Introduction
The antitumor immune response is based on the tight
co-operation between the components of innate and adaptive
immunity, and is strongly influenced by the active role of the
tumor microenvironment manifested by immune cell suppression and
selection of non-immunogenic tumor cell variants (1). In the surveillance of major
histocompatibility complex class I (MHC I)-deficient tumors,
natural killer (NK) cells are primarily involved, but they also
participate in priming the specific, MHC I-restricted immune
responses via IFN-γ secretion leading to upregulation of MHC class
I expression on tumors, potentiating the cytotoxic T
lymphocyte-mediated response (2,3).
To get deeper insight into the mechanisms playing a
role in the antitumor innate and adaptive immunity, we used stable
mouse hybrids of Balb/c and C57BL/6 strains of H-2Db+d-NK1.1neg
(B6-neg) and H-2Db-d+NK1.1high (Balb-high) phenotypes, differing in
the H2-D haplotype and NKC domain. These novel mouse strains
expressed unique features of spontaneous tumor regression in the
MHC-I negative TC-1/A9 experimental model, possibly mediated by the
involvement of NKC polymorphisms and H-2D haplotype in the
effector-target cell interaction. The homozygosity in NKC domain
was proved by PCR genotyping of Nkr-p1 and Ly-49 polymorphic gene
families. The genes shared by Balb and B6 parental mice (i.e.,
Nkr-p1a, Nkr-p1f, Ly-49c, CD69, and Nkg2) were identified in
both hybrid strains. Nkr-p1b and Nkr-p1cBALB gene
isoforms of Balb were present in B6-neg mice, whereas
Nkr-p1d and Nkr-p1cB6 of B6 origin were present in
Balb-high mice; it means that the NKC domain was inherited as a
whole. Our previous results also demonstrated the higher relative
distribution of CD4+ cells in B6-neg and Balb strains (4).
We found that inoculation of B6-neg mice with
TC-1/A9 tumor cells led to temporal tumor growth up to days 10–12,
and then the tumors started to quickly regress. In the Balb-high
mice, the period of tumor growth was prolonged to 20 days,
similarly to the Balb/c parental strain (4). In the prophylactic immunization
experimental settings using irradiated TC-1/A9 tumor cells in
C57BL/6 mice, a remarkable role of NK1.1-positive cells in the
development of immunity was observed (5).
Tumor-infiltrating leukocytes (TIL) represent
important, although controversial, markers associated with the
clinical outcome. Their defectiveness has been attributed to
various mechanisms, including inadequate antigen presentation,
recruiting of inhibitory subpopulations (Treg, MDSC), or
inactivation by immunosuppressive factors in the local tumor
microenvironment or by signaling through co-inhibitory molecules
(6–8).
However, analysis of the TIL presence can provide important
evidence about the antitumor immune reactions in vivo
(9). The novel mouse strains can
serve as a good model for elucidation of the TIL role in tumor
regression.
In this communication, we examined the role of
distinct subpopulations of co-operating in vivo
immunocompetent cells, i.e., CD8+, CD4+,
CD11b+, and NK cells, in the growth and rejection of
TC-1/A9 tumor transplants in the newly established mouse strains.
The results of in vivo experiments were completed by
detailed immunohistological analysis of tumor-infiltrating
leukocytes to show the relationship between depletion of particular
effector cells and tumor growth in the novel mouse strains.
Materials and methods
Experimental animals
Eight-week-old inbred female C57BL/6 (B6) (Charles
River Laboratories, Munich, Germany) and Balb/c (Breading Units of
Animal Facility of IMG CAS, Prague, Czech Republic) mice and the
newly generated mouse strains were housed under natural day/night
conditions (22°C, 55% relative humidity) and fed on a commercial
ST1 diet (Velaz, Prague, Czech Republic) ad libitum. The novel
mouse strains were produced by inbreeding (F30) of
parental C57BL/6 (H2Db+H2Dd-NK1.1high) and Balb/c
(H2Db-H2Dd+NK1.1neg) mice based on the NKC domain gene expression
controlled by DNA analysis, H-2D haplotype and NK1.1 by cytometric
phenotyping to obtain stable H2-Db-Dd+NK1.1high and
H2-Db+Dd-NK1.1neg phenotypes of Balb-high and B6-neg strains,
respectively. The homozygosity in the NKC domain (NK1.1 expression)
and the H2D haplotype of the novel mouse strains was continuously
examined before mating (4). Breading
of mice and all experimental procedures were conducted under SPF
conditions in accordance with the European Convention for the Care
and Use of Laboratory Animals as approved by the Czech Animal Care
and Use Committee.
Tumor cells
The TC-1/A9 (H2-Db-d-) tumor cell line (10) was derived from the TC-1 cell line
(obtained from the ATCC collection) developed by co-transfection of
murine C57BL/6 lung cells with HPV16 E6/E7 genes and activated
(G12V) by Ha-ras plasmid DNA (11).
Briefly, the TC-1 cells were inoculated into mice pre-immunized
with an E7 gene-based DNA vaccine and from tumors developing in a
portion of the animals, cell clones with downregulated MHC class I
surface expression were isolated (TID50=104 tumor
cells/C57BL/6 mouse, s.c.). Temporary growth of MHC class
I-deficient tumor cells in syngeneic mice lead to partial
re-expression of the low MHC class I due to effect of IFN-γ and,
the epigenetic mechanisms (10,12–14). The
TC-1/A9 tumor cell subline, deficient in MHC class I molecules,
escaped due to the selection pressure mediated by the specific
immune response. The TC-1/A9 cell line was maintained in RPMI-1640
medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented
with 10% FCS (PAN-Biotech GmbH, Aidenbach, Germany) and
antibiotics. Cells were cultured at 37°C in humidified atmosphere,
continuously tested for H-2Db, d negativity by FACS before
injection.
Flow cytometry
The efficacy of particular cell depletion from the
spleens of individual mice was verified by flow cytometry using
monoclonal antibodies NK1.1-APC (clone PK136), CD3-BD-Horizon V450,
CD4-PerCP, CD8-APC-eFluor780, NKp46-FITC (BD Biosciences, San Jose,
CA, USA or eBioscience, San Diego, CA, USA). Samples were measured
in a BD LSRII flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA) in a four-laser set-up (405, 488, 561 and 633 nm) and data
were offline-compensated and evaluated based on single-stain
controls in FlowJo version 9 (Tree Star, Ashland, OR, USA). Doublet
exclusion and morphology was based on forward-scatter (FSC), FSC
height and side-scatter (SSC) area; propidium iodide (PI) or
Hoechst33258 (BD Biosciences) was used for exclusion of non-viable
cells.
