Introduction
Approximately one million new cases of early gastric
cancer are reported annually and account for 6.9% of all newly
reported cancer cases (1). Gastric
cancer ranks the fifth worldwide and is the third leading cause of
cancer-related death after lung, breast, colorectal and prostate
cancer (1,2).
According to the depth of infiltration, gastric
cancer is defined as limited infiltration to mucosa or submucosa,
with or without lymphatic metastasis (3). Scholars in Japan and other countries
consider that early detection and treatment is the most effective
way to improve the prognosis of gastric cancer (4,5). Although
gastroscopy is safe for the general population, the application of
gastroscopy in patients with chronic hypertension may cause
arrhythmia, myocardial infarction and complications (6). Therefore, painless gastroscopy can be
performed for patients with chronic hypertension under sedative
conditions to reduce the pain and the incidence of restless
situation.
The current study was carried out to compare the
safety of the application of painless gastroscopy and ordinary
gastroscopy in chronic hypertension patients combined with early
gastric cancer.
Materials and methods
Materials
A total of 123 patients with suspected early gastric
cancer were selected at the Dongying People's Hospital (Shandong,
China) from June, 2014 to August, 2016. The current study was
approved by the Ethics Committee of Dongying People's Hospital.
Signed written informed consent was obtained from the patients.
Patients were randomly divided into the painless
gastroscopy (n=63) and ordinary gastroscopy (n=60) groups. Patients
in the painless gastroscopy group included 30 males and 33 females,
with an average age of of 54.7±7.1 years. Patients in the ordinary
group included 29 males and 31 females, with an average age of
52.7±6.8 years. There was no significant difference in sex, age,
early symptoms of gastric cancer and the history of hypertension
between the two groups (P>0.05) (Table
I).
 | Table I.Comparison of general information of
the two groups. |
Table I.
Comparison of general information of
the two groups.
|
|
|
| Sex | Symptoms (cases) |
|
|---|
|
|
|
|
|
|
|
|---|
| Groups | n | Age years | Male | Female | Anorexia | Nausea and
vomitting | Hematemesis
melena | History of
hypertension (years) |
|---|
| Painless
gastroscopy | 63 | 54.7±7.1 | 30 | 33 | 33 | 29 | 1 | 11.3±6.1 |
| Ordinary
gastroscopy | 60 | 52.7±6.8 | 29 | 31 | 32 | 26 | 2 | 10.5±5.6 |
| t/χ2 | – | 8.72 | 10.11 |
| 12.34 | 8.68 | 9.02 | 13.13 |
| P-value | – | 0.09 | 0.39 |
| 0.28 | 1.02 | 0.58 | 0.34 |
General preparation
Preoperative preparation was performed for all the
patients. Anesthesia and anesthesia-related complications were also
explained to the patients. All the patients signed informed
consent. Medical history was checked and clinical examination was
performed to exclude disease of heart, brain and other vital
organs. Patients were fasted for 8 h and deprivation was performed
for 6 h. Gastroscope, ECG monitor, mask, oxygen supplies and
narcotic drugs were prepared.
Operation methods
Patients were fasted for 8 h and deprivation was
performed for 4 h before treatment. Oral administration of
dacronine hydrochloride (Yangtze River Pharmaceutical Group, Ltd.,
Jiangsu, China) was performed before gastroscopy for mucosal
lubrication and anesthesia. Patients were fixed in left lateral
position, and vital signs were checked. Patients in the painless
gastroscopy were given balanced anesthesia before gastroscopy, and
the specific method employed was: Remifentanil (Yichang Renfu
Pharmaceutical Co., Ltd., Hubei, China) at a dose of 0.5–1 µg/kg
and propofol (Beijing Fresenius Kabi Pharmaceutical Co., Ltd.,
Beijing, China) at a dose of 1.5–2 mg/kg through slow intravenous
injection. Gastroscopy was performed when patients lost
consciousness, relevant reflex disappeared, and muscle relaxed.
Proper drug treatment was performed according to the conditions.
When endoscope mirror body reached the descendant duodenum, images
were captured and examination was stopped. Patients were asked not
to intake any food and only intake liquid diet 2 h after operation.
Balanced anesthesia was not performed for patients in the ordinary
gastroscopy group.
Observation indicators
Changes in arterial pressure, heart rate, and blood
oxygen saturation were recorded and compared before anesthesia,
when the gastroscope passed through the esophageal entrance plane,
and after recovery from anesthesia. The incidence of adverse
reactions including nausea, vomiting, cough, dysphoria and throat
discomfort were recorded and compared.
