Introduction
Paclitaxel is a cytotoxic agent that remains a
first-line chemotherapy in the management of advanced
nasopharyngeal carcinoma (NPC) (1,2). However,
the development of intrinsic or acquired resistance limits the
clinical efficacy of paclitaxel, which ultimately leads to tumor
recurrence and a poor prognosis. Therefore, elucidation of the
underlying molecular mechanisms that lead to paclitaxel resistance
is an essential requirement to identify novel therapeutic
strategies to overcome chemoresistance.
Extracellular pH (pHe) is considerably
more acidic in solid tumors compared with in normal tissue.
Previous studies have identified that acidic pHe is an
important characteristic of tumor tissues and is involved in cancer
progression (3–7). Furthermore, acidic pHe has
been reported to facilitate the development of resistance to
chemotherapeutic drugs, including paclitaxel (8–10).
Previous studies have provided evidence indicating that the
‘ion-trapping’ phenomenon mainly contributes to acidic
pHe-mediated drug resistance (11–13).
However, paclitaxel is not ionizable and therefore the drug
distribution should be unaffected by pHe. Thus, it is
important to understand the underlying molecular mechanisms
responsible for the development of paclitaxel resistance.
Epithelial-mesenchymal transition (EMT) is a complex
series of events, which was initially characterized as being
essential during normal embryonic development (14). Previous studies have demonstrated that
EMT is involved in physiological and pathological processes
(15). Critically, previous studies
have demonstrated an intricate association between chemoresistance
and the changes associated with EMT (16). A previous study in ovarian cancer
discovered that paclitaxel-resistant cells, which develop following
continuous exposure to paclitaxel, exhibited the cellular and
molecular characteristics of EMT (17).
Metadherin (MTDH), also known as astrocyte elevated
gene-1 and lysine-rich carcinoembryonic antigen-related cell
adhesion molecule-1-associated protein is an oncogene that is
expressed in multiple tumors (18).
Aberrant expression and dysfunction of MTDH is involved in tumor
cell proliferation, survival and metastasis activity (19). Previous studies have confirmed that
MTDH expression is associated with the chemoresistance of tumor
cells by the regulation of a series of downstream target genes
(20–24). The association between MTDH expression
and EMT has been established in cervical cancer, breast cancer and
hepatocarcinoma, which may contribute to chemoresistance (25–27).
In the present study, the influence of
pHe on the cytotoxicity of paclitaxel in NPC cells in
vitro was determined, and the associated mechanisms involved
were investigated further. The results indicated that the cytotoxic
efficacy of paclitaxel was markedly decreased at an acidic
pHe. Furthermore, it was revealed that MTDH-mediated EMT
may confer acidic pHe-induced paclitaxel resistance in
NPC.
Materials and methods
Cell culture
The human NPC 5-8F cells were provided by the Cell
Center of Central South University (Changsha, China). 5-8F cells
were maintained as monolayer cultures in RPMI-1640 medium (Hyclone;
GE healthcare, Chicago, IL, USA) supplemented with 10% fetal bovine
serum, 100 IU/ml penicillin and 100 IU/ml streptomycin (all
purchased from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at 37°C in a humidified atmosphere with 5% CO2.
Culture media with different pH values were prepared by the
addition of 20 mM 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic
acid or 20 mM 2-(N-morpholino)ethanesulfonic acid (both purchased
from Thermo Fisher Scientific, Inc.) to RPMI-1640 medium and pH
values were adjusted accordingly, using NaOH or HCl to pH 7.4 or
6.8, respectively. The actual pH in the media was determined prior
to and following each experiment. Cells in the exponential growth
phase were used for all subsequent experiments.
Paclitaxel cytotoxicity assays
The viability of NPC 5-8F cells was analyzed using a
Cell Counting Kit-8 (CCK8; Beyotime Institute of Biotechnology,
