Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most
common subtype of non-Hodgkin lymphomas, constituting up to 40% of
cases globally (1). Global
epidemiological data on DLBCL are limited, but the estimated
incidence is 7 per 100,000 in the USA (2). In Slovenia, the annual incidence of
non-Hodgkin lymphomas was 374 in 2013 (3); 36% of these cases are believed to be
DLBCL (3) Although most commonly
observed in older patients, DLBCL can affect any age group,
including children (4).
DLBCL is an aggressive condition and many patients
have advanced disease at diagnosis (5). The prognosis of a patient with DLBCL can
be predicted using their International Prognostic Index (IPI)
score. The IPI score is calculated based on age, serum lactate
dehydrogenase level, Eastern Cooperative Oncology Group performance
score, disease stage, and the number of extranodal disease sites
(6).
Treatment for patients with DLBCL generally consists
of a combination of chemotherapy [cyclophosphamide, doxorubicin,
vincristine and prednisolone (CHOP)] and rituximab (R-CHOP).
Although many clinical trials have demonstrated the efficacy and
tolerability of R-CHOP in patients with DLBCL, (7–9) less is
known about the use of this regimen in the real-world setting.
Real-world studies can complement the results of
clinical trials and provide additional information that can help
guide physicians making treatment decisions. Clinical trial data
may not be generalizable to the broad range of patients commonly
encountered in the clinical setting, as they may be limited to
younger patients with good baseline characteristics. Patients
encountered in real-world clinical practice may be older and have
comorbidities that would have excluded their participation in
rigorously designed clinical trials. Consequently, real-world
studies can provide a valuable insight into these less widely
studied patients.
We previously described the real-world use of R-CHOP
in patients with DLBCL in Slovenia (10). We now present the results of extended
follow-up in an expanded patient group.
Materials and methods
This was a single-center retrospective
database analysis
Records were searched for all patients with DLBCL
who were treated at the Institute of Oncology Ljubljana between
2004 and 2013 for inclusion in this analysis.
All procedures followed in this study were in
accordance with the ethical standards of the responsible committee
on human experimentation (institutional and national) and the
Helsinki Declaration of 1975, as revised in 2000. Individual
patient consent was not collected for this study as this was a
retrospective database analysis and the institutional informed
consent form for treatment included consent to use the patient's
data, materials and/or test results for research purposes. The
study was approved as such by the institutional review board of the
Institute of Oncology Ljubljana.
Patient characteristics, pathohistological
diagnosis, disease stage, and response to treatment were taken from
patient records. Survival data were retrieved from the Cancer
Registry of the Republic of Slovenia [www.slora.si].
Treatment response was evaluated according to Cheson criteria, with
the exception of criteria regarding positron-emission tomography
(PET) evaluation, which was not routinely used in Slovenia before
January 2016 (11). Progression-free
survival (PFS) and Overall survival (OS) were calculated using
Kaplan-Meier survival curves. PFS, which was determined for
patients receiving first-line treatment only, was defined as the
time from the beginning of treatment to disease progression for
patients achieving complete or partial remission; OS was defined as
the time from the beginning of treatment to the time of death or
the end of observation for all patients.
Patients were categorized according to IPI (6) and revised IPI (R-IPI) (12) scores. We also compared younger
patients (aged <60 years) with good (IPI 0 or 1) vs. poor (IPI
≥2) prognosis and we compared older (aged ≥60 years) vs. younger
(aged <60 years) patients. Statistically significant differences
were calculated using log-rank and χ2 tests.
Results
Patients
Between 2004 and 2013, 624 patients with DLBCL were
treated at the Institute of Oncology Ljubljana, Slovenia. The
diagnosis of DLBCL was established histologically in 523 patients
(84%) and cytologically in 101 patients (16%). Patient
characteristics are summarized in Table
I.
 | Table I.Patient clinical and demographic
characteristics at the start of treatment (n=624). |
Table I.