Statistical analysis
SPSS 11.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for the statistical analyses. Measurement data
were expressed as mean ± SD, and comparisons between groups were
performed using t-test. Countable data were compared using the
χ2 test. P<0.05 was considered to indicate a
statistically significant analysis.
Results
Comparison of mean arterial pressure,
heart rate and blood oxygen saturation between the two groups at
different time points
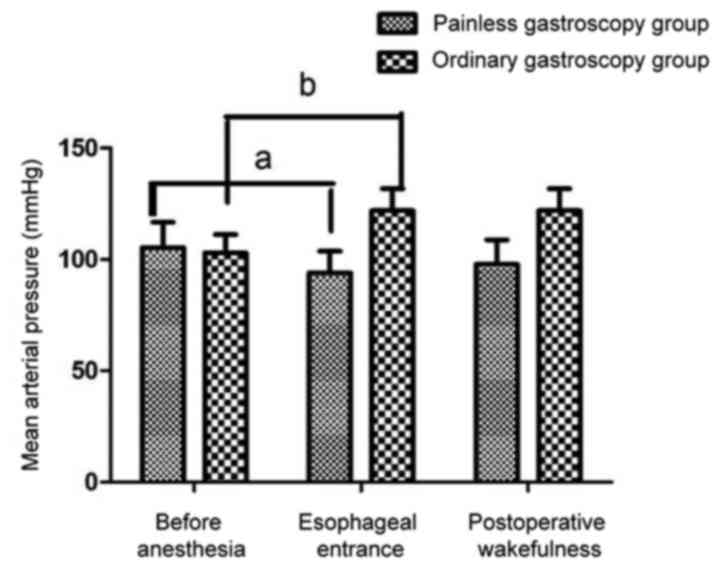
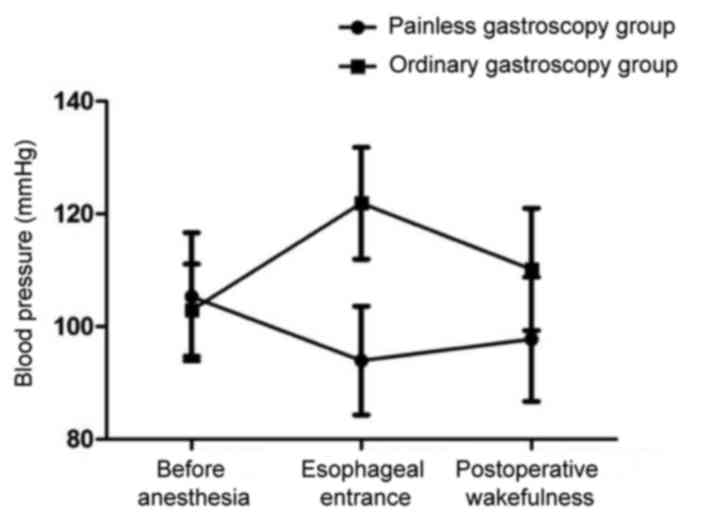
Mean arterial pressure, heart rate and blood oxygen
saturation were significantly reduced in painless gastroscopy when
the gastroscope passed through the esophageal entrance plane
compared with the levels of those factors before anesthesia
(P<0.05). In the ordinary gastroscopy group, compared with the
levels before anesthesia, the mean arterial pressure, heart rate
and blood oxygen saturation were significantly increased when the
gastroscope passed through the esophageal entrance plane
(P<0.05). Blood pressure decreased in the painless gastroscopy
group and increased in the ordinary gastroscopy group after
anesthesia. The decrease in the painless gastroscopy group was
lower than the increase in the ordinary group (Figs. 1–4).
Comparison of intra- and postoperative
adverse reactions between the two groups
The incidence of intra- and postoperative adverse
reactions including nausea, vomiting, cough, dysphoria, throat
discomfort and other adverse reactions was significantly lower in
the painless gastroscopy group compared with the ordinary
gastroscopy group (P<0.05). Thus, painless gastroscopy can
reduce the incidence of postoperative adverse reactions (Table II).
 | Table II.Comparison of intraoperative and
postoperative adverse reactions between the two groups [n (%)]. |
Table II.
