Introduction
Human NIN1/RPN12 binding protein 1 homolog (NOB1)
gene is located on chromosome 16q22.1 and expresses the 50 kDa
protein NOB1. NOB1 is frequently expressed in the liver, lung, and
spleen and mainly located in the nucleus (1,2). It was
reported that NOB1 is abnormally expressed in invasive ductal
carcinoma and may be involved in the occurrence and development of
tumors (3). The expressions of NOB1
messenger RNA (mRNA) and protein in papillary thyroid carcinoma are
significantly higher than those in normal thyroid tissues (4). In non-small cell lung cancer (NSCLC) and
prostate cancer, NOB1 expression is also significantly correlated
with tumor-node-metastasis (TNM) staging, lymph node metastasis and
histological grading (5,6). However, the correlation between NOB1
expression in osteosarcoma and clinicopathological features of
patients has not been reported.
In this study, in order to determine the potential
role of NOB1 in osteosarcoma, reverse transcription-polymerase
chain reaction (RT-PCR) was used to examine the expression of NOB1
in cancer and cancer-adjacent tissues of patients with
osteosarcoma, and the correlation between NOB1 and prognosis was
analyzed. Afterwards, the sensitivity of NOB1 to cisplatin was
examined by the knockout of NOB1 expression in osteosarcoma cells
using small interfering ribonucleic acid (siRNA), and the potential
mechanism was analyzed.
Patients and methods
Clinical data
A total of 74 cases of paired osteosarcoma cancer
and cancer-adjacent tissues were from specimens obtained by
surgical resection from September 2014 to September 2016 in The
Affiliated Hospital of Southwest Medical University (Sichuan,
China) and all specimens were confirmed cases diagnosed by
pathologists. Cancer-adjacent tissues refer to tissues more than 5
cm away from the tumor edge. None of the study patients received
radiotherapy, chemotherapy or other treatment before operation. The
mean age of the patients was 45±12.3 years. According to Ennecking
staging, they were divided into stage I (n=28), stage II (n=30) and
stage III (n=16). Tumors were located in the tibia (n=41), the
femur (n=23) and other parts (n=10). All the participating patients
signed the informed consent form. The study was approved by the
Ethics Committee of The Affiliated Hospital of Southwest Medical
University.
Detection of the expression of NOB1 in
osteosarcoma by RT-PCR
Total RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) and reverse
transcription was performed using a PrimeScript® RT
reagent kit (Takara Biotechnology Co., Ltd., Dalian, China). RT-PCR
was performed using a SYBR® Premix Ex Taq™ II kit
(Takara Biotechnology Co., Ltd.) for 40 amplification cycles at
55°C annealing temperature. The relative quantification of each
gene was analyzed by 2−ΔΔCt with glyceraldehyde
3-phosphate dehydrogenase (GAPDH) as internal reference for
correction. The formula of the relative expression level of mRNA of
each indicator is 2−ΔCt [ΔCt = Ct (target gene) - Ct
(GAPDH)]. NOB1 and GAPDH primers were synthesized by Sangon Biotech
Co., Ltd. (Shanghai, China). Primer sequences are as follows: NOB1
foward, 5-ATCTGCCCTACAAG CCTAAAC-3′ and reverse,
5′-TCCTCCTCCTCCTCCTCAC-3; GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′.
Cell culture
Human osteosarcoma cell line MG-63 was purchased
from the American Type Culture Collection (ATCC; Manassas, VA, USA)
and cultured in Dulbecco's modified Eagle's medium (DMEM)
containing high-level sugar and 10% fetal bovine serum (FBS), then
100 µg/ml streptomycin and 100 IU/ml penicillin were added. The
cell culture flask was placed in an incubator at 37°C containing 5%
CO2 with the humidity of 95%.
siRNA interference
siRNA interference sequences of target NOB1 are
listed in literature (7) and
synthesized by GenePharma (Shanghai, China). Transfection was
performed using siRNA transfection kit (Guangzhou RiboBio Co.,
Ltd., Guangzhou, China). Western blotting was used to validate
silencing efficiency and select the optimal sequence of action.
Three siRNA and si-control sequences are shown below.