Patient clinical and demographic
characteristics at the start of treatment (n=624).
| Characteristic | Value |
|---|
| Sex, n (%) |
|
| Male | 297 (48) |
|
Female | 327 (52) |
| Median age, years
(range) | 67.0 (19–89) |
| <50
years, n (%) | 77 (12) |
| <60
years, n (%) | 208 (33) |
| >65
years, n (%) | 338 (54) |
| >75
years, n (%) | 167 (27) |
| ECOG performance
status, n (%) |
|
| 0 | 299 (48) |
| 1 | 183 (29) |
| 2 | 90 (14) |
| 3 | 33 (5) |
| 4 | 19 (3) |
| Disease stage, n
(%) |
|
| I | 73 (12) |
| II | 178 (29) |
| III | 111 (18) |
| IV | 245 (39) |
| Elevated LDH level, n
(%) | 311 (50) |
| Extranodal
involvement, n (%) | 113 (18) |
| Nodal and extranodal
involvement, n (%) | 326 (52) |
| Nodal involvement, n
(%) | 179 (29) |
| Treatment regimen, n
(%) |
|
| Rituximab +
CHOPa | 575 (92) |
| Rituximab + other
chemotherapyb | 32 (5) |
| Chemotherapy
alonec | 17 (3) |
| IPI score, n
(%)d |
|
| 0 | 63 (10) |
| 1 | 122 (20) |
| 2 | 143 (23) |
| 3 | 141 (23) |
| 4 | 108 (17) |
| 5 | 44 (7) |
All patients received first-line chemotherapy; 607
patients (97%) whose tumors were CD20 positive received rituximab
with their chemotherapy. Chemotherapy consisted of CHOP or a
CHOP-like regimen in 575 patients (92%); a further 32 patients (5%)
received another non-anthracycline chemotherapy regimen in
combination with rituximab and the remaining 17 patients (3%)
received chemotherapy alone. A total of 136 patients (22%) received
second-line therapy and 56 patients (9%) received third-line
therapy. The average number of treatment cycles was 6.7 in the
first line, 3.7 in the second line, and 2.4 in the third line.
Overall, 26 patients (4%) were receiving maintenance or
consolidation treatment with rituximab because of an associated
follicular lymphoma component to their disease.
IPI risk category and R-IPI prognosis category were
assessed in 573 patients. When categorized according to the IPI
system, 170 patients (30%) were considered ‘low’ risk, 134 (23%)
were ‘low-intermediate’ risk, 129 (23%) were ‘high-intermediate’
risk, and 140 (24%) were ‘high’ risk. According to the R-IPI
prognostic system, 59 patients (10%) fell into the ‘very good’
prognosis group, 245 patients (43%) into the ‘good’ prognosis
group, and 269 patients (47%) into the ‘poor’ prognosis group.
Response to treatment
The median follow-up time was 45.3 months (range,