Comparison of intraoperative and
postoperative adverse reactions between the two groups [n (%)].
| Groups | Nausea | Vomiting | Throat
discomfort | Cough | Dysphoria |
|---|
| A | 0 | 0 | 3 (4.8) | 0 | 0 |
| B | 60 | 43 (71.7) | 60 | 48 (80) | 25 (41.7) |
| P-value | 0.047 | 0.038 | 0.026 | 0.034 | 0.049 |
Comparison of the degree of tolerance
between the two groups
The number of patients showing no discomfort to
gastroscopy was significantly smaller in the painless gastroscopy
than in the ordinary gastroscopy (P<0.05) (Table III).
 | Table III.Comparison of degree of tolerance
between the two groups [n (%)]. |
Table III.
Comparison of degree of tolerance
between the two groups [n (%)].
|
|
| Discomfort |
|---|
|
|
|
|
|---|
| Groups | Unbearable | Obviously | Minor |
|---|
| Painless gastroscopy
group | 0 | 0 | 2 |
| Ordinary gastroscopy
group | 15 (25) | 30 (50) | 15v25 |
| P-value | 0.030 | 0.042 | 0.021 |
Discussion
Employing gastroscopy is imperative in the diagnosis
of gastric and upper gastrointestinal diseases, such as bleeding
and ulcers. Due to its invasive nature, gastroscopy leads to pain
in patients (7). Under ordinary
gastroscopy, local irritation caused by the endoscope can induce
nausea and vomiting. In addition, the effects of the
hypothalamus-pituitary-adrenal cortical system lead to, a series of
changes in vital signs of the body (8). Previous findings have shown that
gastroscopy may cause a series of complications in patients with
hypertension, such as myocardial infarction, cardiac arrest and
other complications (9). The
development of endoscopic technology, has led to an increase in the
diagnostic rate of early gastric cancer. However, the incidence of
hypertension is also on the increase. Therefore, the safe use of
this technique has become a focus of current research. The use of
painless gastroscopy, not only significantly reduces pain, but can
also facilitate the diagnosis of gastric cancer.
The morbidity and mortality of gastric cancer are
only lower to lung cancer (10,11).
Additionally, early diagnosis is closely related to the prognosis
of patients (12). Propofol used in
balanced anesthesia before painless gastroscopy can induce
anesthesia rapidly, and the recovery is fast. Propofol can also
inhibit nausea and vomiting. Nevertheless propofol has certain
inhibitory effects on the respiratory and circulatory system,
inducing a decrease in blood pressure and heart rate, albeit these
effects are related to the dose administered (13). Remifentanil is a potent opioid
receptor agonist with rapid action (14,15). The
combined use of propofol and remifentanil utilizes the advantages
of both to maintain vital signs and reduce unnecessary adverse
reactions, in order to facilitate gastroscopy (16–18).
Previous findings have shown that the incidence of
digestive diseases is high in patients with chronic hypertension.
In patients older than 45 years, the risk of cardiovascular disease
can be increased by 2-fold by an increase of 20 mmHg in systolic
blood pressure and 10 mmHg in diastolic blood pressure (19,20). In
patients with chronic hypertension, blood pressure is altered
during gastroscopy, which in turn increases the incidence of
cerebrovascular disease. In the present study, an increase in blood
pressure and heart rate was observed in the ordinary gastroscopy
group, whereas, the drug used in the painless gastroscopy group
reduced the blood pressure. Mean arterial pressure, heart rate and
blood oxygen saturation were significantly reduced in painless
gastroscopy when the gastroscope passed through the esophageal
entrance plane compared with the levels of those factors before
anesthesia (P<0.05). In the ordinary gastroscopy group, compared
with the levels before anesthesia, mean arterial pressure, heart
rate and blood oxygen saturation were significantly increased when
the gastroscope passed through the esophageal entrance plane
(P<0.05). No obvious discomfort, nausea, vomiting, cough,
dysphoria, or pharyngeal discomfort was found in the painless
gastroscopy group.
In conclusion, painless gastroscopy is safer, and
more comfortable and effective for chronic hypertension patients
combined with early gastric cancer. However, contraindications
should be checked and vital signs should be monitored to reduce the
intra- and postoperative bleeding caused by surgery. The
application of painless gastroscopy can significantly increase the
diagnostic rate of early gastric cancer. However, the circulation
should be maintained to reduce complications of anesthesia.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:359–386. 2015.