Shanghai, China) to produce cell viability curves according to the
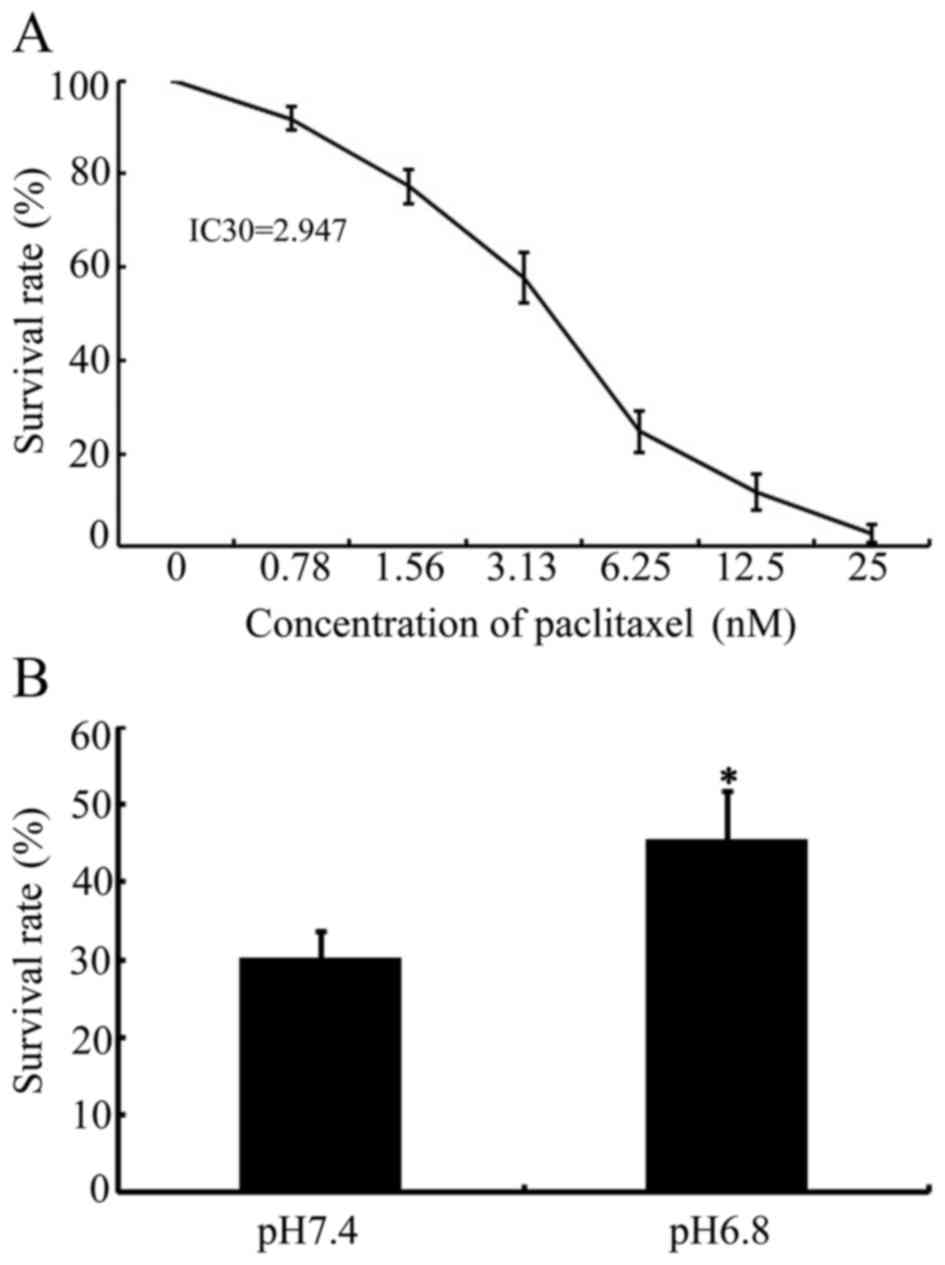
manufacturer's protocol. The IC30 value of paclitaxel in
NPC 5-8F cells was confirmed as 2. 947. IC30 is defined
as the concentration of paclitaxel required to produce 30%
inhibition of 5-8F cells. Subsequently, NPC 5-8F cells were seeded
at a density of 3×103 cells/well into 96-well plates in
triplicate. Following 24 h of culture, the culture medium was
aspirated and replaced with 100 µl medium, buffered to pH 7.4 or
6.8, and supplemented with paclitaxel at its IC30 value.
After 48 h of culture at 37°C, the optical density values of each
group were determined at a wavelength of 490 nm. Each experiment
was representative of three independent repeats.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. In total, 1 µg RNA was used for reverse
transcription to synthesize cDNA using the High Capacity
RNA-to-cDNA Kit (Applied Biosystems; Thermo Fisher Scientific
Inc.). In total, 20 µl of reverse-transcription reaction components
[10 µl 2X RT Buffer, 1 µl 20X RT Enzyme mix, 7 µl Nuclease-free
H2O (all from Applied Biosystems; Thermo Fisher
Scientific, Inc.) and 2 µl RNA] were established and incubated for
60 min at 37°C, 5 min at 95°C and then incubated at 4°C for further
investigation. iQ™SYBR® Green Supermix
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to PCR
amplification in a 20 µl reaction system. The reaction system was
prepared according to the following system: 10 µl
iQ™SYBR® Green Supermix, 0.5 µl PCR forward
primer, 0. 5 µl PCR reverse primer, 8 µl Nuclease-free
H2O (Bio-Rad Laboratories, Inc.) and 1 µl cDNA template.
Primer sequences used in the present study were as follows:
E-cadherin forward, 5′-GCTGGACCGAGAGAGTTTCC-3′ and reverse,
5′-CAAAATCCAAGCCCGTGGTG-3′; vimentin forward,
5′-TGTCCAAATCGATGTGGATGTTTC-3′ and reverse,
5′-TTGTACCATTCTTCTGCCTCCTG-3′; N-cadherin forward,
5′-TGGGAAATGGAAACTTGATGGC-3′ and reverse,
5′-AGTTGCTAAACTTCACTGAAAGGA-3′; MTDH forward,
5′-GATGATGAATGGTCTGGGTTAAA-3′ and reverse,
5′-GACCTTTTGATCATCAGGAATTG-3′; GAPDH forward,
5′-GAGTCAACGGATTTGGTCGT-3′ and reverse, 5′-TTGATTTTGGAGGGATCTCG-3′.
PCR thermocycling conditions were as follows: 3 min at 95°C
followed by 40 cycles of 15 sec at 95°C and 30 sec at 60°C. The
PCRs for each gene were performed in triplicate, and the mean
values of fold changes were used to calculate mRNA expression. The
fold change in expression of each gene was calculated using the
2−ΔΔCq method (28).
Western blot analysis
The western blot assay was performed as described
previously (29,30). In brief, the total protein (50 µg) was
separated by 10% SDS-PAGE and the separated proteins were
transferred to polyvinylidene fluoride membranes (Millipore,
Billerica, MA, USA). Membranes were blocked with 5% skimmed milk at
room temperature for 1 h, then incubated with the following primary
antibodies at 4°C overnight: Primary antibodies used in the present
study were: Mouse monoclonal antibody against E-cadherin (1:400;
cat. no. sc-8426; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
mouse monoclonal antibody against vimentin (1:200; cat. no.
sc-32322; Santa Cruz Biotechnology, Inc.) and rabbit polyclonal
antibody against MTDH (1:800; cat. no. 13860-1-AP, Proteintech
Group, Inc., Chicago, USA). Subsequent to washing three times in
TBST, membranes were incubated with horseradish peroxidase
(HRP)-labeled goat Anti-mouse IgG (H+L; 1:1,000; cat. no. A0216) or
HRP-labeled Goat Anti-rabbit IgG (H+L; 1:4,000; cat. no. A0208)
(both purchased from Beyotime Institute of Biotechnology, Shanghai,
China) for 1 h at room temperature. Bands were visualized using the
BeyoECL Plus Detection System (Beyotime Institute of Biotechnology)
and the images were obtained by X-ray film exposure. For
normalization of protein loading, mouse monoclonal antibody against
β-actin (1:1,000; cat. no. AF0003; Beyotime Institute of
Biotechnology) was used. All antibodies were diluted using primary
antibody dilution buffer or secondary antibody dilution buffer
(both purchased from Beyotime Institute of Biotechnology). Each
experiment was performed in triplicate.