0.1–143.0 months). The overall response rate in all patients was
90%. In the IPI ‘low-risk’ group, 168 patients (99%) had a complete
or partial response. The overall response rates in the
‘low-intermediate’, ‘high-intermediate’, and ‘high-risk’ groups
were 94, 87, and 79%, respectively (χ2 test;
P<0.0001) (Table II). In the
R-IPI ‘very good’ prognosis group, 59 patients (100%) had a
complete or partial response (Table
II). The overall response rates in the R-IPI ‘good’ and ‘poor’
prognosis groups were 96 and 83%, respectively; the difference
between R-IPI groups was statistically significant
(P<0.0001).
 | Table II.Response to treatment. |
Table II.
Response to treatment.
|
| Patients, n (%) |
|---|
|
|
|
|---|
| Group (number of
patients) | CR | PR | SD | PD | Undefined |
|---|
| IPI risk group |
|
|
|
|
|
| Low
(n=170) | 101 (59) | 67 (39) | 0 | 0 | 2 (1) |
|
Low-intermediate (n=134) | 62 (46) | 64 (48) | 1 (1) | 3 (2) | 4 (3) |
|
High-intermediate (n=129) | 56 (43) | 56 (43) | 0 | 4 (3) | 13 (10) |
| High
(n=140) | 50 (36) | 61 (44) | 2 (1) | 7 (5) | 20 (14) |
| R-IPI prognostic
group |
|
|
|
|
|
| Very
good (n=59) | 36 (61) | 23 (39) | 0 | 0 | 0 |
| Good
(n=245) | 127 (52) | 108 (44) | 1 (<1) | 3 (1) | 6 (2) |
| Poor
(n=269) | 106 (39) | 117 (43) | 2 (1) | 11 (4) | 33 (12) |
| All patients
(n=573)a | 269 (47) | 248 (43) | 3 (1) | 14 (2) | 39 (7) |
Progression-free survival
PFS was only determined in patients undergoing
first-line treatment. The median PFS was not reached in the overall
population, as shown in Table III,
nor in any of the subgroups analyzed. PFS according to IPI and
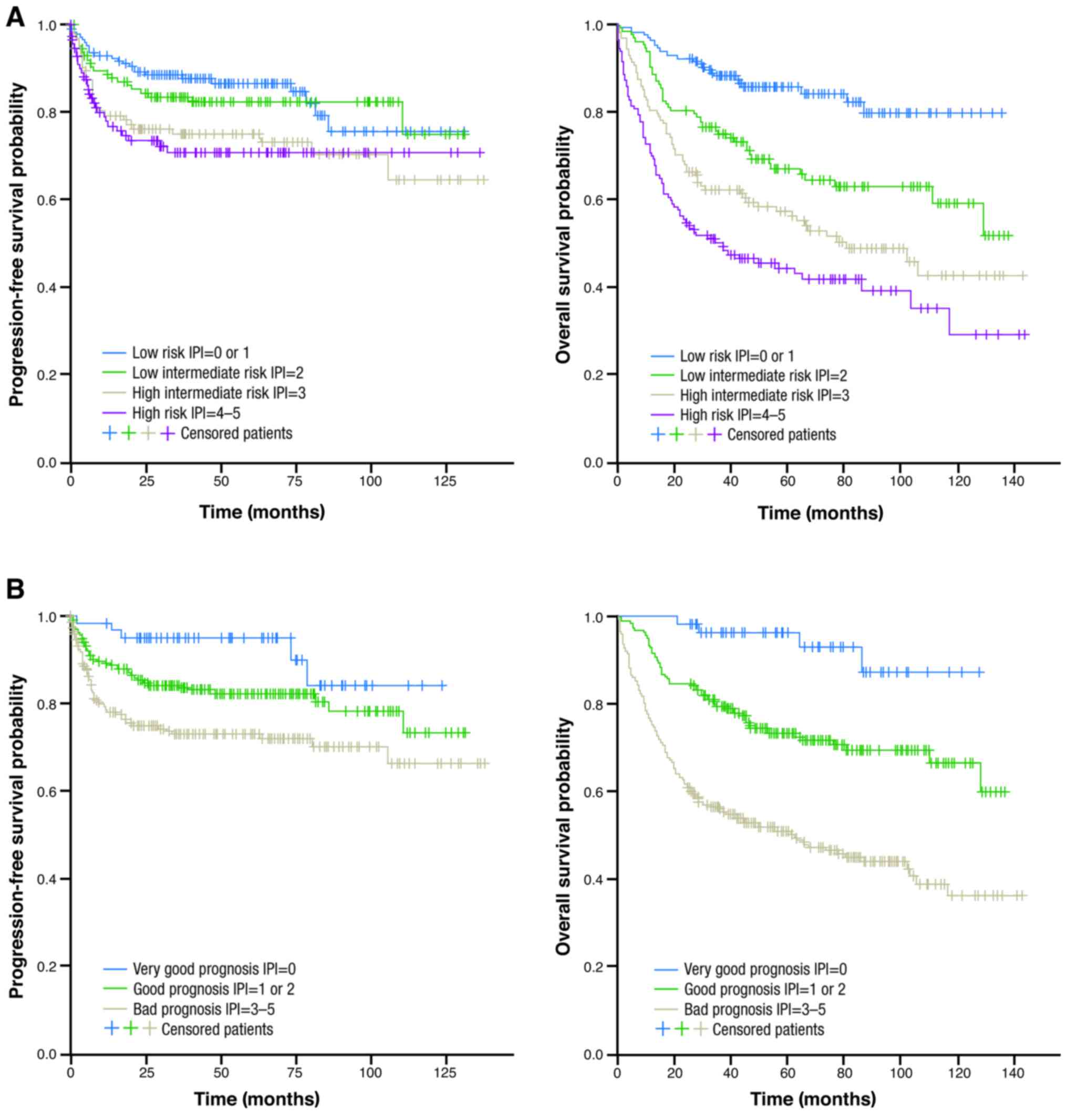
R-IPI category is shown in Fig. 1;
PFS rates are shown in Table III.
Among patients classified as IPI ‘low risk’, 75% were
progression-free 10 years after treatment; 10-year PFS rates in the
‘low-intermediate’, ‘high-intermediate’, and ‘high-risk’ groups
were 75, 64, and 71%, respectively. Among those classified as
having an R-IPI ‘very good’ prognosis, 84% were progression-free at
10 years after treatment; 10-year PFS rates in the good and poor
prognosis groups were 73 and 66%, respectively. The PFS difference
between groups was statistically significant for the IPI (log-rank
P=0.01) and R-IPI groups (P=0.001).
 | Table III.Progression-free survival rates
according to IPI and R-IPI categories. |
Table III.
