Introduction
With the recent advances in endoscopic treatment,
numerous T1 colorectal carcinomas (CRCs) are resected
endoscopically with negative margins (1), and the proportion of early carcinomas
amenable to endoscopic resection has increased (2). Previously identified risk factors for
lymph node metastasis in T1 CRCs include lymphovascular invasion,
histological grade, tumor budding, poor differentiation and degree
of submucosal invasion (3–5). However, the mechanism of these risk
factors and the molecular markers associated with carcinoma cell
invasion remain to be fully elucidated in T1 CRCs.
Matrix metalloproteinases (MMPs) are a family of
Zn2+-dependent proteolytic enzymes involved in the
physiological and pathological remodeling of the extracellular
matrix (ECM) during proliferation, angiogenesis and wound healing
(6). MMP-7 is the smallest
metalloproteinase identified to date (6), and has important roles in the
degradation of ECM proteins (including fibronectin, laminin and
collagen IV) and the regulation of several biochemical processes
such as activation, degradation and shedding of non-ECM proteins
(7). MMP-7 has attracted attention
because previous studies detected overexpression of MMP-7 in
invasive cancers of digestive organs such as the esophagus,
stomach, colon and liver (7). In
addition, overexpression of MMP-7 has been revealed in cancers of
other organs, including the lungs, skin, breast, prostate, head and
neck (7). A previous
immunohistochemical study (8)
revealed a significant correlation between MMP-7 expression at the
invasive front and metastasis or prognosis of advanced CRCs.
However, few studies have focused on T1 CRCs, and no definite
conclusions could be obtained about the implication of MMP-7 with
reference to pathological factors.
In the present study, the ability to examine
three-dimensional ultrastructural changes of carcinoma invasion
using low vacuum-scanning electron microscopy (LV-SEM) was
identified, in addition to routine observation by light microscopy.
To the best of our knowledge, no study has used this new technique
to investigate the morphological and qualitative alterations of the
ECM in CRCs to date. LV-SEM may be able to evaluate
three-dimensional ultrastructural changes of the ECM using the same
sections used for light microscopy, which would be considered as a
novel and useful tool to assess the condition of the carcinoma
invasion front.
Considering the aspects described above, the present
study aimed to clarify the association of MMP-7 expression with
pathological factors of T1 CRCs using immunohistochemistry, and to
examine the three-dimensional ultrastructural changes of carcinoma
invasion using LV-SEM.
Materials and methods
Patients and clinical data
Between April 2008 and December 2009, 211
consecutive cases of T1 CRCs were resected endoscopically or
surgically at Showa University Northern Yokohama Hospital
(Yokohama, Japan). Written informed consent was obtained from all
patients prior to endoscopy or surgery, and the study design was
approved by the Ethical Review Committee of Showa University School
of Medicine Showa (Showa, Japan; approval no. 1929). Patients who
were diagnosed with ulcerative colitis (n=1) were excluded from the
study. Pathological evaluation was not possible for 21 patients due
to damage to or loss of the specimen. In total, 189 cases underwent
only endoscopic treatment (n=52), initial surgery (n=57) or
additional surgery (n=80) with nodal dissection. These procedures
were performed in accordance with the principles of the Japanese
Society for Cancer of the Colon and Rectum (JSCCR) guidelines
(9). The age and sex of the patients,
and the location and size of the lesions, were reviewed from the
electronic records system. The endoscopic morphology of the lesion
was classified as ‘depressed’, ‘flat’ or ‘protruded-type’ based on
the Kudo's morphological/development classification (10).
Pathological evaluations
All resected lesions were retrieved and immediately
fixed in 10% buffered formalin for 24–48 h. Subsequently, the
resected lesions were cut at the point where the deepest invasion
area could be exposed on the cut-end surface. The serial
histological specimens were cut into parallel 2–3 mm-thick slices.
The tumor size was measured upon formalin fixation. The specimens
were examined based on the 2010 World Health Organization (WHO)
criteria (11) and JSCCR guidelines
(9). The histological grade, tumor
budding, desmoplastic reaction (DR) and growth type were
investigated using hematoxylin and eosin (H&E)-stained
specimens. Lymphatic invasion was evaluated using H&E staining
and immunostaining with an anti-D2-40 antibody (Clone D2-40; cat.
no. IR072; dilution, ready to use; Dako; Agilent Technologies,
Inc., Santa Clara, CA, USA), with which the samples were incubated
for 15 min at room temperature. Venous invasion was evaluated using
H&E and Victoria Blue B staining. The histological grade was
based on the least-differentiated tumor component and classified
according to the 2010 WHO criteria (11). In the present study, a poorly
differentiated adenocarcinoma or mucinous carcinoma component was
considered present if any part of the lesion contained either of
these features. Tumor budding was defined as an isolated single
cancer cell or a cluster of <5 carcinoma cells at the invasive
front (12). The field where budding
was most intensive was selected to count the number of buds with a
20X-objective lens. Fields with ≥5 buds were considered ‘positive’
according to Ueno et al (13).