In vivo depletion experiments
For in vivo depletion, monoclonal antibodies
anti-CD8 (clone 2.43), anti-CD4 (clone GK1.5), anti-NK1.1 (clone
PK136) (Exbio, Praha, Czech Republic) and rabbit anti-asialo GM1
polyclonal antibody (Wako Pure Chemicals Inc., Osaka, Japan) were
used. The effectiveness of depletion in vivo was tracked
using flow cytometric analysis. The results are summarized in
Table I.
 | Table I.Expression of CD4+,
CD8+, NK (CD335+) and NK1.1+ specificities on the spleen
cells of control and depleted mice with transplanted TC-1/A9 tumors
(day 21). |
Table I.
Expression of CD4+,
CD8+, NK (CD335+) and NK1.1+ specificities on the spleen
cells of control and depleted mice with transplanted TC-1/A9 tumors
(day 21).
|
|
| Per cent of
positive cells ± SD |
|---|
|
|
|
|
|---|
| Mouse strain | Cell depletion |
CD4+ |
CD8+ | NK (CD335+) | NK1.1+ |
|---|
| B6-neg | Control |
61.00±4.42 |
38.45±4.56 |
3.59±1.40 | – |
|
| Depleted+ |
3.69±3.21a |
4.26±0.86a |
1.90±1.49a | – |
| Balb-high | Control |
55.58±5.33 |
44.20±5.44 |
3.43±1.01 | 6.60±0.74 |
|
| Depleted+ |
0.67±0.39a |
5.28±1.05a |
1.00±0.57a |
1.02±1.20a |
| B6 | Control |
55.76±3.87 |
39.11±3.57 |
3.08±0.55 | 7.55±0.82 |
|
| Depleted+ |
0.51±0.43a |
1.59±0.12a |
1.88±0.89a |
0.60±0.98a |
| Balb/c | Control |
66.06±5.07 |
30.89±1.56 |
5.68±2.17 | – |
|
| Depleted+ |
0.37±0.23a |
4.17±1.08a |
1.22±0.29a | – |
TC-1/A9 tumor cells (5.105 cells) were
transplanted s.c. on day 0. CD8-, CD4-, and NK1.1-positive cells
were depleted with corresponding antibodies on days −7, −5, −2, +4,
+11, +18 (0.1 mg/mouse, i. p.). For NK cell depletion, rabbit
anti-asialo GM1 was applied on days −1, 0, +3, +7 (0.2 mg/mouse,
i.p,), according to the manufacturer's instructions. Mice were
observed twice a week, Two perpendicular diameters of the tumors
were measured with a caliper and the tumor size was expressed as
the tumor area. Tumor area (cm2) was determined by measurement of
the largest diameter of the tumor by the greatest perpendicular
diameter and calculated by the formula: Tumor area (cm2)=largest
diameter (cm) × perpendicular diameter (cm). Mice were euthanized
when tumors reached the maximum size 2.0 cm in the largest
diameter. Mice with multiple tumors were excluded from the
experiments. Mice were euthanized when tumors reached the maximum
size of 2.0 cm in diameter. According to our previous experiences,
the quickly growing TC-1/A9 tumors were a prerequisite of
progressors. These mice were excluded from the depletion
experiments to avoid false results. On day 12, three mice (final
cohorts of 6–8 mice) from each group were sacrificed; tumors were
excised and used for histological analysis. From each mouse 3–4
samples of tumor tissue from the randomly selected distinct parts
of the tumor were collected. The size of the tumors on the day of
analysis was also recorded.
Detection of tumor-infiltrating
leukocytes
According to the procedure described in (15), 5–10 µm thick tumor tissue cryosections
were fixed in 4% paraformaldehyde (30 min at 4°C) and incubated
overnight with rat anti-CD4, rat anti-CD8, rat anti-CD11b or rat
anti-CD335 (NKp46) antibodies (BD Pharmingen). The slides were then
washed with PBS and incubated with goat anti-rat antibody
conjugated with Alexa Fluor 488 (Molecular Probes). After one-hour
incubation, the slides were washed and counterstained with PI (2
µg/ml). All fluorophore-labeled tissue sections were analyzed using
a BioRad MRC 1024 scanning confocal fluorescence microscope
equipped with LaserSharp software. Cryosections of the tumor from
each individual mouse were analyzed and the representative
(average) sections were selected and documented in the figures.
Statistical analyses
For statistical analyses of the growth curves,
analysis of variance (ANOVA) at confidence level 95% followed with
Scheffe's, Tukey-Kramer and Newman-Kreuls tests from the NCSS
(Number Cruncher Statistical System, Kaysville, UT, USA)
statistical package was used. For statistical evaluations of the
bar diagrams, Student's t-test was utilized.
Results
Inoculation of B6-neg mice with TC-1/A9 cells led to
temporal tumor growth up to days 10–12, and then the tumors started
to regress. In the Balb-high mice, the period of tumor growth was
prolonged to 20 days, similarly to the Balb/c parental strain. The
comparison of tumor transplant development is summarized in
Fig. 1A and B. During the inbreeding
process, 10–20% of mice displayed progressively growing tumors and
were used in the experiments comparing the difference in the immune
response of regressors and progressors. Parallel evaluation of the
tumor cryosections on day 12 demonstrated higher infiltration of
the tumor tissue by immunocompetent cells in the regressor mice.
Evaluation of these samples showed an indirect correlation between
the growth of tumor transplants in progressor vs. regressor mice
and the infiltration with CD4+, CD8+, and NK
cells. Cryosections of tumor specimens of regressor B6-neg mice
showed higher infiltration by CD4+ and CD8+
cells. The number of NK and CD11b+ cells remained
unchanged (Fig. 1C). In the Balb-high
regressors, increased numbers of CD4+, CD8+,
as well as NK cells were visible. In the control settings,
infiltration of TC-1/A9 tumors with immunocompetent cells in Balb
(regressors' model) and B6 mice (progressors' model) was compared
with the hybrid strains (Fig. 1C).
Comparing the B6 and Balb parental strains, similar infiltration
with CD4+z, CD8+, and NK cells and markedly
higher numbers of CD11b+ cells in tumor specimens of B6 mice was
observed.