Western blotting
Cells were lysed on ice for 1 h using
radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime
Institute of Biotechnology, Guangzhou, China), and the protein
supernatant was extracted by centrifuging the cells for 1 h at
13,000 × g for 30 min. The protein concentration was measured with
the bicinchoninic acid assay (BCA) protein concentration kit, and
an appropriate amount of loading buffers (both from Beyotime
Institute of Biotechnology), were added at 100°C for 5 min. Each
sample was electrophoresed at an equivalent amount of 40 µg. The
protein was then transferred to a polyvinylidene fluoride (PVDF)
membrane. Using 5% skimmed milk at room temperature for 1 h, rabbit
anti-human NOB1 polyclonal was incubated by the main antibodies
(dilution, 1:1,000; cat. no. 10091-2-AP; Proteintech Group, Inc.,
Chicago, IL, USA), rabbit anti-human caspase-3 and B-cell lymphoma
2 (Bcl-2) polyclonal antibodies (dilution, 1:1,000; cat. nos.
ab13847 and ab59348; Abcam, Cambridge, UK). After membranes were
washed with Tris-buffered saline with Tween-20 (TBST), NOB1 was
incubated by the corresponding goat anti-rabbit horseradish
peroxidase-labeled secondary polyclonal antibody (dilution,
1:5,000; cat. no. A0239; Beyotime Institute of Biotechnology). The
membrane was visualized by the enhanced chemiluminescence (ECL)
detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA),
and grayscale analysis was performed using a gel analyzer. The
relative concentration of the target protein was the ratio of the
target protein to the corresponding intrinsic parameter.
Detection of the sensitivity of MG-63
cells to cisplatin by Cell Counting Kit-8 (CCK-8)
Cells were seeded in 96-well plates at
5×103/well, and the cells were completely adherent 12 h
later. The complete medium in each well was discarded. Two hundred
microliters of prepared cisplatin at different concentrations
(0.001, 0.01, 1, 10, 20 and 50 µM) were added after the culture for
24 h. CCK-8 (20 µl; Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) reagents were added to each well and incubated for 1 h at
37°C in the dark. The optical density (OD) at 450 nm was measured
by a microplate reader. Cell viability = (OD value of the
experimental group - OD value of the blank group)/(OD value of the
control group - OD value of the blank group) × 100%.
Detection of apoptosis by flow
cytometry
In this study, the detection was performed using