Progression-free survival rates
according to IPI and R-IPI categories.
|
| Progression-free
survival rate (%) |
|
|---|
|
|
|
|
|---|
| Patient
category | 1-year | 2-year | 3-year | 5-year | 10-year | Median
progression-free survival (months) |
|---|
| All patients | 86 | 82 | 81 | 80 | 72 | NR |
| IPI risk group |
|
|
|
|
|
|
|
Low | 93 | 90 | 88 | 87 | 75 | NR |
|
Low-intermediate | 89 | 84 | 83 | 82 | 75 | NR |
|
High-intermediate | 79 | 76 | 75 | 75 | 64 | NR |
|
High | 78 | 73 | 71 | 71 | 71 | NR |
| R-IPI prognostic
group |
|
|
|
|
|
|
| Very
good | 95 | 95 | 95 | 95 | 84 | NR |
|
Good | 89 | 85 | 84 | 82 | 73 | NR |
|
Poor | 78 | 75 | 73 | 73 | 66 | NR |
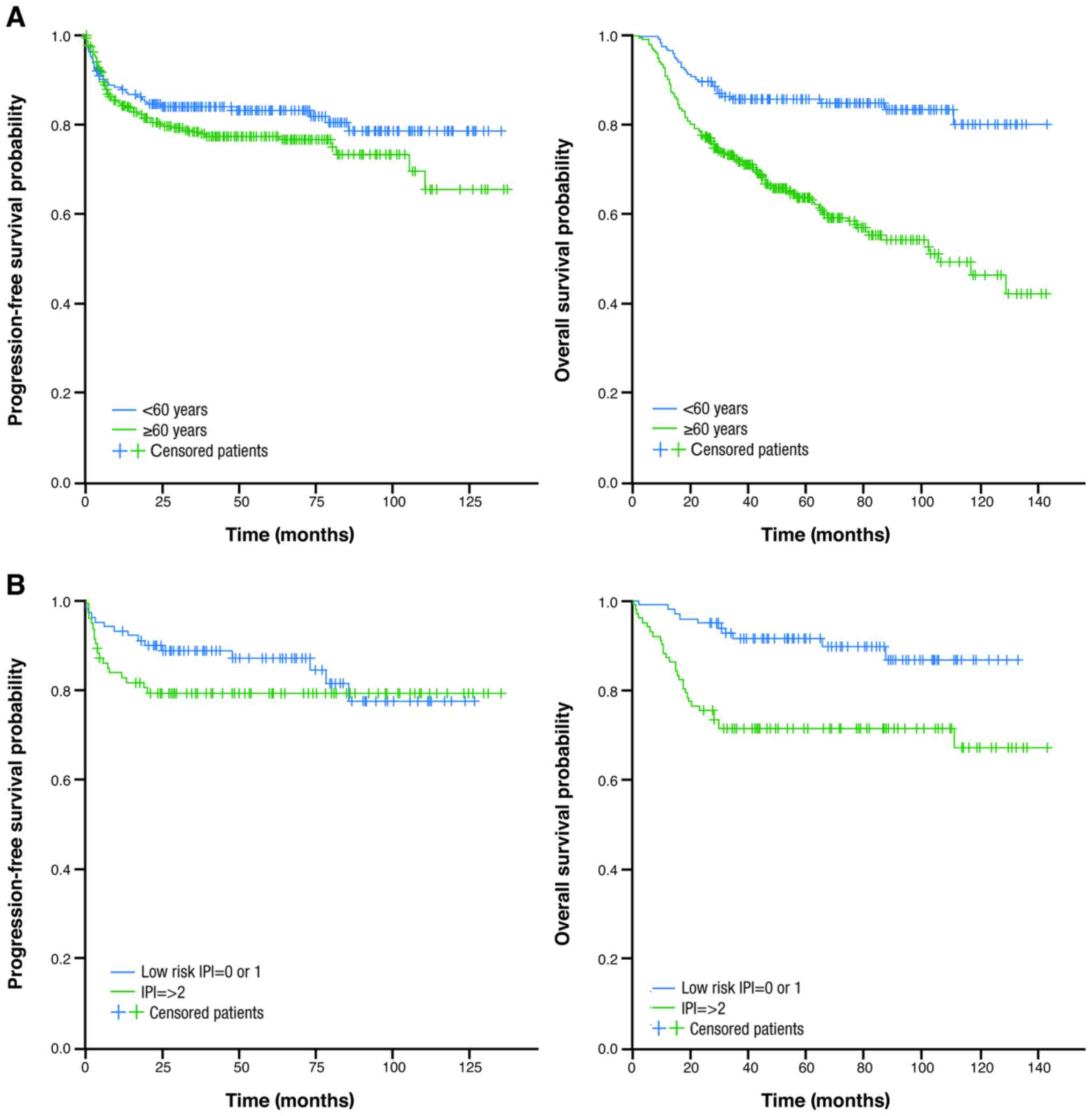
When analyzed according to age alone, no
statistically significant difference was seen in PFS rates between
older and younger patients. Survival outcomes were also evaluated
in younger patients (aged <60 years) with good vs. poor
prognosis (IPI 0 or 1 and IPI ≥2, respectively) (Table IV and Fig.