The growth type was evaluated as polypoid or non-polypoid growth
according to Shimoda et al (14). The vertical invasion depth was
measured based on the JSCCR guidelines (9). The degree of submucosal invasion was
evaluated according to the Kudo's classification of degree of
submucosal invasion (15,16). The status of the muscularis mucosae
(i.e., MM grade) was evaluated using desmin immunostaining (clone
D33; cat. no. IR606; dilution ready to use; Dako; Agilent
Technologies, Inc.) with which the samples were incubated for 15
min at room temperature, and was classified as ‘grade 1’ when the
muscular fibers were maintained or as ‘grade 2’ when the muscle
fibers had fragmented or disappeared (17).
Immunohistochemistry of MMP-7
Serial sections (4-µm thick) were newly cut from the
slice with the deepest invasion area. The sections were stained
using an automated immunohistochemistry staining device (BOND-III;
Leica Microsystems, Inc., Buffalo Grove, IL, USA). In brief, the 4
µm-thick sections were transferred onto poly-L-lysine-coated
adhesive slides and dried at 62°C for 30 min. The sections were
then incubated with the primary anti-MMP-7 antibody (clone ID-2;
cat. no. M0785; dilution 1:2,000; Merck KGaA, Darmstadt, Germany)
with which the samples were incubated for 15 min at room
temperature, using a Bond Polymer Refine Detection kit (Leica
Biosystems Newcastle, Newcastle, UK).
Evaluation of MMP-7 expression
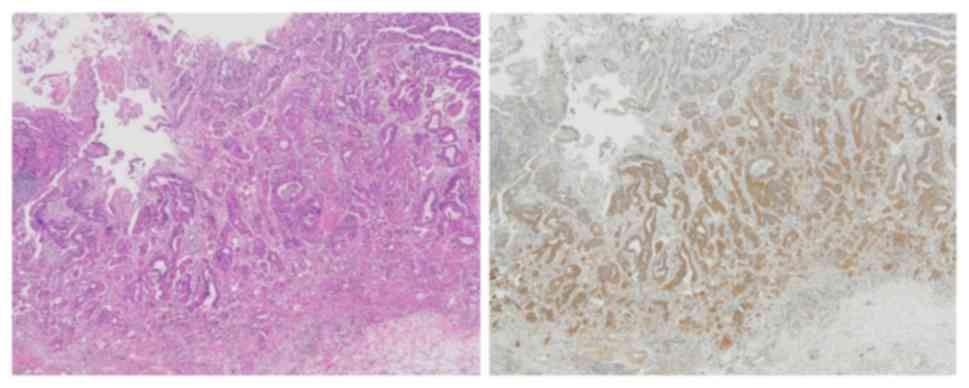
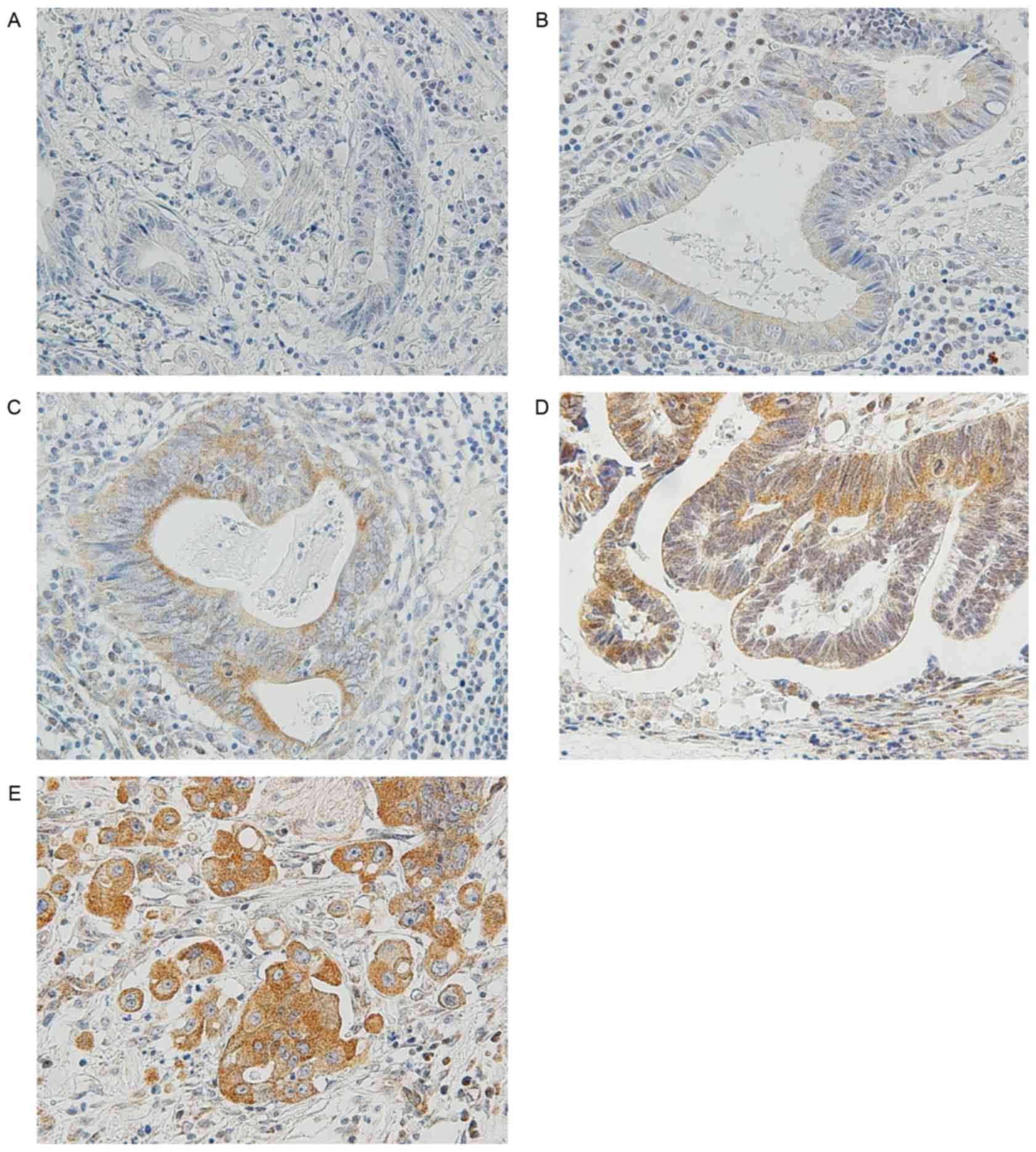
MMP-7 is unique because its expression is restricted
to tumor cells, and can be stained in the cytoplasm and cell
membranes (Fig. 1) (8). In accordance with the procedure used in
previous studies (18–21), MMP-7 immunostaining signals were
counted in five fields at the invasive front. Scores were
calculated as the percentage of tumor cells expressing MMP-7
divided by the total number of carcinoma cells in each field. The
specimens were then classified as follows: ‘Negative’ (−); ‘low
expression’ for <10% cells expressing MMP-7 (1+); ‘moderate
expression’ for 10–50% cells expressing MMP7 (2+); ‘high
expression’ for 50–80% cells expressing MMP-7 (3+); and ‘intense
expression’ for >80% cells expressing MMP-7 (4+). Tumor cells