cell apoptosis kits (BD Biosciences, San Jose, CA, USA) for
testing. Cells were digested and centrifuged 2 days after
transfection with siRNA, and then washed twice with cold
phosphate-buffered saline (PBS). Afterwards, they were resuspended
in 100 µl of 1X binding buffer, and after 5 µl propidium iodide
(PI) and Annexin V were added, respectively, they were incubated at
room temperature for 15 min. Then, the cells were sent to the
Scientific Research Center of our hospital for detection by
apparatus within 1 h. Apoptosis rate = early apoptosis rate + late
apoptosis rate.
Statistical analysis
The results were analyzed by GraphPad Prism software
(version 5.01; GraphPad Software Inc., La Jolla, CA, USA).
Differences between two groups were compared by Student's t-test.
Intergroup comparison of indicators was conducted using one-way
analysis of variance (ANOVA). P<0.05 was considered to indicate
a statistically significant difference.
Results
Detection of the expression of NOB1
mRNA in cancer and cancer-adjacent tissues of patients with
osteosarcoma by RT-PCR
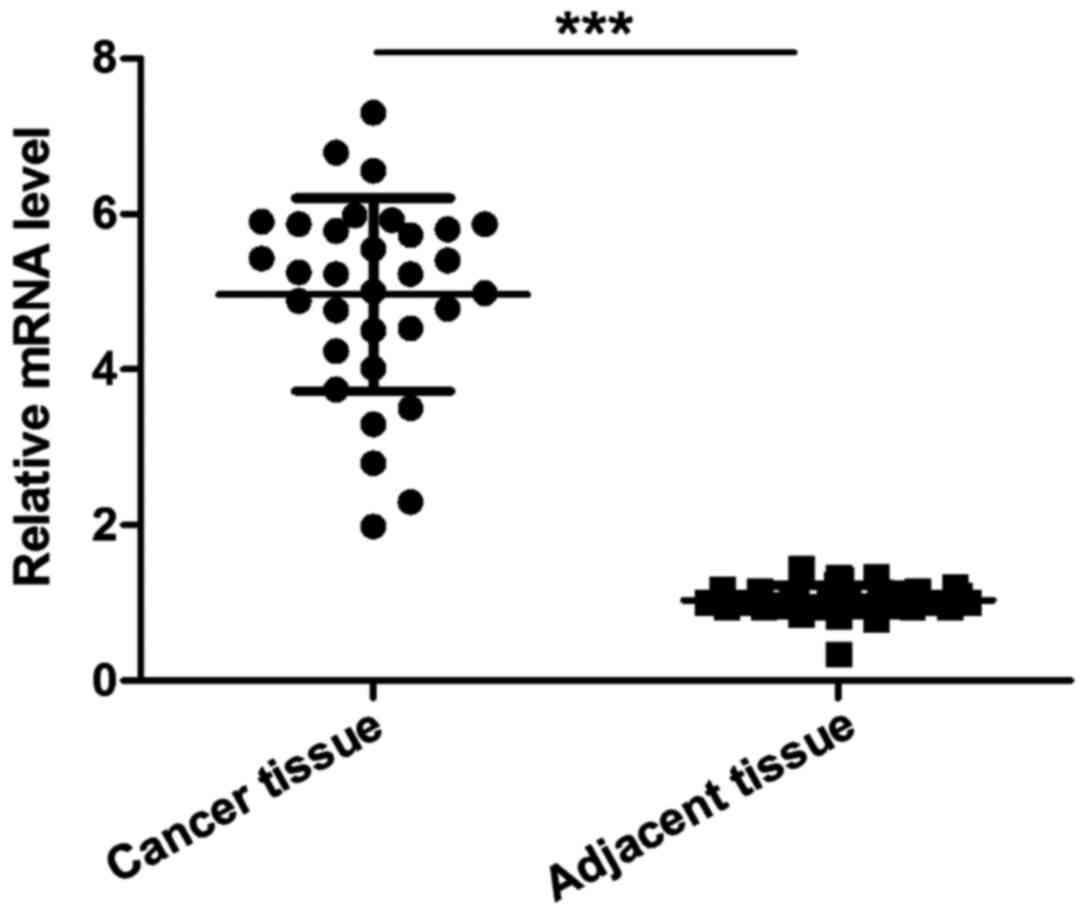
The level of NOB1 mRNA in cancer tissues was
significantly higher than that in cancer-adjacent tissues of
patients with osteosarcoma, and the difference was statistically
significant (p<0.001).
Correlation between the expression of
NOB1 and the prognosis of patients with osteosarcoma (mean ±
SD)
As shown in Fig. 1,
the expression of NOB1 was not related to the age, sex, tumor
location and lung metastasis (p>0.05) but correlated with
Ennecking staging and tumor size, and the difference was
statistically significant (p<0.05), suggesting that NOB1 was
associated with the condition of patients with osteosarcoma.
Verification of the efficiency of
three siRNAs in interfering with the expression of NOB1 in
osteosarcoma cells (MG-63) by western blotting
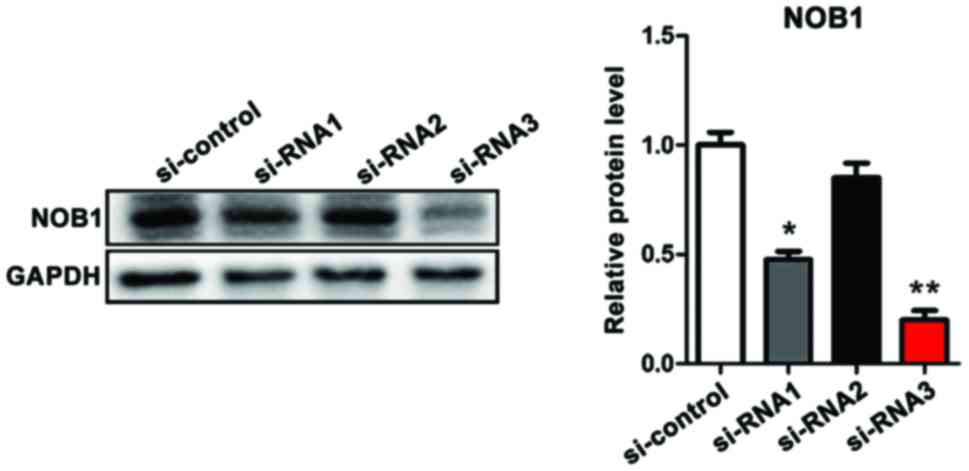
Clinical experiments suggested that NOB1 mRNA was
upregulated in cancer tissues of patients with osteosarcoma and was
significantly associated with poor prognosis. In order to further
study the chemosensitivity of NOB1 and osteosarcoma to cisplatin,
three siRNAs were constructed for target interference with NOB1
expression in MG-63 cells. Western blotting results showed that
siRNA2 had the highest efficiency in downregulating NOB1, and in
the follow-up study, siRNA3 was studied (Fig. 2).