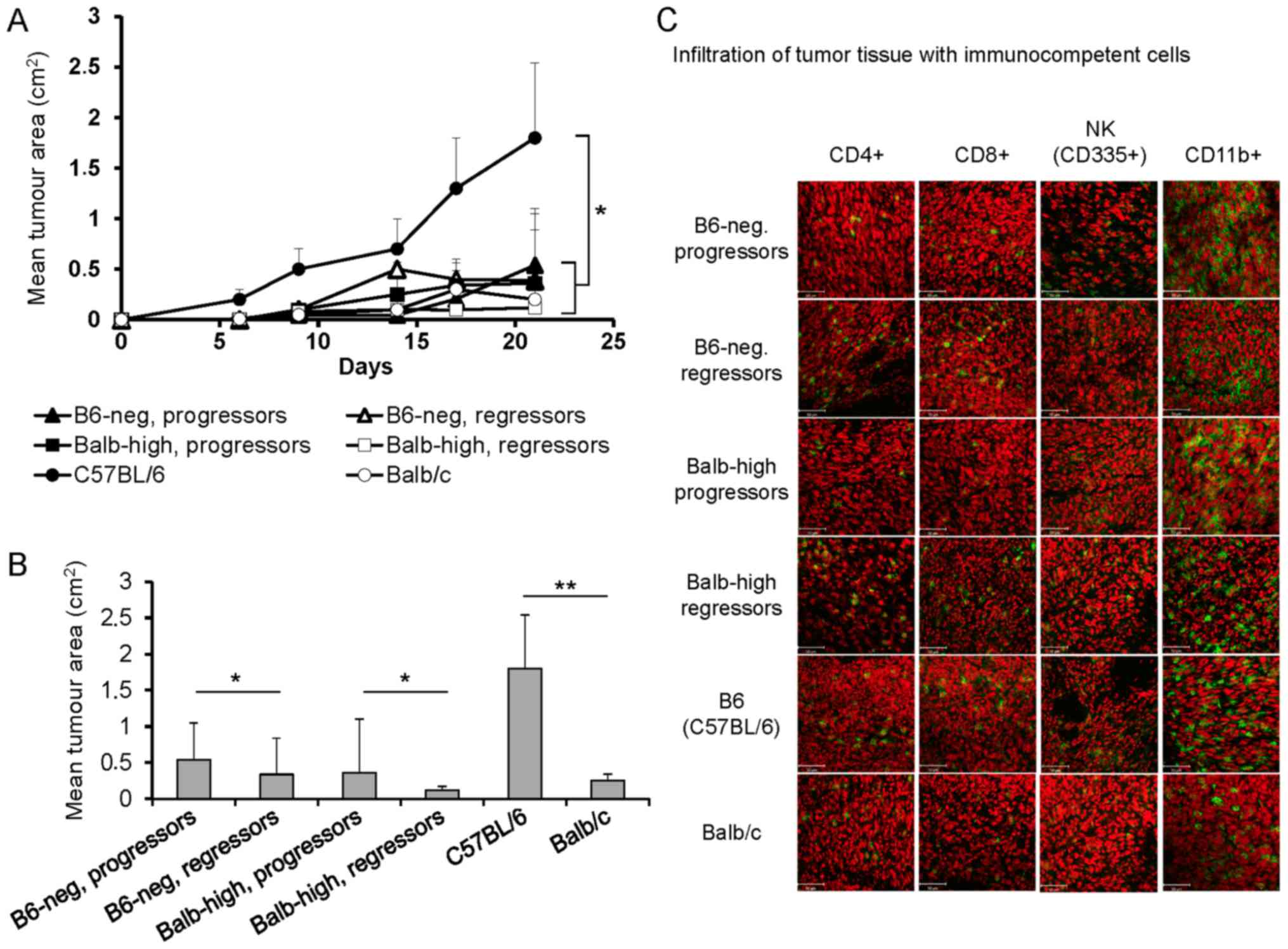 | Figure 1.Development of TC-1/A9 tumors in
parental and hybrid mouse strains. (A) Growth of TC-1/A9 tumor
transplants, n=10, *P<0.05, ANOVA, B6 vs. Balb/c; ANOVA, B6-neg,
progressors vs. B6-neg, regressors and Balb-high, progressors vs.
Balb-high, regressors. (B) Mean tumor area of TC-1/A9 on day 21,
n=10; **P<0.01, B6 vs. Balb/c; *P<0.05, B6-neg, progressors
vs. B6-neg, regressors and Balb-high, progressors vs. Balb-high,
regressors, t-test. (C) Infiltration of the TC-1/A9 tumor
microenvironment (regressors × progressors) with immunocompetent
cells (cryosections, day 12, n=3). Parental strains-B6 and Balb,
hybrid strains-Balb-high and B6-neg. Figure shows representative
data of two experiments performed. Ex vivo tumor samples
were obtained from the mice inoculated with TC-1/A9 cells in the
parallel in vivo experiments. |
Following the results summarized in Fig. 1, the next sets of experiments were
focused on the role of CD8+, CD4+, and NK
cells in the TC-1/A9 tumor elimination in regressor B6-neg and
Balb-high mice employing in vivo depletion of particular
populations. Fig. 2 shows the results
of the experiments documenting the development of TC-1/A9 tumors
inoculated after pretreatment of mice with anti-CD8 mAb. The
CD8+ cells depletion enhanced growth of the TC-1/A9
tumor transplants in both hybrid strains (P<0.05, ANOVA Fig. 2A, P<0.05-B6-neg mice,
P<0.01-Balb-high mice, t-test, Fig.
2B, day 21). The analysis of tumor cryosections demonstrated
that intraperitoneal administration of monoclonal anti-CD8 antibody
diminished the infiltration of tumors by CD8+ cells in
both novel mouse strains. Further, the number of CD11b+ cells
increased, that of CD4+ cells decreased, and the number
of NK cells was not changed in B6-neg mice. In the Balb-high
strain, the tumor infiltration by immunocompetent cells remained
unchanged, except for missing CD8+ cells after the
depletion (Fig. 2C).
