirus-mediated NK4 gene
suppresses peritoneal dissemination of pancreatic cancer cell line
AsPC-1 in nude mice. Cancer Gene Ther. 9:799–806. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Houghton JL, Zeglis BM, Abdel-Atti D,
Aggeler R, Sawada R, Agnew BJ, Scholz WW and Lewis JS:
Site-specifically labeled CA19.9-targeted immunoconjugates for the
PET, NIRF, and multimodal PET/NIRF imaging of pancreatic cancer.
Proc Natl Acad Sci USA. 112:pp. 15850–15855. 2015; View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takiguchi S, Inoue K, Matsusue K, Furukawa
M, Teramoto N and Iguchi H: Crizotinib, a MET inhibitor, prevents
peritoneal dissemination in pancreatic cancer. Int J Oncol.
51:184–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fukushima T, Kawaguchi M, Yamasaki M,
Tanaka H, Yorita K and Kataoka H: Hepatocyte growth factor
activator inhibitor type 1 suppresses metastatic pulmonary
colonization of pancreatic carcinoma cells. Cancer Sci.
102:407–413. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beltran PJ, Mitchell P, Chung YA, Cajulis
E, Lu J, Belmontes B, Ho J, Tsai MM, Zhu M, Vonderfecht S, et al:
AMG 479, a fully human anti-insulin-like growth factor receptor
type I monoclonal antibody, inhibits the growth and survival of
pancreatic carcinoma cells. Mol Cancer Ther. 8:1095–1105. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gaya A and Tse V: A preclinical and
clinical review of aflibercept for the management of cancer. Cancer
Treat Rev. 38:484–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bruns CJ, Harbison MT, Kuniyasu H, Eue I
and Fidler IJ: In vivo selection and characterization of metastatic
variants from human pancreatic adenocarcinoma by using orthotopic
implantation in nude mice. Neoplasia. 1:50–62. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishikawa F, Yasukawa M, Lyons B, Yoshida
S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD and
Harada M: Development of functional human blood and immune systems
in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood.
106:1565–1573. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shultz LD, Ishikawa F and Greiner DL:
Humanized mice in translational biomedical research. Nat Rev
Immunol. 7:118–130. 2007. View
Article : Google Scholar : PubMed/NCBI
|