Detection of the sensitivity of MG-63
cells to cisplatin by CCK-8
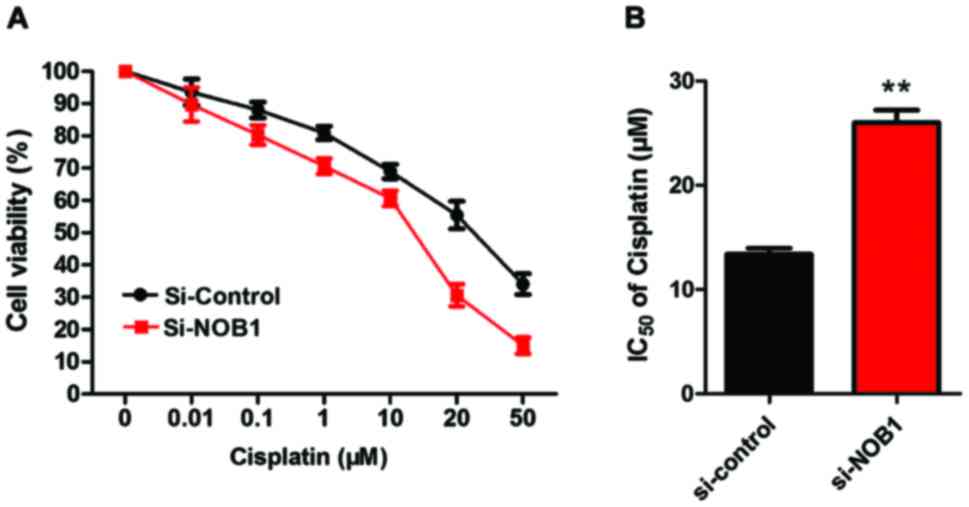
As shown in Fig. 3,
the sensitivity of MG-63 cells in the NOB1 downregulation group was
significantly higher than that in the control group (p<0.01),
suggesting that the expression of NOB1 in osteosarcoma cells was
negatively correlated with the sensitivity to cisplatin.
Detection of the effect of NOB1
downregulation on apoptosis of MG-63 cells by flow cytometry
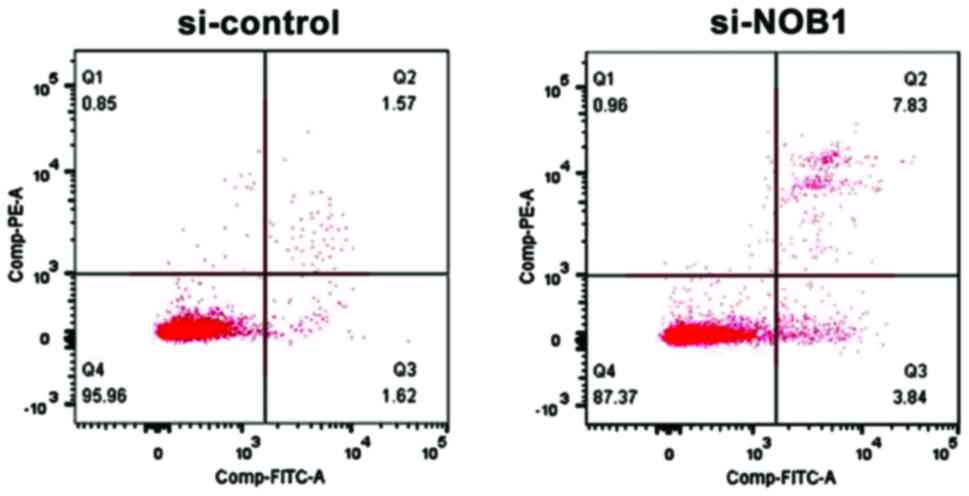
Annexin V and PI staining were used to mark early
and late apoptosis cells. The results showed that NOB1 knockdown
significantly increased the apoptosis rate of osteosarcoma MG-63
cells: si-NOB1 vs. si-control = 12.54±1.58 vs. 4.24±1.03
(p<0.05), suggesting that NOB1 was closely related to the
apoptosis level of osteosarcoma cells (Fig. 4).