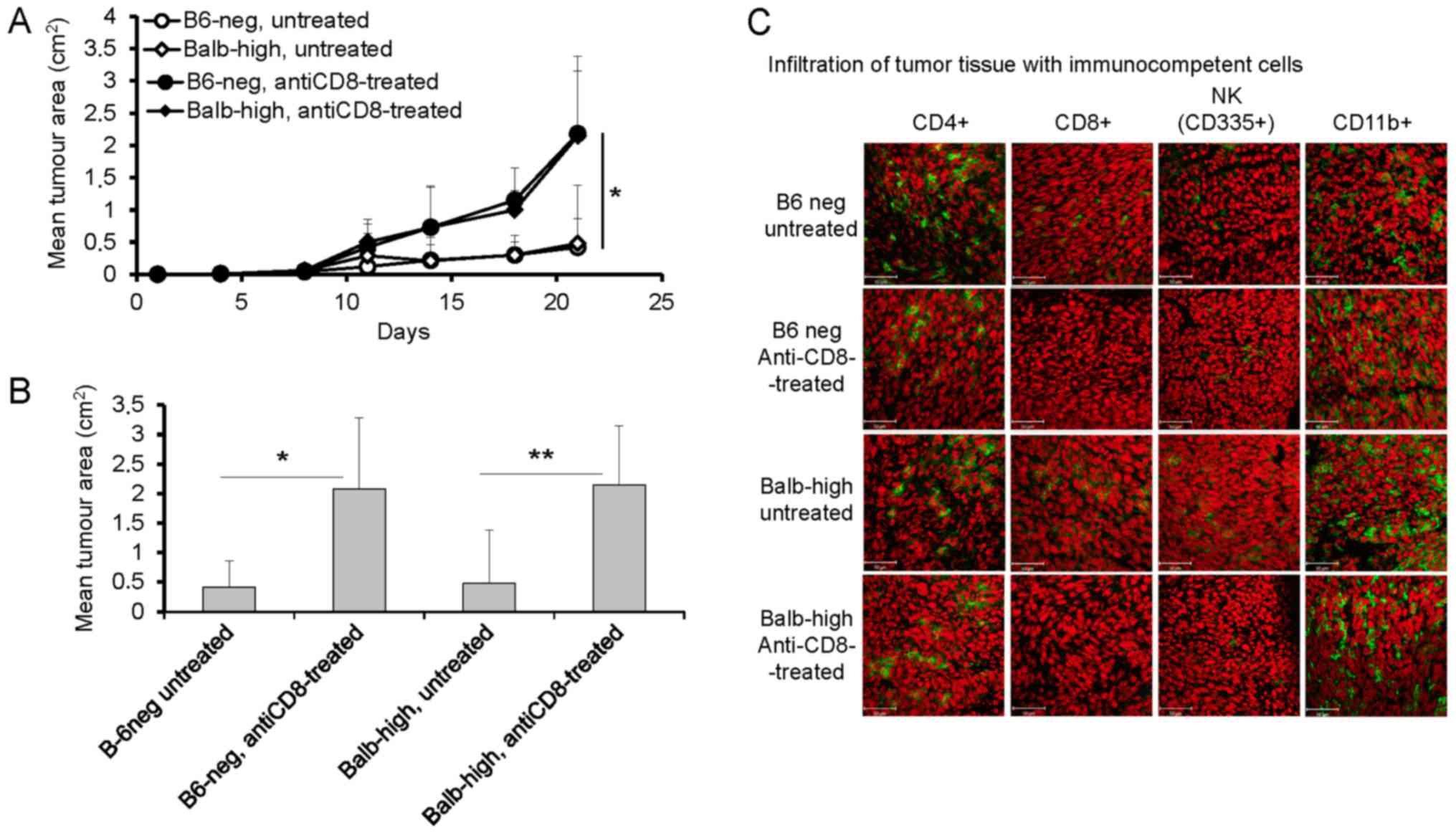 | Figure 2.Enhanced growth of TC-1/A9 tumors in
Balb-high and B6-neg mice after CD8+ cell depletion. (A)
Growth of tumor transplants; n=6, *P<0.05, ANOVA, B6-neg,
untreated vs. B6-neg, anti-CD8-treated, Balb-high untreated vs.
Balb-high, anti-CD8-treated. (B) Effect of CD8-positive cell
depletion on day 21, n=6; *P<0.05. B6-neg, untreated vs. B6-neg,
anti-CD8-treated, **P<0.01 (Balb-high, untreated vs. Balb-high,
anti-CD8-treated, t-test.), t-test. (C) Infiltration of tumor
tissue with immunocompetent cells (cryosections), day 12, n=3.
Monoclonal antibodies: anti-CD4, anti-CD8, anti-CD11b, anti-NKp46.
Ex vivo tumor samples were obtained from the mice inoculated
with TC-1/A9 cells in the parallel in vivo experiments.
Representative data from three independent experiments with similar
results are depicted. |
Depletion of CD4+ cells had a different
effect on the growth of tumors in B6-neg compared to Balb-high
mice. Application of anti-CD4 antibodies significantly enhanced
growth of the TC-1/A9 tumor transplants in B6-neg mice (P<0.05,
ANOVA, Fig. 3A, P<0.01, t-test,
Fig. 3B, day 21). The effect was
accompanied by decreased infiltration of tumor tissue by
CD4+ cells, while other immunocompetent cells
(CD8+, NK, CD11b+) were not influenced (Fig. 3C). Interestingly, elimination of
CD4+ cells in Balb-high mice had no significant effect
on the growth and immune cell infiltration of TC-1/A9 tumors (data
not shown).