expressing MMP-7 were considered as ‘positive’ if the median of the
scores in the five fields was 3+ or 4+. Representative findings are
shown in Fig. 2.
Immunohistochemistry of collagen
IV
Collagen IV is the principal structural component of
the ECM in the vascular basement membrane (22). In the present study, to examine the
morphological and qualitative alterations of the injured vascular
basement membrane using immunostaining for collagen IV, a number of
MMP-7-positive samples were selected from the 189 cases of T1 CRC.
As a normal control, areas where the carcinoma had not invaded to
the submucosa were also prepared. Tissue sections (4-µm thick) from
MMP-7-positive cases and normal controls were stained with an
anti-collagen IV antibody (clone CIV22; cat. no., MAB13414;
dilution 1:50; Dako; Agilent Technologies, Inc.) with which the
samples were incubated for 15 min at room temperature using the
aforementioned BOND-III automated immunohistochemistry staining
device and a Bond Polymer Refine Detection kit. The positive
staining (brown) of collagen IV was identified in vessel walls.
LV-SEM
Sections with Masson's trichrome staining were
examined without a mounting coverslip under LV-SEM using a tabletop
microscope (TM3000; Hitachi Ltd., Tokyo, Japan). The present study
compared the same area in MMP-7-positive samples and normal
controls using serial sections (4-µm thick) subjected to
immunostaining for collagen IV and stained with Masson's trichrome
staining for observation under LV-SEM. During the staining process,
10-µm thick sections were firstly stained with the anti-MMP-7
antibody visualized by 3,3′-Diaminobenzidine and incubated for 15
min at room temperature, then secondly stained with Victoria Blue B
for light microscopy examination. Upon demounting, these sections
were stained with Masson's trichrome staining for LV-SEM
observation.
Statistical analysis
The association between MMP-7 expression and the
pathological factors mentioned above was examined using the
Fisher's exact test. P<0.05 was considered to indicate a
statistically significant difference. All analyses were conducted
using R statistical software version 2.10.0 (R Foundation, Vienna,
Austria; available at http://www.r-project.org).