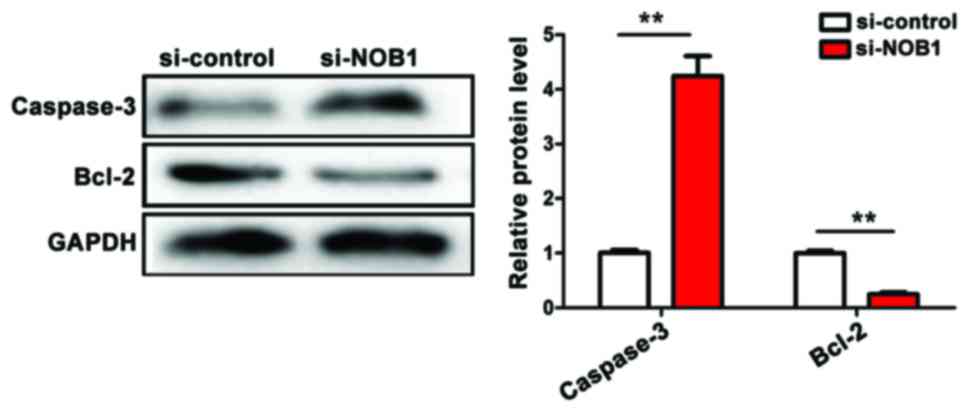
Detection of the effect of NOB1
downregulation on apoptosis indicators of MG-63 cells by western
blotting
The downregulation of NOB1 expression in
osteosarcoma MG-63 cells activated the apoptosis pathway of
caspase-3 [(si-NOB1 vs. si-control = 4.32±0.56 vs. 1.02±0.03
(p<0.01)] and inhibited the expression of anti-apoptotic
indicator Bcl-2 [(si-NOB1 vs. si-control = 0.22±0.02 vs. 1.03±0.02
(p<0.01)]. The downregulation of NOB1 expression promoted
apoptosis of MG-63 cells, and the difference was statistically
significant (Fig. 5).
(p<0.05).
Discussion
Osteosarcoma originates from mesenchymal cells
formed by original bones, and is the most common primary bone
malignancy (8). It mostly occurs in
adolescents under the age of 20 years and children. In children and
adolescents, 8.9% of deaths are caused by bone and joint
malignancies. Studies have shown that the long-term survival rate
of patients who only underwent surgical resection of osteosarcoma
was <20% (9). At present,
cisplatin-based multidrug chemotherapy has greatly improved the
prognosis of patients, and the 5-year survival rate with
non-metastasis is 60–70% (10).
However, chemotherapy resistance is still a problem in clinical
treatment of osteosarcoma.
In normal cells, the 20S and 26S proteasomes are
important constituents of the proteolytic system of cytosolic and
nuclear proteasomes (11). NOB1 has
been proven to be involved in two key cellular biological
processes. First, NOB1 accomplishes degradation of 20S protease by
regulating the biosynthesis of the 26S proteasome, which means that
NOB1 participates in ubiquitin-regulated protein degradation
processes (12,13). Second, at the nuclear outlet, NOB1 and
40S ribosome activate the 20S ribosomal ribonucleic acid (rRNA)
precursor at the D site so as to promote its formation of mature
18S rRNA (13–15), indicating that NOB1 participates in
the protein synthesis process. The two functions show that NOB1
plays an important role in the balance of proteins. Abnormal
expression of NOB1 in cells may lead to dysfunction in protein
synthesis and protein degradation. Rapidly growing cancer cells
require more proteins for deoxyribonucleic acid (DNA) replication
and cell division, so NOB1 are often abnormally activated and plays
a biological role in a variety of tumor cells.
Apoptosis is an important event that affects cell
growth, and it is also an important way to kill tumor cells by
radiotherapy and chemotherapy. Liu et al and Meng et
al reported that silencing NOB1 enhances the sensitivity of
thyroid tumor cells to radiotherapy and the anticancer activity of
chemotherapeutic agents (16,17). Recent studies have shown that the
downregulation of NOB1 induces apoptosis in colon and lung cancer
(18,19). Bcl-2/Bax opens permeability transition
pore of mitochondria and releases cytochrome c to activate
caspase-9, thus inducing apoptosis. In this study, it was found
that NOB1 expression level in cancer tissues was higher than that
in cancer-adjacent tissues of patients with osteosarcoma, which is
consistent with current studies and reports. siRNA knockout was
used to silence NOB1, and it was found that apoptosis of MG-63
cells were significantly increased. Further mechanism studies have
shown that NOB1 knockdown activates caspase-3 expression and
inhibits anti-apoptotic Bcl-2 expression. In addition, the
sensitivity of MG-63 cells to cisplatin is also significantly
enhanced, suggesting that NOB1 may be a potential target for the
treatment of osteosarcoma.