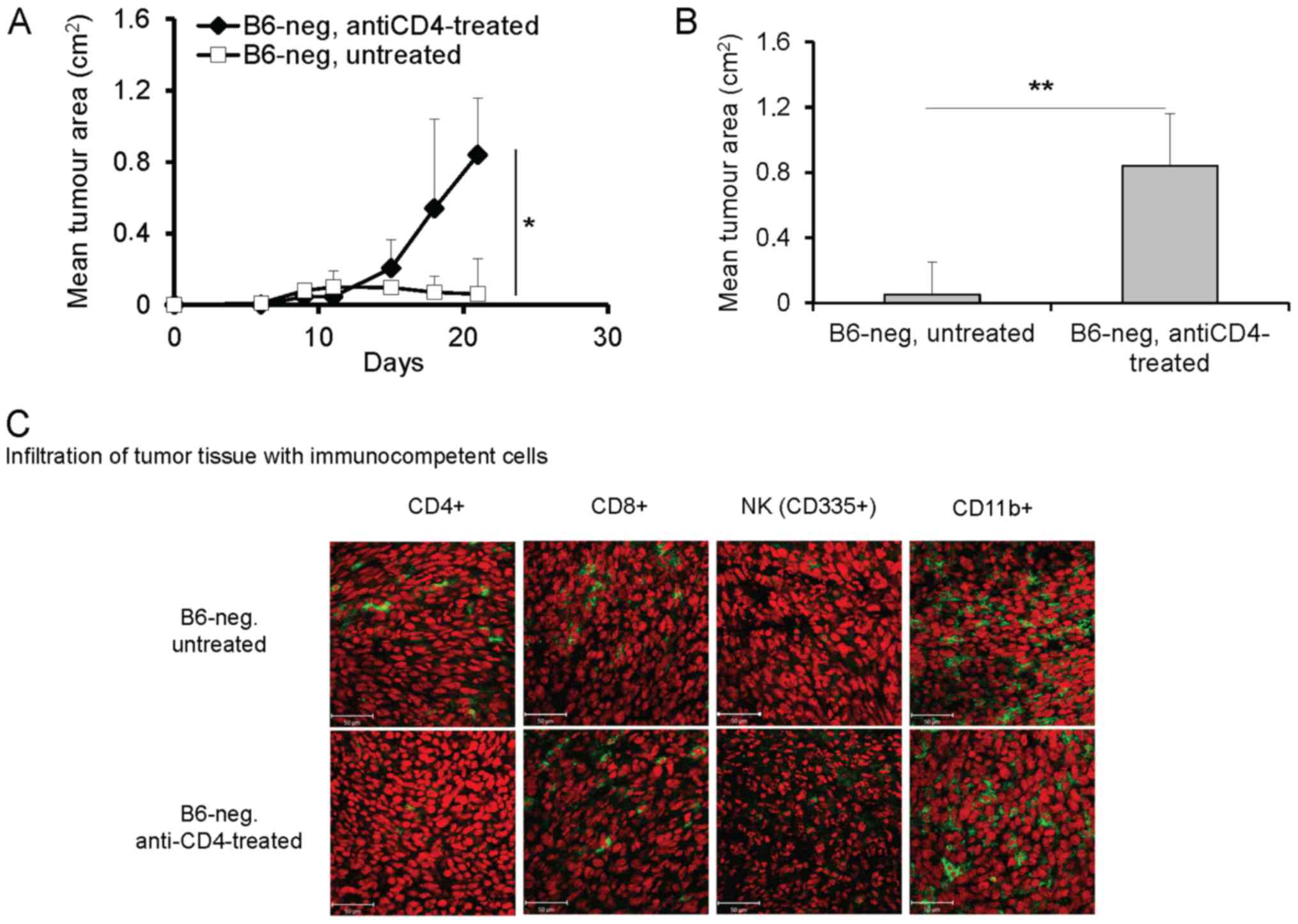 | Figure 3.Enhanced growth of TC-1/A9 tumor
transplants in B6-neg mice after CD4+ cell depletion.
(A) Growth of tumor transplants. n=6; *P<0.05, ANOVA, B6-neg,
untreated vs. B6-neg, anti-CD8-treated. (B) Effect of
CD4+ cell depletion on day 21, n=6; **P<0.01 t-test,
B6-neg, untreated vs. B6-neg, anti-CD8-treated. (C) Infiltration of
tumor tissue with immunocompetent cells (cryosection) on day 12
detected by monoclonal antibodies: anti-CD4, anti-CD8, anti-CD11b,
anti-NKp46, n=3 Ex vivo tumor samples were obtained from the
mice inoculated with TC-1/A9 cells in the parallel in vivo
experiments. Representative data from three independent experiments
with similar results are shown. |
Depletion of NK cells with anti-asialo GM1 antibody
in the Balb-high mice was assessed (Fig.
4) Depletion of NK cells with anti-asialo GM1 antibody in the
Balb-high mice led to enhancement of TC-1/A9 tumor growth
(P<0.05, ANOVA, Fig. 4A, and
P<0.05 t-test, day 21, Fig. 4B),
but not in B6-neg mice (Fig. 4A and
B). The tumor growth-promoting effect was accompanied by a
decrease in NK cells, increased infiltration by CD11b+ cells, and
no changes in CD4+ and CD8+ cells in both
strains (Fig. 4E). Reduction of NK
cells in Balb-high mice was achieved by depletion of NK1.1+ cells
using PK 136 antibody. The depletion of NK1.1+ cells induced rapid
growth of TC-1/A9 tumors (P<0.05, ANOVA, Fig. 4C, and P<0.05, t-test, day 21,
Fig. 4D). These results, together
with the accompanied diminishing of NK1.1-positive cells
infiltration (Fig. 4F) have proved
that the NK1.1+ subpopulation plays an important role in the
TC-1/A9 tumor rejection (Fig. 4F).
The effectiveness of depletion of the respective cells was proved
by FACS analysis of splenocytes on day 12 post TC-1/A9 inoculation,
which showed good results in the case of CD8+ and
CD4+ cells. The NK cell depletion was generally limited
when anti-asialo GM1 was used compared to the results obtained with
the PK 136 antibody in the Balb-high strain (Table I). We can conclude that NK cells in
the NK1.1-negative strain (B6-neg) do not influence the TC-1/A9
regression process, but take part in the regulation of the
infiltration of the tumor microenvironment by CD11b+ cells.