Results
Clinical characteristics
As shown in Table I,
endoscopic or surgical treatment was administered to 189 patients,
of which 126 were male and 63 were female. The patients had a mean
age of 66.0±10.9 years (range, 31–89 years). The mean tumor size
was 21.0±11.5 mm (range, 4–65 mm). There were 35 depressed-type,
and 154 flat and protruded-type lesions. In total, 143 lesions were
in the colon and 46 lesions were in the rectum. The depth of
invasion was 3,078.0±2,994.4 µm (range, 0–21,200 µm).
 | Table I.Clinicopathological factors of 189 CRC
cases. |
Table I.
Clinicopathological factors of 189 CRC
cases.
| Clinicopathological
factors | T1 CRC, n
(n=189) |
|---|
| Age (mean ± SD),
years | 66.0±10.9 |
| Sex |
|
|
Female | 63 |
| Male | 126 |
| Tumor size (mean ±
SD), mm | 21.0±11.5 |
| Location |
|
|
Rectum | 46 |
|
Colon | 143 |
| Tumor budding |
|
| + | 22 |
| − | 167 |
| Lymphatic
invasion |
|
| + | 45 |
| − | 144 |
| Venous invasion |
|
| + | 53 |
| − | 136 |
| Por/Muc
component |
|
| + | 31 |
| − | 158 |
| Growth type |
|
| PG | 123 |
| NPG | 66 |
| Morphology |
|
|
Depressed | 35 |
|
Non-depressed | 154 |
| DR on the
superficial layer |
|
| + | 36 |
| − | 153 |
| Depth of invasion,
µm |
|
|
<1,000 | 49 |
|
≥1,000 | 140 |
| Kudo's
classification of degree of submucosal invasion |
|
| sm1a,
1b | 38 |
| sm1c,
2, 3 | 151 |
| MM grade |
|
| 2 | 165 |
| 1 | 24 |
| Depth of invasion
(mean ± SD), µm |
3,078.0±2,994.4 |
Association of MMP-7 expression with
pathological factors
The expression of MMP-7 was positive in 104 (55.0%)
of the 189 examined lesions. As shown in Table II, MMP-7 expression was positive in
38 (71.7%) of 53 lesions with venous invasion and in 66 (48.5%) of
136 lesions without venous invasion. This difference was
statistically significant (P=0.0054, Fisher's exact test). MMP-7
expression was not significantly associated with tumor budding,
lymphatic invasion, histological differentiation, growth type,
morphology, DR on the superficial layer, depth of invasion, the
Kudo's classification of degree of submucosal invasion, or the MM
grade (17).
 | Table II.Association between various
pathological factors and MMP-7 expression. |
Table II.
Association between various
pathological factors and MMP-7 expression.
|
| MMP-7 expression at
the invasive front |
|
|
|---|
|
|
|
|
|
|---|
| Pathological
factors | Positive
(n=104) | Negative
(n=85) | OR (95% CI) |
P-valuea |
|---|
| Tumor budding |
|
| 1.20
(0.45–3.38) | 0.821 |
| + | 13 | 9 |
|
|
| − | 91 | 76 |
|
|
| Lymphatic
invasion |
|
| 1.16
(0.56–2.43) | 0.733 |
| + | 26 | 19 |
|
|
| − | 78 | 66 |
|
|
| Venous
invasion |
|
| 2.67
(1.29–5.74) | 0.005 |
| + | 38 | 15 |
|
|
| − | 66 | 70 |
|
|
| Por/Muc
component |
|
| 0.85
(0.36–1.99) | 0.697 |
| + | 16 | 15 |
|
|
| − | 88 | 70 |
|
|
| Growth type |
|
| 1.24
(0.65–2.37) | 0.540 |
| PG | 70 | 53 |
|
|
|
NPG | 34 | 32 |
|
|
| Morphology |
|
| 1.48
(0.66–3.45) | 0.350 |
|
Depressed type | 22 | 13 |
|
|
|
Non-depressed type | 82 | 72 |
|
|
| DR on the