2). PFS rates were similar in both groups of patients.
 | Table IV.Outcomes according to age and
prognosis. |
Table IV.
Outcomes according to age and
prognosis.
|
| Survival rate
(%) |
|
|---|
|
|
|
|
|---|
| Outcome | 1-year | 2-year | 3-year | 5-year | 10-year | Median survival
(months) |
|---|
| Progression-free
survival |
|
|
|
|
|
|
| Age,
<60 years | 88 | 85 | 84 | 83 | 79 | NR |
| Age.
≥60 years | 84 | 80 | 79 | 78 | 70 | NR |
| Age,
<60 years, IPI 0 or 1 | 93 | 90 | 89 | 87 | 77 | NR |
| Age,
<60, IPI ≥2 | 83 | 79 | 79 | 79 | 79 | NR |
| Overall
survival |
|
|
|
|
|
|
| Age,
<60 years | 93 | 85 | 81 | 81 | 76 | NR |
| Age,
≥60 years | 82 | 70 | 64 | 56 | 41 | 80.1 |
| Age,
<60 years, IPI 0 or 1 | 98 | 95 | 91 | 91 | 87 | NR |
| Age,
<60, IPI ≥2 | 87 | 76 | 71 | 71 | 67 | NR |
Overall survival
OS was determined in all patients undergoing
treatment. The median OS was 124 months in the overall population.
OS according to IPI and R-IPI category is shown in Fig. 1; OS rates are shown in Table V. Among patients classified as having
an IPI ‘low risk’, 80% were alive at 10 years; the 10-year OS rates
in the ‘low-intermediate’, ‘high-intermediate’, and ‘high-risk’
groups were 60, 43, and 30%, respectively. Ten-year OS for the
R-IPI ‘very good prognosis’ group was 87%; 10-year rates in the
good and poor prognosis groups were 67 and 37%, respectively.
Between-group differences were statistically significant for OS for
the IPI (log-rank test P<0.0001) and R-IPI groups (log-rank test
P<0.0001).
 | Table V.Overall survival rates according to
IPI and R-IPI categories. |
Table V.
Overall survival rates according to
IPI and R-IPI categories.
|
| Overall
survival |
|
|---|
|
|
|
|
|---|
| Patient
category | 1-year | 2-year | 3-year | 5-year | 10-year | Median overall
survival (months) |
|---|
| All patients | 85 | 74 | 69 | 63 | 51 | 123.7 |
| IPI risk group |
|
|
|
|
|
|
|
Low | 97 | 92 | 88 | 86 | 80 | NR |
|
Low-intermediate | 90 | 81 | 75 | 67 | 60 | NR |
|
High-intermediate | 81 | 68 | 63 | 58 | 43 | 80.6 |
|
High | 71 | 56 | 50 | 45 | 30 | 35.9 |
| R-IPI prognostic
group |
|
|
|
|
|
|
| Very
good | 100 | 98 | 96 | 96 | 87 | NR |
|
Good | 93 | 85 | 79 | 73 | 67 | NR |
|
Poor | 76 | 62 | 56 | 51 | 37 | 62.3 |
Median OS was not reached in younger patients and
was 81 months in older patients. The difference in OS rates between
the younger and older groups was statistically significant
(P<0.0001). Regarding OS in younger patients (aged <60
years), outcomes were statistically significantly better in those
with a good vs. poor prognosis (IPI 0 or 1 vs. IPI ≥2,
respectively) (P=0.001; Table IV and
Fig. 2).