Transient transfection
NPC 5-8F cells were transiently transfected with
MTDH small interfering (si)RNA (cat. no. sc-77797) or control siRNA
(cat. no. sc-37007; Santa Cruz Biotechnology, Inc.), according to
the manufacturer's protocol. MTDH siRNA is a pool of three
target-specific 19-25 nt siRNAs designed to knock down MTDH
expression. Control siRNA is a non-targeting 20–25 nt siRNA
designed as a negative control. 5-8F cells (2×105 cells)
were seeded on a six well plate 24 h prior to transfection. 8 µl of
siRNA duplex (80 pM siRNA) or control siRNA was diluted into 100 µl
siRNA transfection medium (cat. no. sc-36868; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) as solution A. Furthermore, 6
µl of siRNA transfection reagent (cat. no. sc-29528; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) was diluted into 100 µl siRNA
transfection medium as solution B. Solution A and B were mixed and
incubated for 45 min at room temperature. In total, 0. 8 ml siRNA
transfection medium was added to each tube containing the siRNA
transfection reagent mixture (solution A and B), and overlayed onto
the washed cells. Cells were incubated for 5–7 h at 37°C in a 5%
CO2 incubator. To determine the efficiency of the siRNA
knockdown, the transfected cells were collected 3 days after
transfection and the protein levels of MTDH were assessed using
western blotting, as aforementioned.
Statistical analysis
All statistical analyses were conducted using SPSS
17. 0 software (SPSS, Inc., Chicago, IL, USA). Quantitative data
are expressed as the mean ± standard deviation. Statistical
differences between groups were compared using two-tailed unpaired
Student's t-tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Acidic pHe decreases the
cytotoxicity of paclitaxel in NPC cells
To confirm the association between acidic
pHe and the sensitivity of NPC to paclitaxel, the
IC30 value for paclitaxel in NPC 5-8F cells was
confirmed as 2.947 (Fig. 1A). The
viability of NPC 5-8F cells, incubated in normal (pH 7.4) or acidic
(pH 6.8) medium, following paclitaxel stimulation at its
IC30 for 48 h, was evaluated using CCK-8 assays. As
presented in Fig. 1B, the survival
rate of NPC 5-8F cells was 30.32±3.34 compared with 45.58±6.34
(P<0.05), incubated in normal (pH 7.4) or acidic (pH 6.8)
medium, respectively. These results indicated that acidic medium
enhanced the survival rate of NPC 5-8F cells and that acidic
pHe significantly decreased the cytotoxicity of
paclitaxel in NPC cells.
EMT-like features of NPC cells grown
in acidified medium
In order to investigate whether EMT is involved in
acidic pHe-induced paclitaxel resistance, the key
markers of EMT were measured in NPC 5-8F cells grown in acidified
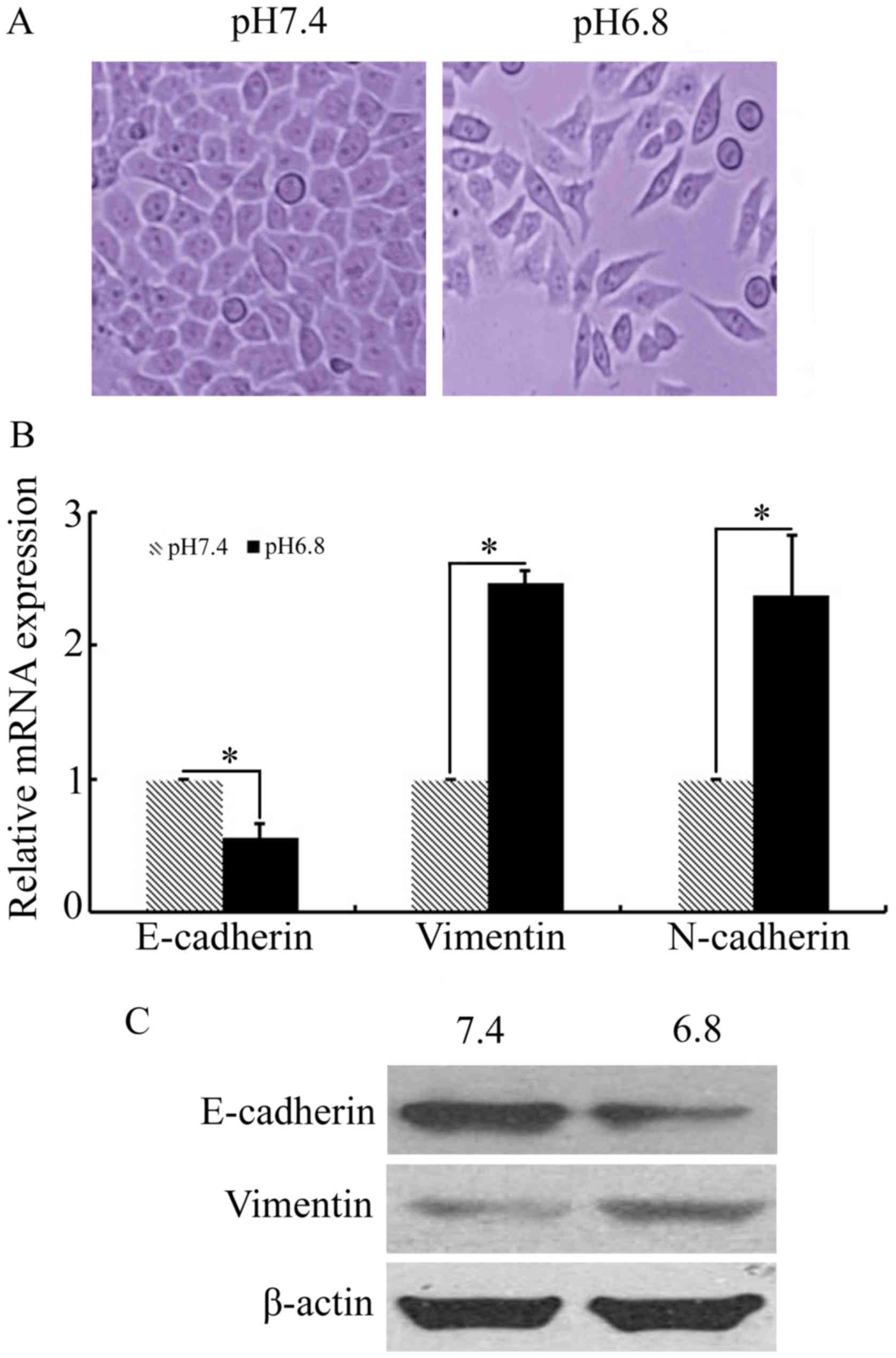
medium for 48 h. Phase-contrast microscopy revealed that NPC 5-8F
cells cultured in acidic medium underwent several morphological
changes, with some loss of adherence and cell-to-cell contact and
the induction of a spindle-like form (Fig. 2A). The results of qPCR and western
blot analysis demonstrated that expression of the characteristic
mesenchymal markers vimentin and N-cadherin were upregulated in NPC
5-8F cells cultured at pHe 6.8, compared with that of
pHe 7.4 (Fig. 2B and C).