References
|
1
|
Zhou GJ, Zhang Y, Wang J, Guo JH, Ni J,
Zhong ZM, Wang LQ, Dang YJ, Dai JF and Yu L: Cloning and
characterization of a novel human RNA binding protein gene PNO1.
DNA Seq. 15:219–224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y, Ni J, Zhou G, Yuan J, Ren W, Shan
Y, Tang W, Yu L and Zhao S: Cloning, expression and
characterization of the human NOB1 gene. Mol Biol Rep. 32:185–189.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li XY, Luo QF, Li J, Wei CK, Kong XJ,
Zhang JF and Fang L: Clinical significance of NOB1 expression in
breast infiltrating ductal carcinoma. Int J Clin Exp Pathol.
6:2137–2144. 2013.PubMed/NCBI
|
|
4
|
Lin S, Meng W, Zhang W, Liu J, Wang P, Xue
S and Chen G: Expression of the NOB1 gene and its clinical
significance in papillary thyroid carcinoma. J Int Med Res.
41:568–572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu K, Gu MM, Chen HL and You QS: NOB1 in
non-small-cell lung cancer: Expression profile and clinical
significance. Pathol Oncol Res. 20:461–466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu G, Shen D, Jiao L and Sun Y: Nin one
binding protein expression as a prognostic marker in prostate
carcinoma. Clin Transl Oncol. 16:843–847. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao X, Wang J, Bai W, Ji W and Wang L:
NOB1 silencing inhibits the growth and metastasis of laryngeal
cancer cells through the regulation of JNK signaling pathway. Oncol
Rep. 35:3313–3320. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Friedman MA and Carter SK: The therapy of
osteogenic sarcoma: Current status and thoughts for the future. J
Surg Oncol. 4:482–510. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawahara M, Takahashi Y, Takazawa K,
Tsuchiya H, Tomita K, Yokogawa K and Miyamoto K: Caffeine
dose-dependently potentiates the antitumor effect of cisplatin on
osteosarcomas. Anticancer Res. 28(3A): 1–1685. 2008.PubMed/NCBI
|
|
11
|
Reinheckel T, Ullrich O, Sitte N and Grune
T: Differential impairment of 20S and 26S proteasome activities in
human hematopoietic K562 cells during oxidative stress. Arch
Biochem Biophys. 38:65–68. 2000. View Article : Google Scholar
|
|
12
|
Tone Y1 and Toh E: A Nob1p is required for
biogenesis of the 26S proteasome and degraded upon its maturation
in Saccharomyces cerevisiae. Genes Dev. 16:3142–3157. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lamanna AC and Karbstein K: Nob1 binds the
single-stranded cleavage site D at the 3′-end of 18S rRNA with its
PIN domain. Proc Natl Acad Sci USA. 106:pp. 14259–14264. 2009;
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fatica A, Oeffinger M, Dlakić M and
Tollervey D: Nob1p is required for cleavage of the 3′ end of 18S
rRNA. Mol Cell Biol. 23:1798–1807. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fatica A, Tollervey D and Dlakić M: PIN
domain of Nob1p is required for D-site cleavage in 20S pre-rRNA.
RNA. 10:1698–1701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu J, Dong BF, Wang PS, Ren PY, Xue S,
Zhang XΝ, Han Z and Chen G: Silencing NOB1 enhances doxorubicin
antitumor activity of the papillary thyroid carcinoma in vitro and
in vivo. Oncol Rep. 33:1551–1559. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meng W, Wang PS, Liu J, Xue S, Wang GM,
Meng XY and Chen G: Adenovirus-mediated siRNA targeting NOB1
inhibits tumor growth and enhances radiosensitivity of human
papillary thyroid carcinoma in vitro and in vivo. Oncol Rep.
32:2411–2420. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He XW, Feng T, Yin QL, Jian YW and Liu T:
NOB1 is essential for the survival of RKO colorectal cancer cells.
World J Gastroenterol. 21:868–877. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Ma C, Qian M, Wen Z, Jing H and Qian
D: Downregulation of NOB1 suppresses the proliferation and tumor
growth of non-small cell lung cancer in vitro and in vivo. Oncol
Rep. 31:1271–1276. 2014. View Article : Google Scholar : PubMed/NCBI
|