Discussion
Long-term follow-up data for patients with DLBCL are
scarce, particularly among patients treated in the real-world
setting. We have described the use of rituximab-based regimens in
over 600 patients of varying ages and disease stages over a
prolonged follow-up period. To the best of our knowledge, no other
studies have assessed outcomes in patients with DLBCL in the
real-world setting.
In our patient population, an overall 10-year PFS
rate of 72% was observed, ranging from 64% in patients classed
according to IPI as ‘high-intermediate’ risk (IPI 3) to 75% in
patients with low risk (IPI 0 or 1) and from 66% in patients with
R-IPI poor prognosis (R-IPI 3–5) to 84% in those with very good
prognosis (R-IPI 0). Median PFS was not reached in any of the IPI
risk categories. PFS rates were numerically but not statistically
significantly higher in patients aged <60 years compared with
those aged ≥60 years, with 79% of younger patients and 70% of older
patients free from progression after 10 years. Coiffier et
al reported a 10-year PFS rate of 37% in their group of
patients aged 60–80 years in the LNH-98.5 trial (13) lower than the 10-year rates seen in our
older patients. The median PFS was 4.8 years in the LNH-98.5 trial;
median PFS has not yet been reached in our group of patients after
a median follow-up of 45 months. However, differences between the
two patient populations and study designs call for caution when
comparing the results of the two studies.
With regard to OS, we observed a 10-year OS rate of
51% in the overall population and 41% in older patients, the latter
being comparable with the 43.5% reported for patients aged ≥60
years in the LNH-98.5 study (13).
Purroy et al reported a 10-year OS rate of 64% in their
group of patients with DLBCL, with little difference between older
and younger patients (64% for those aged <60 years and 63% for
those aged ≥60 years) in contrast to the present study (14). They also reported a 10-year OS rate of
59% in patients with high-risk disease (IPI score ≥3), somewhat
higher than the rates we observed in our patients. Once again,
however, cross-study comparisons are complicated by many factors,
including differences in the treatment settings and patient
populations.
We observed a statistically significant difference
in OS between younger patients with good vs. poor prognosis, with
10-year OS rates of 87 and 67%, respectively. This was in line with
our observations at the 5-year timepoint (10). Younger, high-risk patients clearly
represent a population for whom better treatment options are
needed. Intensive regimens such as R-CHOEP-14 (rituximab,
cyclophosphamide, doxorubicin, vincristine, etoposide, and
prednisolone) and megaCHOEP (high-dose cyclophosphamide,
doxorubicin, vincristine, etoposide, and prednisolone) (15,16) and
R-ACVBP (rituximab, doxorubicin, vindesine, cyclophosphamide,
bleomycin, and prednisolone) (17)
are frequently used (18) but have
not been shown to be superior to R-CHOP in this patient population
(19). Consequently, European
treatment guidelines currently recommend recruitment into clinical
trials for young ‘high-risk’ and ‘high-intermediate’ risk patients
(18). In the activated B-cell
subtype of DLBCL that is more commonly seen in older patients, the
addition of bortezomib, lenalidomide, or ibrutinib to standard
therapy has provided encouraging indications of activity in early
(20,21). Results from ongoing studies such as
ROBUST (NCT02285062) and PHOENIX (NCT01855750) will provide an
indication as to whether this is a valid approach for this
poor-prognosis group of patients.
A plateau was observed in our PFS curves according
to IPI category, with few relapses after 6 years in patients with
‘low-intermediate’ and ‘high-risk’ disease. This mirrors
observations in the LNH-98.5 study (13). In contrast, an analysis of the SWOG
S8736 and S0014 studies, which only included patients with
limited-stage disease, revealed a pattern of late relapse in those
patients (22). Patients with
limited-stage disease comprised 40% of our patient population; in
our group of ‘low-risk’ patients, some evidence of late disease
progression was evident at 5–7 years post therapy, highlighting the
need for continued observation of these patients.