In addition, a significant decrease in the expression of the
epithelial maker E-cadherin was observed when NPC 5-8F cells were
cultured in acidic medium (Fig. 2B and
C). These results indicated that the acidic
pHe-induced paclitaxel-resistant NPC cells acquired the
EMT phenotype.
Acidic pHe enhances the
expression of MTDH in NPC cells
In the present study, the potential molecular
mechanism responsible for the EMT-like phenotypic changes in acidic
pHe-induced paclitaxel resistance in NPC cells was
explored. In a previous study, it was demonstrated that MTDH was
increased and promoted EMT in squamous cell carcinoma of the head
and neck (SCCHN) (30). Another
previous report revealed that increased MTDH expression is also
associated with drug resistance, including that of paclitaxel, in
breast cancer, hepatocellular carcinoma and prostate cancer
(23,31). Therefore, the differences in MTDH
expression in NPC cells cultured at a pHe of 6.8
compared with that at a pHe of 7.4 were investigated
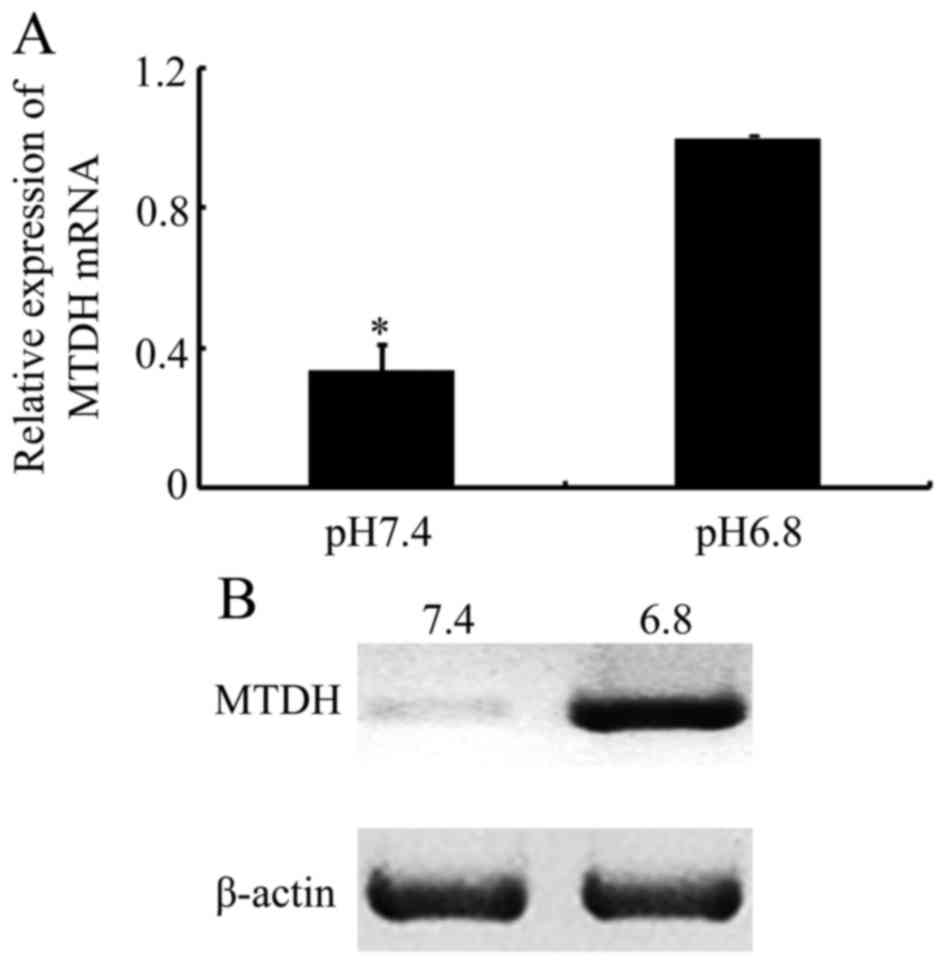
further. As presented in Fig. 3A,
MTDH mRNA levels were increased when cultured in acidic medium. In
addition, western blot analysis demonstrated that MTDH protein
expression was significantly increased in response to an acidic
culture environment (Fig. 3B). The
results from the present study indicated that MTDH may be
associated with an acidic pHe-mediated EMT and
paclitaxel resistance in NPC cells.