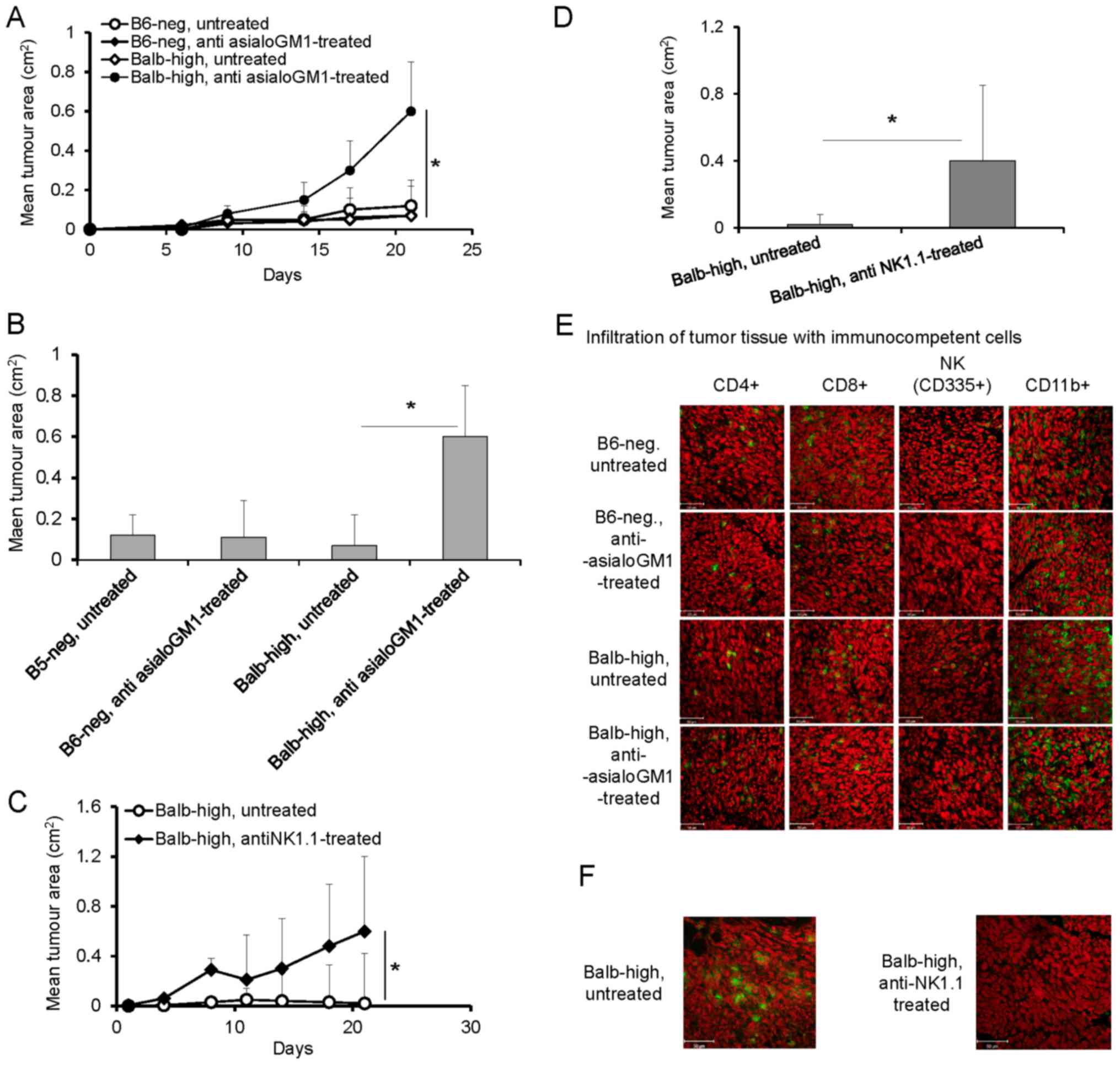 | Figure 4.(A-F) Depletion of NK cells in
TC-1/A9-inoculated Balb-high and B6-neg mice by polyclonal rabbit
anti-asialo-GM1 (A, B and E) and monoclonal anti-NK1.1 (PK136)
antibody (C, D and F). Effect of NK cell depletion (A) on the
growth of tumor transplants. n=6, ANOVA, B6-neg, untreated, vs.
B6-neg, anti asialoGM1-treated. *P<0.05, ANOVA, Balb-high,
untreated vs. Balb-high, anti asialoGM1-treated. (B) Effect of anti
asialoGM1 antibody on the tumor size on day 21, t-test, B6-neg
untreated, vs. B6-neg, anti asialoGM1-treated; *P<0.05 t-test,
Balb-high untreated vs. Balb-high, anti asialoGM1-treated. (E)
Infiltration of tumor tissue with immunocompetent cells
(cryosection) on day 12 was detected by monoclonal antibodies:
anti-CD4, anti-CD8, anti-CD11b, anti-NKp46, n=3. (C) Depletion of
NK1.1+ cells in Balb-high mice by PK136 antibody enhanced the
growth of TC-1/A9 tumor transplants. n=6; *P<0.05, ANOVA,
Balb-high, untreated vs. Balb-high, antiNK1.1-treated. (D) Effect
of NK1.1+ cell depletion on the size of tumors was in correlation
with enhanced tumor growth. *P<0.05, t-test, Balb-high,
untreated, vs. Balb-high, antiNK1.1-treated. (F) Cryosections of
tumor specimens, day 12, n=3. Ab: anti-NK1.1 (PK 136);
representative data of three independent experiments are shown.
Ex vivo tumor samples were obtained from the mice inoculated
with TC-1/A9 cells in the parallel in vivo experiments.
Representative data of three independent experiments with similar
results are shown. |
Discussion
Stable mouse hybrids of Balb/c and C57BL/6 strains,
B6-neg and Balb-high, differing in the NKC domain and H2-D
haplotype, were used for getting insight into the participation of
CD8+, CD4+, CD11b+, and NK cell
subpopulations in the rejection of MHC class I-deficient, HPV16
E6/E7-associated TC-1/A9 tumors. The results presented here
indicate the essential role of CD8+ T cells, as well as
of in vivo co-operation of CD4+, CD8+ and
CD11b+ cells with NK cells, during the process of tumor
rejection. However, the engagement of particular cells was
different in the two hybrid strains. In B6-neg mice, co-operation
of CD8+ and CD4+ cells is required, whereas
in Balb-high mice, mainly CD8+ and NK (NK1.1+) cells are
important.
The TC-1/A9 tumor displayed higher infiltration of
leukocytes, which was probably associated with the MHC-I deficiency
of TC-1/A9 cells that does not correlate with the in vitro
cytotoxic effector function of spleen cells in B6 mice (6). In our experimental settings, the in
vivo depletion of CD4+, CD8+, or NK1.1+
cells led to elimination of these cells from both the tumor tissue
and spleen, resulting in significant enhancement of the TC-1/A9
tumor growth.
The antitumor immune response, based on the tight
co-operation between the components of innate and adaptive
immunity, is also strongly influenced by the active role of the
tumor microenvironment manifested by immunosuppression and
selection of non-immunogenic tumor cell variants (1). The presence or lack of infiltrating
immunocompetent cells (TILs) in the tumor tissue is considered one
of the key characteristics and predictive factors of tumor
regression or expansion. In the neoplastic process, the tumor
microenvironment, which is largely regulated by inflammatory cells,
is an indispensable participant fostering the migration, survival
and proliferation of TILs. However, the frequent functional
defectiveness of TILs has been attributed to various mechanisms,
including inadequate antigen-presenting and/or costimulatory
capacity of tumor cells leading to T cell ignorance or anergy and
production of immune suppressive factors by the tumors (16).
The important role of CD8+ T cells
responding to antigens presented by MHC I molecules could be
diminished by partial renewal of the H2-Db marker on the tumor cell
surface under the pressure induced by the growing tumor transplants
in B6 and B6-neg mice (14) (Indrová,
unpublished). In our study, we found that after elimination of
CD8+ cells, the CD4+ cell infiltration
increased in the tumor tissue. Although CD4+ T cells
along with CD8+ T cells represent the majority of T
lymphocytes, they can differentiate into specific subpopulations
mediating the immune response through secretion of specific
cytokines. However, depending on the cytokine milieu in the tumor
microenvironment, they play dual roles: They may activate or
suppress the immune reactions (17,18).