Five-year OS rates ranged from 86% in IPI ‘low-risk’
patients to 45% in IPI ‘high-risk’ patients. Our OS rates were
higher than the 5-year OS rates reported for patients in a large
validation study by Olszewski et al those authors reported
5-year OS of 74, 58, 49, and 33% for ‘low-risk’,
‘low-intermediate’, ‘high-intermediate’ and ‘high-risk’ patients,
compared with 86, 67, 58, and 45%, respectively, in the present
study (23). Five-year OS rates in
our study were higher in all three R-IPI categories compared with
the validation study (‘very good’ prognosis: 96% vs. 87%; ‘good’
prognosis: 73% vs. 64%; ‘poor’ prognosis: 51% vs. 41%,
respectively), differences that may have been due to the
characteristics of the two patient populations.
Some limitations of the present study should be
considered in addition to those inherent in retrospective
observational studies. PET was not used for disease staging until
2016; consequently, some patients may have been understaged at
diagnosis. Since the introduction of diagnostic PET, we have
observed discrete paraspinal lymphomatous masses in some patients
that might have been overlooked with computed tomography. This has
the potential to affect our survival data.
Although many studies have examined the efficacy of
R-CHOP and similar regimens in patients with DLBCL, real-world data
are scarce for these patients. We have shown that many patients
treated with R-CHOP and R-CHOP-like regimens in the real-world
setting can have excellent outcomes; however, accurate disease
staging is essential to confidently assign prognostic scores and
assess likely outcomes for patients.
Acknowledgements
The present study was supported by the Slovenian
Ministry of Higher Education, Science and Technology (grant no.
P3-0321). Support for third-party editorial assistance for this
manuscript was provided by F. Hoffmann-La Roche, Ltd.
References
|
1
|
World Health Organization, . Diffuse large
B-cell lymphoma. http://www.who.int/selection_medicines/committees/expert/20/applications/DiffuseLargeBCellLymphoma.pdf16–December.
2016
|
|
2
|
Li Y, Wang Y, Wang Z, Yi D and Ma S:
Racial differences in three major NHL subtypes: Descriptive
epidemiology. Cancer Epidemiol. 39:8–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cancer in Slovenia 2013, . Ljubljana:
Institute of Oncology Ljubljana, Epidemiology and Cancer Registry.
http://www.onko-i.si/fileadmin/onko/datoteke/dokumenti/RRS/LP_2013.pdf8–March.
2017
|
|
4
|
Cairo MS and Perkins SL: Hematological
Malignancies in Children, Adolescents and Young Adults. First
edition. World Scientific Publishing Co.; Singapore: 2012,
View Article : Google Scholar
|
|
5
|
Sehn LH and Gascoyne RD: Diffuse large
B-cell lymphoma: Optimizing outcome in the context of clinical and
biologic heterogeneity. Blood. 125:22–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
International Non-Hodgkin's Lymphoma
Prognostic Factors Project: A predictive model for aggressive
non-Hodgkin's lymphoma. N Engl J Med. 329:987–994. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coiffier B, Lepage E, Brier J, Herbrecht
R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G,
Gaulard P, et al: CHOP chemotherapy plus rituximab compared with
CHOP alone in elderly patients with diffuse large-B-cell lymphoma.
N Engl J Med. 346:235–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Habermann TM, Weller EA, Morrison VA,
Gascoyne RD, Cassileth PA, Cohn JB, Dakhil SR, Woda B, Fisher RI,
Peterson BA and Horning SJ: Rituximab-CHOP versus CHOP alone or
with maintenance rituximab in older patients with diffuse large
B-cell lymphoma. J Clin Oncol. 24:3121–3127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pfreundschuh M, Trümper L, Österborg A,
Pettengell R, Trneny M, Imrie K, Ma D, Gill D, Walewski J, Zinzani
PL, et al: CHOP-like chemotherapy plus rituximab versus CHOP-like
chemotherapy alone in young patients with good-prognosis diffuse
large-B-cell lymphoma: A randomised controlled trial by the
MabThera International Trial (MInT) Group. Lancet Oncol. 7:379–391.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gregoric B, Zadnik V and Novakovic
Jezersek B: The diffuse large B-cell lymphoma-where do we stand now
in everyday clinical practice. Radiol Oncol. 46:153–159. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheson BD, Fisher RI, Barrington SF,
Cavalli F, Schwartz LH, Zucca E and Lister TA: Alliance,
Australasian Leukaemia and Lymphoma Group; Eastern Cooperative
Oncology Group; European Mantle Cell Lymphoma Consortium:
Recommendations for initial evaluation, staging, and response
assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano
classification. J Clin Oncol. 32:3059–3068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sehn LH, Berry B, Chhanabhai M, Fitzgerald
C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J,
et al: The revised international prognostic index (R-IPI) is a
better predictor of outcome than the standard IPI for patients with
diffuse large B-cell lymphoma treated with R-CHOP. Blood.