Silencing of MTDH reversed the EMT
phenotype and sensitized NPC cells to paclitaxel in an acidic
pHe environment
To determine whether increased MTDH expression was
associated with EMT in NPC cells, siRNA was used to downregulate
MTDH expression in NPC cells cultured at a pHe of 6.8.
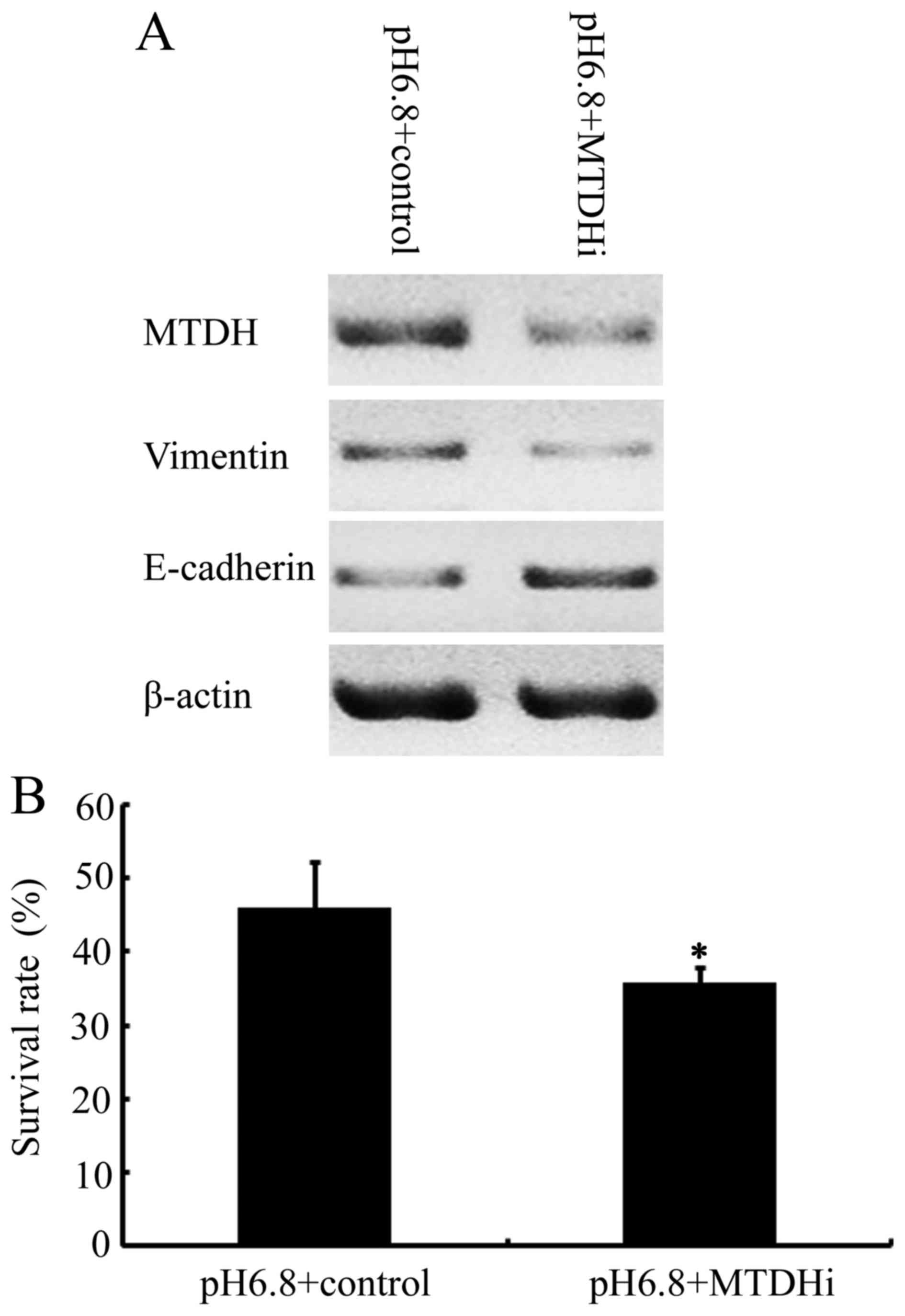
Cells transfected with MTDH siRNA demonstrated decreased MTDH
expression, accompanied by decreased vimentin expression and
increased E-cadherin expression, compared with NPC 5-8F cells
transfected with the control vector (Fig.
4A). The results demonstrated that E-cadherin downregulation
and vimentin upregulation, induced by acidic pHe were
attenuated by MTDH RNA interference in NPC 5-8F cells. This is
indicative of a crucial function for MTDH in the induction of the
EMT phenotype in NPC cells, stimulated by an acidic pHe
environment. Finally, the effects of inhibiting MTDH on the
cytotoxicity of paclitaxel in NPC cells in acidic pHe
environment were investigated. As presented in Fig. 4B, the cell survival rate of NPC 5-8F
cells at an acidic pHe in the presence of paclitaxel at
its IC30 was 45.92±6.3, compared with 35.72±2.27 in the
MTDH-downregulated 5-8F cells (P<0.05). These results revealed
that MTDH knockdown significantly restored the sensitivity of NPC
5-8F cells to paclitaxel, previously decreased in response to an
acidic pHe environment.
Discussion
NPC is one of the most common malignant tumors in
southern China and Southeast Asia, with ~70% of newly diagnosed NPC
cases, and thus is classified as locoregionally advanced disease
(32). Paclitaxel is now routinely
used to treat advanced NPC. However, the development of paclitaxel
resistance is a significant barrier to the treatment of NPC.
Therefore, elucidation of the molecular mechanisms underlying
paclitaxel resistance is crucial to enhance the efficacy of
treatment and to improve the survival rates of patients with
NPC.