The results obtained after NK cell in vivo
depletion also indicate a relevant role for NK cells in the
development and growth of TC-1/A9 tumor transplants. NK cells also
participate in priming specific, MHC I-restricted immune responses
via IFN-γ secretion leading to up-regulation of MHC class I
expression on tumor cells. This process can diminish the
surveillance of (MHC I)-deficient tumors because it influences the
lytic activity of cytotoxic T lymphocytes (1,3). For NK
cell depletion, two types of antibodies were used, polyclonal
anti-asialo GM1 and monoclonal anti-NK1.1 (PK136), which are
commonly used to elucidate the in vivo functions of NK cells
in mice. Anti-asialo GM1-mediated NK cell depletion is effective in
a variety of mouse strains, whereas anti-NK1.1-mediated NK cell
depletion acts only in certain strains such as B6 but not in
strains lacking the NK1.1 allotype, for example Balb. Of note, the
expression of asialo-GM1 is not strictly confined to NK cells among
hematopoietic cells and is detected on a subpopulation of NKT,
CD8+ T, and γδ T cells and some activated form of
CD4+ T cells, macrophages, and eosinophils under certain
experimental conditions. At least NKT cells, characterized by
expression of T cell and NK cell receptors, are important immune
regulators that can either promote or suppress antitumor immunity
and could play a role in the growth and regression of TC-1/A9
tumors (19–21). Nevertheless, anti-asialo GM1-mediated
NK cell depletion still remains a powerful tool to analyze the
in vivo functions of NK cells (22). The anti-asialo GM1 antibody
administration or elimination of NK1.1 cells with anti-NK1.1
antibody (PK136) resulted in significant enhancement of tumor
growth in the Balb-high strain. On the other hand, the anti-asialo
GM1 antibody administration did not affect the tumor growth in the
B6-neg tumor strain. These findings, indicating the crucial role of
NK1.1+ NK cell subpopulation in the development and growth of
transplanted TC-1/A9 tumors, correspond to those obtained in our
laboratory in the B6 mouse model, which showed an important role of
NK1.1+ cells in the development of protective immunity against
TC-1/A9 tumors (5). Activity of NK
cells is downregulated by Tregs, having important role in the
resulting anti-tumor immunity (12,23). NK
cells contribute also to adaptive immunity through interaction with
dendritic cells (DC). NK-DC communication regulates T cell-mediated
immune responses and DC itself has been considered in adjuvant
therapeutic modality of tumors. Such effect could be direct, T
cell-mediated, or indirect when APC were treated. (24). Further, Zalli et al showed that
neural regulation of immune response, namely through −2 adrenergic
receptor, which potentiate NK killing (25). This is in correlation with our
preliminary results using differential expression (DE) analysis of
RNA isolated from splenocytes of tumor regressors in comparison
with progressor mice that showed the involvement −2 adrenergic
receptors. These findings need to be verified by further molecular
analysis.
In this study, the increased infiltration of tumors
with CD11b+ cells in anti-asialo GM1-treated mice is probably
related to the regulatory role of NK cells in the monocyte (MDSC)
number (26).
In both newly established mouse strains, the high
infiltration of CD11b+ monocytes was confirmed. CD11b, an integrin
family member, is considered to be a pan-macrophage marker, which
is expressed on a huge variety of leukocytes and can be upregulated
on activated cells irrespective of their naive expression status.
CD11b is also a marker for myeloid-derived suppressor cells (MDSC).
MDSCs are a heterogeneous population of undifferentiated cells
characterized in mice by markers of monocytes (CD11b) and
neutrophils (Gr-1). Among leukocyte-infiltrating tumors, MDSCs
represent one of the key players mediating immunosuppression
(27–31). Therefore, the number of CD11b+
infiltrating cells did not itself exhibit any effect on the
development, growth and rejection of TC-1/A9 tumors in novel mouse
strains.
Taken together, these results extend the findings
published earlier (4) and provide
more detailed information about the changes in the repertoire of
immune cells during the development of transplanted TC-1/A9
(H2-Db-d-) tumors in the novel mouse strains differing in the H-2D
haplotype and NKC domain. We have demonstrated the important role
of NK1.1+ NK cells in the development, growth and rejection of
TC.1/A9 tumors and in the regulation of antitumor immunity in
general.
Acknowledgements
The present study was supported by the grant
14-10100S awarded by the Czech Science Foundation, in part by the
Ministry of Education, Youth and Sports (MEYS, LM2015040; Czech
Centre for Phenogenomics), Academy of Sciences of the Czech
Republic (RVO 68378050), by project ‘BIOCEV-Biotechnology and
Biomedicine Centre of the Academy of Sciences and Charles
University’ (CZ.1.05/1.1.00/02.0109) and ‘Higher quality and
capacity for transgenic models’ (CZ.1.05/2.1.00/19.0395) funded by
the Ministry of Education, Youth and Sports and the European
Regional Development Fund, and, in part, by the Bilateral Agreement
between Polish Academy of Sciences and the Czech Academy of
Sciences. The authors are grateful to Milan Reiniš, PhD, for
reviewing the manuscript, Mrs. Renáta Turečková for skillful
technical assistance, and Mrs. Šárka Takáčová for editorial
help.
References
|
1
|
Swann JB and Smyth MJ: Immune surveillance
of tumors. J Clin Invest. 117:1137–1146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu D, Gu P, Pan PY, Li Q, Sato AI and Chen
SH: NK and CD8+ T cell-mediated eradication of poorly
immunogenic B16-F10 melanoma by the combined action of IL-12 gene
therapy and 4-1BB costimulation. Int J Cancer. 109:499–506. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ghiringhelli F, Apetoh L, Housseau F,
Kroemer G and Zitvogel L: Links between innate and cognate tumor
immunity. Curr Opin Immunol. 19:224–231. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fišerová A, Richter J, Čapková K, Bieblová
J, Mikyšková R, Reiniš M and Indrová M: Resistance of novel mouse
strains different in MHC class I and the NKC domain to the
development of experimental tumors. Int J Oncol. 49:763–772. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Indrová M, Símová J, Bieblová J, Bubeník J
and Reinis M: NK1.1+ cells are important for the development of
protective immunity against MHC I-deficient, HPV16-associated
tumours. Oncol Rep. 25:281–288. 2011.PubMed/NCBI
|
|
6
|
Indrová M, Bieblová J, Rossowska J,
Kuropka P, Pajtasz-Piasecka E, Bubeník J and Reinis M: HPV
16-associated tumours: IL-12 can repair the absence of cytotoxic
and proliferative responses of tumour infiltrating cells after
chemotherapy. Int J Oncol. 34:173–179. 2009.PubMed/NCBI
|
|
7
|
Mikyšková R, Indrová M, Vlková V, Bieblová
J, Šímová J, Paračková Z, Pajtasz-Piasecka E, Rossowska J and
Reiniš M: DNA demethylating agent 5-azacytidine inhibits
myeloid-derived suppressor cells induced by tumor growth and
cyclophosphamide treatment. J Leukoc Biol. 95:743–753. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kodumudi KN, Siegel J, Weber AM, Scott E,
Sarnaik AA and Pilon-Thomas S: Immune checkpoint blockade to
improve tumor infiltrating lymphocytes for adoptive cell therapy.