superficial layer |
|
| 1.18
(0.53–2.66) | 0.712 |
| + | 21 | 15 |
|
|
| − | 83 | 70 |
|
|
| Depth of invasion,
µm |
|
| 1.00
(0.49–2.01) | 1.000 |
|
<1,000 | 27 | 22 |
|
|
|
≥1,000 | 77 | 63 |
|
|
| Kudo's
classification of degree of submucosal invasion |
|
| 1.13
(0.52–2.45) | 0.856 |
| sm1a,
1b | 20 | 18 |
|
|
| sm1c,
2, 3 | 84 | 67 |
|
|
| MM grade |
|
| 1.52
(0.59–4.00) | 0.383 |
| 2 | 93 | 72 |
|
|
| 1 | 11 | 13 |
|
|
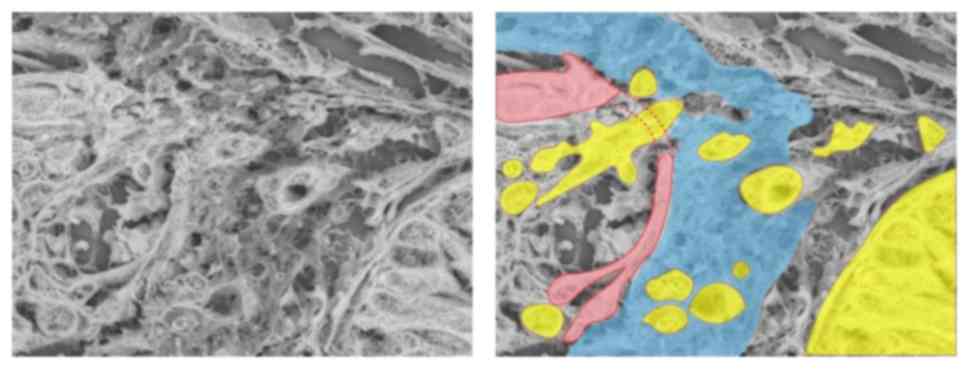
Ultrastructural alteration of venous
invasion as determined by LV-SEM
Alterations of veins were examined by Victoria Blue
B staining, collagen IV staining and LV-SEM, and the findings were
compared (Fig. 3A-H). Normal veins
were stained as a two-layered structure by collagen IV and Victoria
Blue B staining. The structure of collagen IV staining was inside
the veins, and exhibited a strong and thick linear pattern
(Fig. 3A and C). Ultrastructural
alterations of the veins were examined by LV-SEM. Normal veins were
characterized by smooth bundles of elastic fibers in the adventitia
and a mesh-like structure of collagen fibers in the intima
(Fig. 3E and G). In samples with
positive MMP-7 expression, venous invasion was frequently observed
at the invasive front. In veins invaded by tumor cells, Victoria
Blue B staining was thinner, and collagen IV staining was weaker,
compared with normal veins (Fig. 3B and
D). Under LV-SEM, veins invaded by tumor cells were
characterized by an irregular surface that reflected the rupture of
the two-layered structure of the vein (Fig. 3F). In addition, LV-SEM revealed that
the thin part of collagen IV staining was characterized by partial
disappearance of the mesh-like structure of collagen fibers in the
intima. The invading tumor cells in the isolated and clustered form
were visible within the altered layers of elastic and collagen
bundles (Figs. 3H and 4).
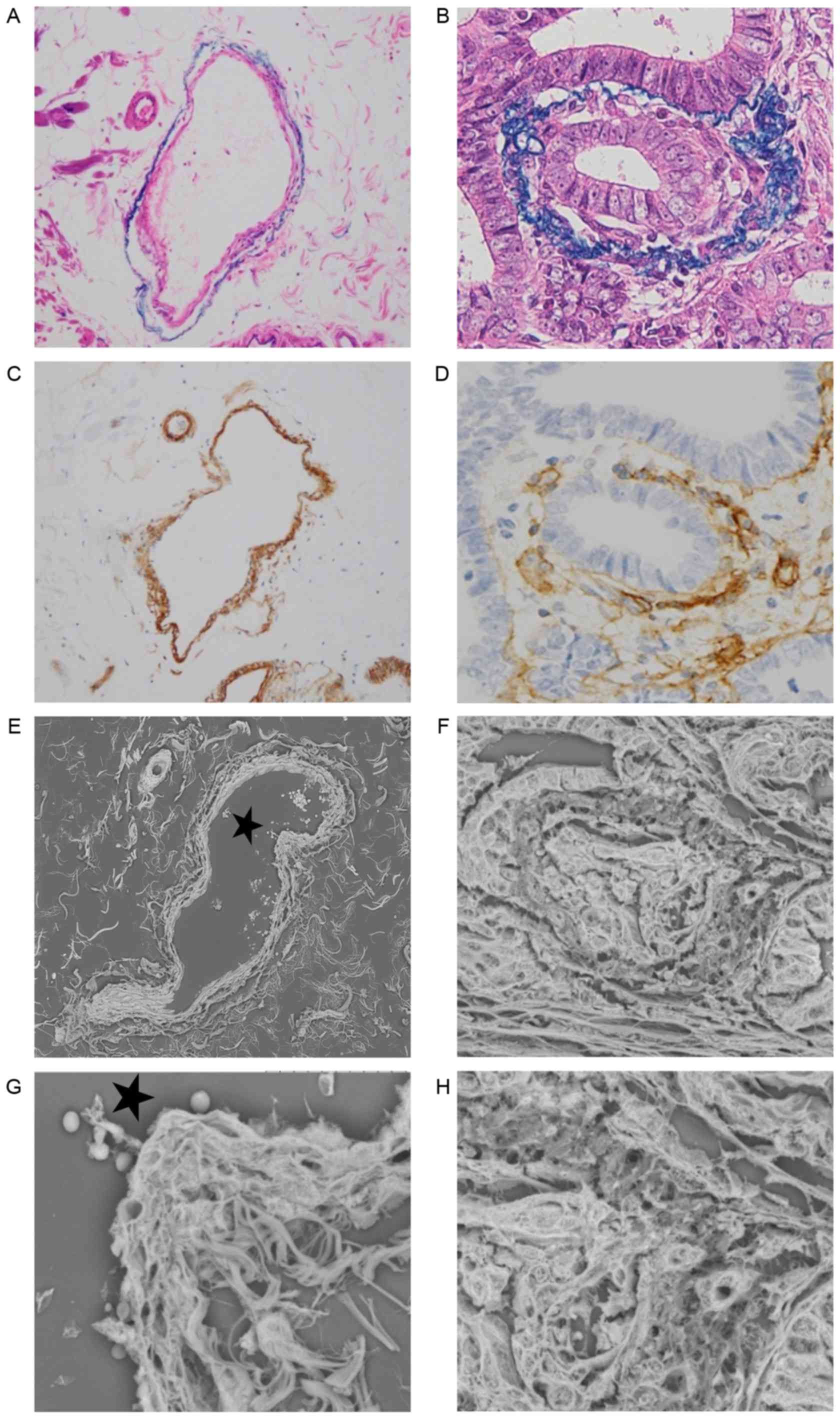 | Figure 3.Histological findings of (A and B)
hematoxylin and eosin, Victoria Blue B and (C and D) collagen IV
staining, and (E-H) three-dimensional ultrastructural findings in
LV-SEM. Panels A, C, E and G show a normal vein in serial sections,
while panels B, D, F and H show a vein invaded by tumor cells with
positive matrix metalloproteinase-7 expression in serial sections.
(A) Victoria Blue B staining was specifically visible in the
elastic fibers of the adventitia (magnification, ×200). (B)
Victoria Blue B staining of adventitia was thinner on the invasion
side (magnification, ×400). (C) Collagen IV staining was visible in
the intima. Collagen IV of the normal vein was stained as a strong
and thick linear pattern (magnification, ×200). (D) Collagen IV
staining of the intima was weak and interrupted by the invading
tumor cells (magnification, ×200). (E) Serial section stained with
phosphotungstic acid (Masson's trichrome staining) and assessed by
LV-SEM (magnification, ×300). (F) Veins invaded by tumor cells were
characterized by an irregular change in the two vascular layers in