Acidic pHe, a hallmark of solid tumors,
is thought to decrease the efficacy of chemotherapeutic regimens
(33–35). However, the exact function of acidic
pHe in mediating chemotherapeutic resistance in NPC
remains unclear. In the present study, it was demonstrated that
acidic pHe decreased the cytotoxicity of paclitaxel in
NPC 5-8F cells in vitro. The results from the present study
are consistent with those of a previous study that identified that
acidic pHe leads to decreased paclitaxel sensitivity in
murine EMT6 cells and the human bladder carcinoma cell line MGH-Ul
(36). However, other previous in
vitro studies have revealed no significant differences in the
paclitaxel toxicity of MCF-7 cells cultured at a pHe of
6.8 compared with 7.4 (12,13). The discrepancies in the results
between distinct cancer cell lines indicated that the effects of
acidic pHe on paclitaxel resistance may be cell
line-specific.
Low pHe forms a physiological drug
barrier, namely ion trapping, which has a negative effect on the
efficacy of weakly basic chemotherapies, yet is more suited to
weaker acidic therapeutics (9,12).
Previous studies have demonstrated that ion trapping mainly
contributes to acidic pHe-mediated chemoresistance.
Owing to the complex structure of paclitaxel, which is composed of
acidic and basic domains, the drug is not ionizable under
physiological conditions, and thus its efficacy should be
unaffected by ion trapping (12,37). EMT
is considered to be an essential feature of epithelial malignant
tumor cells and is accompanied by increased vimentin and N-cadherin
expression, and decreased E-cadherin expression (38–40).
Previous studies have revealed that acidic pHe induces
EMT-like changes in human melanoma cells and Lewis lung carcinoma,
which further promotes tumor progression (41,42). In
the present study, the transformation of fibroblast-like
morphology, epithelial marker E-cadherin downregulation, and
upregulation of mesenchymal markers vimentin and N-cadherin, were
observed in NPC 5-8F cells cultured at a pHe of 6.8,
suggesting that acidic pHe may induce EMT-like changes
in NPC cells.
EMT is thought to be involved in wound healing, stem
cell behavior, development and the progression of cancer. Emerging
evidence has revealed a strong link between resistance to
chemotherapy and the induction of EMT in cancer (43). A number of studies have indicated that
chemoresistant cancer cells undergo morphological and molecular
changes similar to EMT. In colorectal cancer, oxaliplatin-resistant
cells acquired the ability to migrate and invade with phenotypic
changes resembling those of EMT (44). In pancreatic and ovarian cancer,
stable cell lines resistant to gemcitabine and paclitaxel
established by continuous exposure were able to undergo EMT with
increased Snail and Twist expression (17,45). A
previous study also revealed that EMT is necessary for acquired
resistance to cisplatin and increases the metastatic potential of
NPC cells (46). In addition, another
in vitro and in vivo study demonstrated that
paclitaxel-resistant NPC cells exhibited characteristic EMT
phenotypes, and forkhead box C2 promoted chemoresistance in NPC via
the induction of EMT (47). Taken
together, this evidence, together with the results of the present
study supports the conclusion that EMT is responsible for acidic
pHe-induced paclitaxel resistance in NPC cells.
Previous results indicated that acidic
pHe was able to promote tumor progression through the
increase in the expression of specific genes, including those
encoding vascular endothelial growth factor, interleukin-8 and
matrix metalloproteinase-9 (48–51). MTDH,
a novel oncogene, is prevalently expressed in numerous solid
tumors, including SCCHN, and is involved in multiple cellular
behaviors associated with malignant cells, including malignant cell
transformation, proliferation, angiogenesis, invasion and
metastasis (30,52–55). There
is further evidence to indicate that MTDH modulates the sensitivity
of cancer cells to chemotherapeutic agents (20,56). In
the present study, the results demonstrated that MTDH expression
was significantly enhanced in NPC cells cultured in an acidic
medium. In a previous study, our group identified that MTDH
promoted EMT in SCCHN (30). In the
present study, the potential function of MTDH in acidic
pHe-mediated EMT was investigated further. The data
demonstrated that knockdown of MTDH abrogated the acidic
pHe-induced suppression of E-cadherin and increased
vimentin expression in NPC 5-8F cells; which indicated that MTDH is
involved in acidic pHe-mediated EMT-like changes in NPC
cells. Furthermore, the cytotoxicity of paclitaxel in NPC cells
recovered, whereas MTDH was knocked down, under acidic conditions.
Taken together, these results supported the existence of an
association between acidic pHe-induced paclitaxel
resistance and MTDH upregulation-mediated EMT in NPC cells.
In conclusion, the results of the present study
indicated that MTDH-mediated EMT may be an alternative mechanism
through which acidic pHe promotes paclitaxel resistance
in NPC. Thus, normalization of pHe may be a reasonable
strategy for tumor therapy. Furthermore, targeting MTDH may provide
a novel strategy for overcoming chemoresistance in NPC therapy.
However, further in vivo experiments are required to confirm
whether MTDH is a viable target for therapy.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant no. 81402232).
References
|
1
|
Leong SS, Wee J, Tay MH, Toh CK, Tan SB,
Thng CH, Foo KF, Lim WT, Tan T and Tan EH: Paclitaxel, carboplatin,
and gemcitabine in metastatic nasopharyngeal carcinoma: A Phase II
trial using a triplet combination. Cancer. 103:569–575. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leong SS, Wee J, Rajan S, Toh CK, Lim WT,
Hee SW, Tay MH, Poon D and Tan EH: Triplet combination of
gemcitabine, paclitaxel, and carboplatin followed by maintenance
5-fluorouracil and folinic acid in patients with metastatic
nasopharyngeal carcinoma. Cancer. 113:1332–1337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vaupel P, Kallinowski F and Okunieff P:
Blood flow, oxygen and nutrient supply, and metabolic
microenvironment of human tumors: A review. Cancer Res.
49:6449–6465. 1989.PubMed/NCBI
|
|
4
|
Gatenby RA and Gillies RJ: A
microenvironmental model of carcinogenesis. Nat Rev Cancer.
8:56–61. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang JS, Gillies RD and Gatenby RA:
Adaptation to hypoxia and acidosis in carcinogenesis and tumor
progression. Semin Cancer Biol. 18:330–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hashim AI, Zhang X, Wojtkowiak JW,
Martinez GV and Gillies RJ: Imaging pH and metastasis. NMR Biomed.