PLoS One. 11:e01530532016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu P and Fu YX: Tumor-infiltrating T
lymphocytes: Friends or foes? Lab Invest. 86:231–245. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smahel M, Síma P, Ludvíková V, Marinov I,
Pokorná D and Vonka V: Immunisation with modified HPV16 E7 genes
against mouse oncogenic TC-1 cell sublines with downregulated
expression of MHC class I molecules. Vaccine. 21:1125–1136. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin KY, Guarnieri FG, Staveley-O'Carroll
KF, Levitsky HI, August JT, Pardoll DM and Wu TC: Treatment of
established tumors with a novel vaccine that enhances major
histocompatibility class II presentation of tumor antigen. Cancer
Res. 56:21–26. 1996.PubMed/NCBI
|
|
12
|
Mikysková R, Bubeník J, Vonka V, Smahel M,
Indrova M, Bieblová J, Símová J and Jandlová T: Immune escape
phenotype of HPV16-associated tumours: MHC class I expression
changes during progression and therapy. Int J Oncol. 26:521–527.
2005.PubMed/NCBI
|
|
13
|
Símová J, Bubeník J, Bieblová J, Rosalia
RA, Fric J and Reinis M: Depletion of T (reg) cells inhibits
minimal residual disease after surgery of HPV16-associated tumours.
Int J Oncol. 29:1567–1571. 2006.PubMed/NCBI
|
|
14
|
Manning J, Indrova M, Lubyova B, Pribylova
H, Bieblova J, Hejnar J, Simova J, Jandlova T, Bubenik J and Reinis
M: Induction of MHC class I molecule cell surface expression and
epigenetic activation of antigen-processing machinery components in
a murine model for human papilloma virus 16-associated tumours.
Immunology. 123:218–227. 2008.PubMed/NCBI
|
|
15
|
Rossowska J, Pajtasz-Piasecka E, Szyda A,
Zietara N and Duś D: Tissue localization of tumor antigen-loaded
mouse dendritic cells applied as an anti-tumor vaccine and their
influence on immune response. Folia Histochem Cytobiol. 45:349–355.
2007.PubMed/NCBI
|
|
16
|
Hadrup S, Donia M and Thor Straten P:
Effector CD4 and CD8 T cells and their role in the tumor
microenvironment. Cancer Microenviron. 6:123–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luckheeram RV, Zhou R, Verma AD and Xia B:
CD4+T cells: Differentiation and functions. Clin Dev
Immunol. 2012:9251352012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koretzky GA: Multiple roles of CD4 and CD8
in T cell activation. J Immunol. 185:2643–2644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smyth MJ, Crowe NY and Godfrey DI: NK
cells and NKT cells collaborate in host protection from
methylcholanthrene-induced fibrosarcoma. Int Immunol. 13:459–463.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bendelac A, Savage PB and Teyton L: The
biology of NKT cells. Annu Rev Immunol. 25:297–336. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Símová J, Indrová M, Bieblová J, Mikysková
R, Bubeník J and Reinis M: Therapy for minimal residual tumor
disease: Beta-galactosylceramide inhibits the growth of recurrent
HPV16-associated neoplasms after surgery and chemotherapy. Int J
Cancer. 126:2997–3004. 2010.PubMed/NCBI
|
|
22
|
Nishikado H, Mukai K, Kawano Y, Minegishi
Y and Karasuyama H: NK cell-depleting anti-asialo GM1 antibody
exhibits a lethal off-target effect on basophils in vivo. J
Immunol. 186:5766–5771. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tomar MS, Kumar S, Kumar S, Gautam PK,
Singh RK, Verma PK, Singh SP and Acharya A: NK cell effector
functions regulation by modulating nTreg cell population during
progressive growth of Dalton's lymphoma in mice. Immunol Invest.
11:1–17. 2017.
|
|
24
|
da Cunha A, Antoniazi Michelin M and
Cândido Murta EF: Phenotypic profile of dendritic and T cells in
the lymph node of Balb/C mice with breast cancer submitted to
dendritic cells immunotherapy. Immunol Lett. 177:25–37. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zalli A, Bosch JA, Goodyear O, Riddell N,
McGettrick HM, Moss P and Wallace GR: Targeting β2 adrenergic
receptors regulate human T cell function directly and indirectly.
Brain Behav Immun. 45:211–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sato Y, Shimizu K, Shinga J, Hidaka M,
Kawano F, Kakimi K, Yamasaki S, Asakura M and Fujii SI:
Characterization of the myeloid-derived suppressor cell subset
regulated by NK cells in malignant lymphoma. Oncoimmunology.
4:e9955412015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mikyšková R, Indrová M, Símová J, Bieblová
J, Bubeník J and Reiniš M: Genetically modified tumour vaccines
producing IL-12 augment chemotherapy of HPV16-associated tumours
with gemcitabine. Oncol Rep. 25:1683–1689. 2011.PubMed/NCBI
|
|
29
|
Gabrilovich DI, Ostrand-Rosenberg S and
Bronte V: Coordinated regulation of myeloid cells by tumours. Nat
Rev Immunol. 12:253–268. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Umansky V and Sevko A: Tumor
microenvironment and myeloid-derived suppressor cells. Cancer
Microenviron. 6:169–177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sevko A and Umansky V: Myeloid-derived
suppressor cells interact with tumors in terms of myelopoiesis,
tumorigenesis and immunosuppression: Thick as thieves. J Cancer.
4:3–11. 2013. View Article : Google Scholar : PubMed/NCBI
|


