LV-SEM (magnification, ×800). (G) A normal vein was characterized
by a two-layered structure with smooth elastic fibers of the
adventitia and a mesh-like structure of collagen fibers in the
intima by LV-SEM (magnification, ×1,500). (H) The thin portion of
collagen IV staining was characterized by disappearance of the
intimal mesh-like structure of collagen fibers in LV-SEM
(magnification, ×1,500). The invading tumor cells in an isolated
and clustered form were visible within the altered venous walls in
LV-SEM (magnification, ×1,500). The stars in (E) and (G) indicate
the same lumen. LV-SEM, low vacuum-scanning electron
microscopy. |
Discussion
T1 CRC is the initial step in tumor spread, and may
precisely reflect the metastatic potential of the primary tumor
(19). The purpose of the present
study was to investigate the association between MMP-7 expression
at the invasive front and the risk factors of metastasis in T1
CRCs. A previous study on T1 CRCs (19) revealed that positive basilar and/or
cytoplasmic MMP-7 immunostaining at the invasive front was
significantly associated with the depth of invasion, tumor budding,
lymphatic invasion and venous invasion. However, another study
(20) indicated that the frequency of
MMP-7 expression was significantly different among different
histological grades, and that the frequency of MMP-7 expression at
the invasive front differs depending on the selected microscopic
field of the sections. Therefore, in the present study, MMP-7
immunostaining was evaluated by the median of the scores in five
microscopic fields at the invasive front. Furthermore, objective
discrimination between lymphatic and blood vessels was performed
using immunostaining with an anti-D2-40 antibody, and double
staining with H&E and Victoria Blue B staining. Based on the
above aspects, the present results revealed that the expression of
MMP-7 was significantly associated with venous invasion in T1 CRCs.
In a prospective surveillance program for early-stage CRC, venous
invasion was a risk factor with a significant effect on
disease-free survival, overall survival and disease recurrence
(local or distant) (22). In
addition, using LV-SEM, the present study observed morphological
changes of collagen IV in veins. These findings may be
histologically plausible because MMP-7 has important roles in the
degradation of the principal structural components of veins such as
collagen IV (7). A previous study
(23) also reported that tumor cells
expressing MMP-9 (which, similarly to MMP-7, degrades collagen IV)
have significant positive correlations with venous invasion and
liver metastasis in pancreatic cancer.
A previous study has shown expression of MMP-2 and
MMP-9 in the glomerular basement membrane (GBM), which primarily
consists of collagen IV, in immunoglobulin A (IgA) nephropathy
(24). Another study (25) reported that morphological and
qualitative alterations of type IV collagen on the GBM in IgA
nephropathy are characterized by fractures of the GBM in LV-SEM.