24:582–591. 2011.PubMed/NCBI
|
|
7
|
Peppicelli S, Bianchini F and Calorini L:
Extracellular acidity, a ‘reappreciated’ trait of tumor environment
driving malignancy: Perspectives in diagnosis and therapy. Cancer
Metastasis Rev. 33:823–832. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raghunand N and Gillies RJ: pH and drug
resistance in tumors. Drug Resist Updat. 3:39–47. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wojtkowiak JW, Verduzco D, Schramm KJ and
Gillies RJ: Drug resistance and cellular adaptation to tumor acidic
pH microenvironment. Mol Pharm. 8:2032–2038. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trédan O, Galmarini CM, Patel K and
Tannock IF: Drug resistance and the solid tumor microenvironment. J
Natl Cancer Inst. 99:1441–1454. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Parks SK, Chiche J and Pouysségur J:
Disrupting proton dynamics and energy metabolism for cancer
therapy. Nat Rev Cancer. 13:611–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mahoney BP, Raghunand N, Baggett B and
Gillies RJ: Tumor acidity, ion trapping and chemotherapeutics. I.
Acid pH affects the distribution of chemotherapeutic agents in
vitro. Biochem Pharmacol. 66:1207–1218. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raghunand N, Mahoney BP and Gillies RJ:
Tumor acidity, ion trapping and chemotherapeutics. II. pH-dependent
partition coefficients predict importance of ion trapping on
pharmacokinetics of weakly basic chemotherapeutic agents. Biochem
Pharmacol. 66:1219–1229. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thiery JP: Epithelial-mesenchymal
transitions in development and pathologies. Curr Opin Cell Biol.
15:740–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Z, Li Y, Ahmad A, Azmi AS, Kong D,
Banerjee S and Sarkar FH: Targeting miRNAs involved in cancer stem
cell and EMT regulation: An emerging concept in overcoming drug
resistance. Drug Resist Updat. 13:109–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kajiyama H, Shibata K, Terauchi M,
Yamashita M, Ino K, Nawa A and Kikkawa F: Chemoresistance to
paclitaxel induces epithelial-mesenchymal transition and enhances
metastatic potential for epithelial ovarian carcinoma cells. Int J
Oncol. 31:277–283. 2007.PubMed/NCBI
|
|
18
|
Lee SG, Kang DC, DeSalle R, Sarkar D and
Fisher PB: AEG-1/MTDH/LYRIC, the beginning: Initial cloning,
structure, expression profile, and regulation of expression. Adv
Cancer Res. 120:1–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Emdad L, Das SK, Dasgupta S, Hu B, Sarkar
D and Fisher PB: AEG-1/MTDH/LYRIC: Signaling pathways, downstream
genes, interacting proteins, and regulation of tumor angiogenesis.
Adv Cancer Res. 120:75–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu G, Chong RA, Yang Q, Wei Y, Blanco MA,
Li F, Reiss M, Au JL, Haffty BG and Kang Y: MTDH activation by 8q22
genomic gain promotes chemoresistance and metastasis of
poor-prognosis breast cancer. Cancer Cell. 15:9–20. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoo BK, Gredler R, Vozhilla N, Su ZZ, Chen
D, Forcier T, Shah K, Saxena U, Hansen U, Fisher PB and Sarkar D:
Identification of genes conferring resistance to 5-fluorouracil.
Proc Natl Acad Sci USA. 106:pp. 12938–12943. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoo BK, Chen D, Su ZZ, Gredler R, Yoo J,
Shah K, Fisher PB and Sarkar D: Molecular mechanism of
chemoresistance by astrocyte elevated gene-1. Cancer Res.
70:3249–3258. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meng X, Thiel KW and Leslie KK: Drug
resistance mediated by AEG-1/MTDH/LYRIC. Adv Cancer Res.
120:135–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J, Zhang Y, Liu S, Zhang Q, Wang Y,
Tong L, Chen X, Ji Y, Shang Q, Xu B, et al: Metadherin confers
chemoresistance of cervical cancer cells by inducing autophagy and
activating ERK/NF-κB pathway. Tumour Biol. 34:2433–2440. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Wang D, Liu H, Feng Y, Zhu T, Zhang
L, Zhu B and Zhang Y: Knockdown of astrocyte elevated gene-1
(AEG-1) in cervical cancer cells decreases their invasiveness,
epithelial to mesenchymal transition, and chemoresistance. Cell
Cycle. 13:1702–1707. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ward A, Balwierz A, Zhang JD, Küblbeck M,
Pawitan Y, Hielscher T, Wiemann S and Sahin Ö: Re-expression of
microRNA-375 reverses both tamoxifen resistance and accompanying
EMT-like properties in breast cancer. Oncogene. 32:1173–1182. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng J, Li C, Wu X, Liu M, Sun X, Yang Y,
Hao M, Sheng S, Sun Y, Zhang H, et al: Huaier polysaccharides
suppresses hepatocarcinoma MHCC97-H cell metastasis via
inactivation of EMT and AEG-1 pathway. Int J Biol Macromol.
64:106–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu C, Liu Y, Huang D, Dai Y, Cai G, Sun J,
Xu T, Tian Y and Zhang X: TGF-β1 mediates epithelial to mesenchymal
transition via the TGF-β/Smad pathway in squamous cell carcinoma of
the head and neck. Oncol Rep. 25:1581–1587. 2011.PubMed/NCBI
|
|
30
|
Yu C, Liu Y, Tan H, Li G, Su Z, Ren S, Zhu
G, Tian Y, Qiu Y and Zhang X: Metadherin regulates metastasis of
squamous cell carcinoma of the head and neck via AKT signalling
pathway-mediated epithelial-mesenchymal transition. Cancer Lett.