View Article : Google Scholar
|
|
3
|
Katai H and Sano T: Early gastric cancer:
Concepts, diagnosis, and management. Int J Clin Oncol. 10:375–383.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crew KD and Neugut AI: Epidemiology of
gastric cancer. World J Gastroenterol. 12:354–362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsubono Y and Hisamichi S: Screening for
gastric cancer in Japan. Gastric Cancer. 3:9–18. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yeoh KG: How do we improve outcomes for
gastric cancer? J Gastroenterol Hepatol. 22:970–972. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ross R and Newton JL: Heart rate and blood
pressure changes during gastroscopy in healthy older subjects.
Gerontology. 50:182–186. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maki S, Yao K, Nagahama T, Beppu T, Hisabe
T, Takaki Y, Hirai F, Matsui T, Tanabe H and Iwashita A: Magnifying
endoscopy with narrow-band imaging is useful in the differential
diagnosis between low-grade adenoma and early cancer of superficial
elevated gastric lesions. Gastric Cancer. 16:140–146. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miskovitz P: Sleisenger & Fordtran's
gastrointestinal and liver
disease-pathophysiology/diagnosis/management. Gastrointest Endosc.
49:A21999. View Article : Google Scholar
|
|
10
|
Takeno S, Hashimoto T, Maki K, Shibata R,
Shiwaku H, Yamana I, Yamashita R and Yamashita Y: Gastric cancer
arising from the remnant stomach after distal gastrectomy: A
review. World J Gastroenterol. 20:13734–13740. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujiwara S, Yao K, Nagahama T, Uchita K,
Kanemitsu T, Tsurumi K, Takatsu N, Hisabe T, Tanabe H, Iwashita A,
et al: Can we accurately diagnose minute gastric cancers (≤5 mm)?
Chromoendoscopy (CE) vs magnifying endoscopy with narrow band
imaging (M-NBI). Gastric Cancer. 18:590–596. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tao G, Xing-Hua L, Ai-Ming Y, Wei-Xun Z,
Fang Y, Xi W, Li-Yin W, Chong-Mei L, Gui-Jun F, Hui-Jun S, et al:
Enhanced magnifying endoscopy for differential diagnosis of
superficial gastric lesions identified with white-light endoscopy.
Gastric Cancer. 17:122–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Disma N, Astuto M, Rizzo G, Rosano G, Naso
P, Aprile G, Bonanno G and Russo A: Propofol sedation with fentanyl
or midazolam during oesophagogastroduodenoscopy in children. Eur J
Anaesthesiol. 22:848–852. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dias-Silva D, Pimentel-Nunes P, Magalhaes
J, Magalhaes R, Veloso N, Ferreira C, Figueiredo P, Moutinho P and
Dinis-Ribeiro M: The learning curve for narrow-band imaging in the
diagnosis of precancerous gastric lesions by using web-based video.
Gastrointest Endosc. 79:910–920. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Numata N, Oka S, Tanaka S, Kagemoto K,
Sanomura Y, Yoshida S, Arihiro K, Shimamoto F and Chayama K: Risk
factors and management of positive horizontal margin in early
gastric cancer resected by en bloc endoscopic submucosal
dissection. Gastric Cancer. 18:332–338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ichikawa D, Komatsu S, Kubota T, Okamoto
K, Shiozaki A, Fujiwara H and Otsuji E: Long-term outcomes of
patients who underwent limited proximal gastrectomy. Gastric
Cancer. 17:141–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ozcelik M, Guclu C, Bermede O, Baytas V,
Altay N, Karahan MA, Erdogan B and Can O: The administration
sequence of propofol and remifentanil does not affect the ED50 and
ED95 of rocuronium in rapid sequence induction of anesthesia: A
double-blind randomized controlled trial. Eur Rev Med Pharmacol
Sci. 20:1479–1489. 2016.PubMed/NCBI
|
|
18
|
Hwang SW and Lee DH: Is endoscopic
ultrasonography still the modality of choice in preoperative
staging of gastric cancer? World J Gastroenterol. 20:13775–13782.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao P, Xiao SM, Tang LC, Ding Z, Zhou X
and Chen XD: Proximal gastrectomy with jejunal interposition and
TGRY anastomosis for proximal gastric cancer. World J
Gastroenterol. 20:8268–8273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amornyotin S, Lertakayamanee N,
Wongyingsinn M, Pimukmanuskit P and Chalayonnavin V: The
effectiveness of intravenous sedation in diagnostic upper
gastrointestinal endoscopy. J Med Assoc Thai. 90:301–306.
2007.PubMed/NCBI
|