Prior to the present study, upon demounting the sections, these
were stained with Masson's trichrome staining, periodic
acid-methenamine-silver or Platinum Blue staining, which contain
heavy metals (namely phosphotungstic acid, silver and platinum,
respectively). Based on those preliminary results, Masson's
trichrome staining was considered as the most effective method for
improving the observation of colonic samples by LV-SEM (data not
shown). A benefit of LV-SEM was the examination of the same veins
observed by double staining of MMP-7 and Victoria Blue B. Following
examination by double staining, the same sections were stained with
Masson's trichrome staining and then assessed by LV-SEM. In the
present study, the changes observed with collagen IV and Victoria
Blue B staining in the invaded venous walls were consistent with
the ultrastructural findings characterized by the rupture and
disappearance of the normal structure of collagen and elastic
fibers of veins by LV-SEM. These findings may be associated with
MMP-7 expression in tumor cells at the front of venous
invasion.
A limitation of the present study should be
acknowledged. The association between MMP-7 expression and tumor
budding in the current study was not significant. Basolateral
staining of MMP-7 tended to occur in the cytoplasm of the invading
tumor cells in the isolated and clustered form at the invasive
front. However, since only five fields were observed, it is
possible that the frequency of MMP-7 expression was underestimated.
A recent study (26) revealed an
association between the function of MMP-7 and its intracellular
localization. Basolateral localization of MMP-7 has been associated
with cell adhesion molecules and basement membrane components of
basolaterally located substrates of tumor cells, such as E-cadherin
and collagen IV (7). Such degradation
by MMP-7 may initiate cancer invasion (20).
In conclusion, MMP-7 expression by tumor cells at
the invasive front is significantly correlated with venous invasion
in T1 CRCs. This finding may provide important information to
evaluate the invasive potential of T1 CRCs. The expression of MMP-7
would be clinically useful as a prognostic marker of additional
therapies and/or close surveillance.
Acknowledgements
The authors thank Mrs. T. Nagai and Mr. Y. Sasaki,
medical technicians, of the Department of Pathology, Showa
University School of Medicine (Tokyo) for their valuable assistance
with immunohistochemical analysis of tissue samples, and Mrs. N.
Takenaka, illustrator, of Nikko memorial hospital (Hokkaido) for
creating the figures.
References
|
1
|
Kobayashi N, Saito Y, Uraoka T, Matsuda T,
Suzuki H and Fujii T: Treatment strategy for laterally spreading
tumors in Japan: Before and after the introduction of endoscopic
submucosal dissection. J Gastroenterol Hepatol. 24:1387–1392. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beaton C, Twine CP, Williams GL and
Radcliffe AG: Systematic review and meta-analysis of
histopathological factors influencing the risk of lymph node
metastasis in early colorectal cancer. Colorectal Dis. 15:788–797.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suzuki T, Sadahiro S, Mukoyama S, Ishikawa
K, Yasuda S, Tajima T, Makuuchi H and Murayama C: Risk of lymph
node and distant metastases in patients with early invasive
colorectal cancer classified as Haggitt's level 4 invasion: Image
analysis of submucosal layer invasion. Dis Colon Rectum.
46:203–208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ueno H, Hashiguchi Y, Kajiwara Y, Shinto
E, Shimazaki H, Kurihara H, Mochizuki H and Hase K: Proposed
objective criteria for ‘grade 3’ in early invasive colorectal
cancer. Am J Clin Pathol. 134:312–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benizri EI, Bereder JM, Rahili A, Bernard
JL, Vanbiervliet G, Filippi J, Hébuterne X and Benchimol D:
Additional colectomy after colonoscopic polypectomy for T1 colon
cancer: A fine balance between oncologic benefit and operative
risk. Int J Colorectal Dis. 27:1473–1478. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roeb E, Arndt M, Jansen B, Schumpelick V
and Matern S: Simultaneous determination of matrix
metalloproteinase (MMP)-7, MMP-1, −3, and −13 gene expression by
multiplex PCR in colorectal carcinomas. Int J Colorectal Dis.
19:518–524. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ii M, Yamamoto H, Adachi Y, Maruyama Y and
Shinomura Y: Role of matrix metalloproteinase-7 (matrilysin) in
human cancer invasion, apoptosis, growth, and angiogenesis. Exp
Biol Med (Maywood). 231:20–27. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adachi Y, Yamamoto H, Itoh F, Arimura Y,
Nishi M, Endo T and Imai K: Clinicopathologic and prognostic
significance of matrilysin expression at the invasive front in
human colorectal cancers. Int J Cancer. 95:290–294. 2001.PubMed/NCBI
|
|
9
|
Watanabe T, Itabashi M, Shimada Y, Tanaka
S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et
al: Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2010 for the treatment of colorectal cancer. Int J Clin
Oncol. 17:1–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kudo S, Lambert R, Allen JI, Fujii H,
Fujii T, Kashida H, Matsuda T, Mori M, Saito H, Shimoda T, et al:
Nonpolypoid neoplastic lesions of the colorectal mucosa.