343:258–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang C, Li HZ, Qian BJ, Liu CM, Guo F and
Lin MC: MTDH/AEG-1-based DNA vaccine suppresses metastasis and
enhances chemosensitivity to paclitaxel in pelvic lymph node
metastasis. Biomed Pharmacother. 70:217–226. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chua MLK, Wee JTS, Hui EP and Chan ATC:
Nasopharyngeal carcinoma. Lancet. 387:1012–1024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rotin D, Robinson B and Tannock IF:
Influence of hypoxia and an acidic environment on the metabolism
and viability of cultured cells: Potential implications for cell
death in tumors. Cancer Res. 46:2821–2826. 1986.PubMed/NCBI
|
|
34
|
Tannock IF and Rotin D: Acid pH in tumors
and its potential for therapeutic exploitation. Cancer Res.
49:4373–4384. 1989.PubMed/NCBI
|
|
35
|
Yamagata M and Tannock IF: The chronic
administration of drugs that inhibit the regulation of
intracellular pH: In vitro and anti-tumour effects. Br J Cancer.
73:1328–1334. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vukovic V and Tannock IF: Influence of low
pH on cytotoxicity of paclitaxel, mitoxantrone and topotecan. Br J
Cancer. 75:1167–1172. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huizing MT, Misser VH, Pieters RC, ten
Bokkel Huinink WW, Veenhof CH, Vermorken JB, Pinedo HM and Beijnen
JH: Taxanes: A new class of antitumor agents. Cancer Invest.
13:381–404. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gomes LR, Terra LF, Sogayar MC and
Labriola L: Epithelial-mesenchymal transition: Implications in
cancer progression and metastasis. Curr Pharm Biotechnol.
12:1881–1890. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Savagner P: The epithelial-mesenchymal
transition (EMT) phenomenon. Ann Oncol. 21 Suppl 7:vii89–vii92.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lim J and Thiery JP:
Epithelial-mesenchymal transitions: Insights from development.
Development. 139:3471–3486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Peppicelli S, Bianchini F, Torre E and
Calorini L: Contribution of acidic melanoma cells undergoing
epithelial-to-mesenchymal transition to aggressiveness of
non-acidic melanoma cells. Clin Exp Metastasis. 31:423–433. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Suzuki A, Maeda T, Baba Y, Shimamura K and
Kato Y: Acidic extracellular pH promotes epithelial mesenchymal
transition in Lewis lung carcinoma model. Cancer Cell Int.
14:1292014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang AD, Fan F, Camp ER, van Buren G, Liu
W, Somcio R, Gray MJ, Cheng H, Hoff PM and Ellis LM: Chronic
oxaliplatin resistance induces epithelial-to-mesenchymal transition
in colorectal cancer cell lines. Clin Cancer Res. 12:4147–4153.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shah AN, Summy JM, Zhang J, Park SI,
Parikh NU and Gallick GE: Development and characterization of
gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol.
14:3629–3637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang P, Liu H, Xia F, Zhang QW, Zhang YY,
Zhao Q, Chao ZH, Jiang ZW and Jiang CC: Epithelial-mesenchymal
transition is necessary for acquired resistance to cisplatin and
increases the metastatic potential of nasopharyngeal carcinoma
cells. Int J Mol Med. 33:151–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhou Z, Zhang L, Xie B, Wang X, Yang X,
Ding N, Zhang J, Liu Q, Tan G, Feng D and Sun LQ: FOXC2 promotes
chemoresistance in nasopharyngeal carcinomas via induction of
epithelial mesenchymal transition. Cancer Lett. 363:137–145. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fukumura D, Xu L, Chen Y, Gohongi T, Seed
B and Jain RK: Hypoxia and acidosis independently up-regulate
vascular endothelial growth factor transcription in brain tumors in
vivo. Cancer Res. 61:6020–6024. 2001.PubMed/NCBI
|
|
49
|
Shi Q, Abbruzzese JL, Huang S, Fidler IJ,
Xiong Q and Xie K: Constitutive and inducible interleukin 8
expression by hypoxia and acidosis renders human pancreatic cancer
cells more tumorigenic and metastatic. Clin Cancer Res.
5:3711–3721. 1999.PubMed/NCBI
|
|
50
|
Shi Q, Xiong Q, Le X and Xie K: Regulation
of interleukin-8 expression by tumor-associated stress factors. J
Interferon Cytokine Res. 21:553–566. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kato Y, Ozawa S, Tsukuda M, Kubota E,
Miyazaki K, St-Pierre Y and Hata R: Acidic extracellular pH
increases calcium influx-triggered phospholipase D activity along
with acidic sphingomyelinase activation to induce matrix
metalloproteinase-9 expression in mouse metastatic melanoma. FEBS
J. 274:3171–3183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hu G, Wei Y and Kang Y: The multifaceted
role of MTDH/AEG-1 in cancer progression. Clin Cancer Res.
15:5615–5620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yoo BK, Emdad L, Lee SG, Su ZZ,
Santhekadur P, Chen D, Gredler R, Fisher PB and Sarkar D: Astrocyte
elevated gene-1 (AEG-1): A multifunctional regulator of normal and
abnormal physiology. Pharmacol Ther. 130:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu Y, Su Z, Li G, Yu C, Ren S, Huang D,
Fan S, Tian Y, Zhang X and Qiu Y: Increased expression of
metadherin protein predicts worse disease-free and overall survival
in laryngeal squamous cell carcinoma. Int J Cancer. 133:671–679.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhu GC, Yu CY, She L, Tan HL, Li G, Ren
SL, Su ZW, Wei M, Huang DH, Tian YQ, et al: Metadherin regulation
of vascular endothelial growth factor expression is dependent upon
the PI3K/Akt pathway in squamous cell carcinoma of the head and
neck. Medicine (Baltimore). 94:e5022015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu H, Song X, Liu C, Xie L, Wei L and Sun
R: Knockdown of astrocyte elevated gene-1 inhibits proliferation
and enhancing chemo-sensitivity to cisplatin or doxorubicin in
neuroblastoma cells. J Exp Clin Cancer Res. 28:192009. View Article : Google Scholar : PubMed/NCBI
|