Gastrointest Endosc. 68(4 Suppl): S3–S47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamilton SR, Bosman FT, Boffetta P, Ilyas
M, Morreau H and Nakamura SI: WHO classification of tumours of the
digestive systemCarcinoma of the colon and rectum. Bosman FT,
Carneiro F, Hruban RH and Theise ND: International Agency for
Research on Cancer (IARC); Lyon: pp. 134–146. 2010
|
|
12
|
Ueno H, Murphy J, Jass JR, Mochizuki H and
Talbot IC: Tumour ‘budding’ as an index to estimate the potential
of aggressiveness in rectal cancer. Histopathology. 40:127–132.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ueno H, Mochizuki H, Hashiguchi Y,
Shimazaki H, Aida S, Hase K, Matsukuma S, Kanai T, Kurihara H,
Ozawa K, et al: Risk factors for an adverse outcome in early
invasive colorectal carcinoma. Gastroenterology. 127:385–394. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shimoda T, Ikegami M, Fujisaki J, Matsui
T, Aizawa S and Ishikawa E: Early colorectal carcinoma with special
reference to its development de novo. Cancer. 64:1138–1146. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kudo S: Endoscopic mucosal resection of
flat and depressed types of early colorectal cancer. Endoscopy.
25:455–461. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kashida H and Kudo SE: Early colorectal
cancer: Concept, diagnosis, and management. Int J Clinical Oncol.
11:1–8. 2006. View Article : Google Scholar
|
|
17
|
Miyachi H, Kudo SE, Ichimasa K, Hisayuki
T, Oikawa H, Matsudaira S, Kouyama Y, Kimura YJ, Misawa M, Mori Y,
et al: Management of T1 colorectal cancers after endoscopic
treatment based on the risk stratification of lymph node
metastasis. J Gastroenterol Hepatol. 31:1126–1132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Masaki T, Matsuoka H, Sugiyama M, Abe N,
Goto A, Sakamoto A and Atomi Y: Matrilysin (MMP-7) as a significant
determinant of malignant potential of early invasive colorectal
carcinomas. Br J Cancer. 84:1317–1321. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kurokawa S, Arimura Y, Yamamoto H, Adachi
Y, Endo T, Sato T, Suga T, Hosokawa M, Shinomura Y and Imai K:
Tumour matrilysin expression predicts metastatic potential of stage
I (pT1) colon and rectal cancers. Gut. 54:1751–1758. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tamahashi U, Kumagai J, Takizawa T, Sekine
M and Eishi Y: Expression and intracellular localization of matrix
metalloproteinases in intraductal papillary mucinous neoplasms of
the pancreas. Virchows Arch. 453:79–87. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barros SS, Henriques ÁC, Pereira KM, de
Medeiros AM, Galvão HC and Freitas Rde A: Immunohistochemical
expression of matrix metalloproteinases in squamous cell carcinoma
of the tongue and lower lip. Arch Oral Biol. 56:752–760. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gilardoni E, Bernasconi DP, Poli S,
Garancini M, Luperto M, Zucchini N, Bovo G, Totis M, Bugatti A and
Gianotti L: Surveillance for early stages of colon cancer:
Potentials for optimizing follow-up protocols. World J Surg Oncol.
13:2602015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho K, Matsuda Y, Ueda J, Uchida E, Naito
Z and Ishiwata T: Keratinocyte growth factor induces matrix
metalloproteinase-9 expression and correlates with venous invasion
in pancreatic cancer. Int J Oncol. 40:1040–1048. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sekiuchi M, Kudo A, Nakabayashi K,
Kanai-Azuma M, Akimoto Y, Kawakami H and Yamada A: Expression of
matrix metalloproteinases 2 and 9 and tissue inhibitors of matrix
metalloproteinases 2 and 1 in the glomeruli of human glomerular
diseases: The results of studies using immunofluorescence, in situ
hybridization, and immunoelectron microscopy. Clin Exp Nephrol.
16:863–874. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Masuda Y, Yamanaka N, Ishikawa A, Kataoka
M, Arai T, Wakamatsu K, Kuwahara N, Nagahama K, Ichikawa K and
Shimizu A: Glomerular basement membrane injuries in IgA nephropathy
evaluated by double immunostaining for α5(IV) and α2(IV) chains of
type IV collagen and low-vacuum scanning electron microscopy. Clin
Exp Nephrol. 19:427–435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harrell PC, McCawley LJ, Fingleton B,
McIntyre JO and Matrisian LM: Proliferative effects of apical, but
not basal, matrix metalloproteinase-7 activity in polarized MDCK
cells. Exp Cell Res. 303:308–320. 2005. View Article : Google Scholar : PubMed/NCBI
|