109:1857–1861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coiffier B, Thieblemont C, Van Den Neste
E, Lepeu G, Plantier I, Castaigne S, Lefort S, Marit G, Macro M,
Sebban C, et al: Long-term outcome of patients in the LNH-98.5
trial, the first randomized study comparing rituximab-CHOP to
standard CHOP chemotherapy in DLBCL patients: A study by the Groupe
d'Etudes des Lymphomes de l'Adulte. Blood. 116:2040–2045. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Purroy N, Bergua J, Gallur L, Prieto J,
Lopez LA, Sancho JM, García-Marco JA, Castellví J, Montes-Moreno S,
Batlle A, et al: Long-term follow-up of dose-adjusted EPOCH plus
rituximab (DA-EPOCH-R) in untreated patients with poor prognosis
large B-cell lymphoma. A phase II study conducted by the Spanish
PETHEMA Group. Br J Haematol. 169:188–198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Holte H, Leppä S, Björkholm M, Fluge O,
Jyrkkiö S, Delabie J, Sundström C, Karjalainen-Lindsberg ML,
Erlanson M, Kolstad A, et al: Dose-densified chemoimmunotherapy
followed by systemic central nervous system prophylaxis for younger
high-risk diffuse large B-cell/follicular grade 3 lymphoma
patients: Results of a phase II Nordic Lymphoma Group study. Ann
Oncol. 24:1385–1392. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmitz N, Nickelsen M, Ziepert M, Haenel
M, Borchmann P, Schmidt C, Viardot A, Bentz M, Peter N, Ehninger G,
et al: Conventional chemotherapy (CHOEP-14) with rituximab or
high-dose chemotherapy (MegaCHOEP) with rituximab for young,
high-risk patients with aggressive B-cell lymphoma: An open-label,
randomised, phase 3 trial (DSHNHL 2002–1). Lancet Oncol.
13:1250–1259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fitoussi O, Belhadj K, Mounier N, Parrens
M, Tilly H, Salles G, Feugier P, Ferme C, Ysebaert L, Gabarre J, et
al: Survival impact of rituximab combined with ACVBP and upfront
consolidation autotransplantation in high-risk diffuse large B-cell
lymphoma for GELA. Haematologica. 96:1136–1143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tilly H, da Silva Gomes M, Vitolo U, Jack
A, Meignan M, Lopez-Guillermo A, Walewski J, André M, Johnson PW,
Pfreundschuh M, et al: Diffuse large B-cell lymphoma (DLBCL): ESMO
clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 5 Suppl 26:v116–v125. 2015. View Article : Google Scholar
|
|
19
|
Le Gouill S, Milpied JN, Lamy T, Delwail
V, Gressin R, Guyotat D, Damaj GL, Foussard C, Cartron G,
Maisonneuve H, et al: First-line rituximab (R) high-dose therapy
(R-HDT) versus R-CHOP14 for young adults with diffuse large B-cell
lymphoma: Preliminary results of the GOELAMS 075 prospective
multicenter randomized trial. J Clin Oncol. 29 Suppl 15:S80032011.
View Article : Google Scholar
|
|
20
|
Wilson WH, Young RM, Schmitz R, Yang Y,
Pittaluga S, Wright G, Lih CJ, Williams PM, Shaffer AL, Gerecitano
J, et al: Targeting B cell receptor signalling with ibrutinib in
diffuse large B cell lymphoma. Nat Med. 21:922–926. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hong JY, Suh C and Kim WS: Evolution of
frontline treatment of diffuse large B-cell lymphoma: A brief
review and recent update. F1000Res. 5:pii: F1000 Faculty Rev-1933.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stephens DM, Li H, LeBlanc ML, Puvvada SD,
Persky D, Friedberg JW and Smith SM: Continued risk of relapse
independent of treatment modality in limited-stage diffuse large
B-cell lymphoma: Final and long-term analysis of Southwest Oncology
Group Study S8736. J Clin Oncol. 34:2997–3004. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Olszewski AJ, Winer ES and Castillo JJ:
Validation of clinical prognostic indices for diffuse large B-cell
lymphoma in the national cancer data base. Cancer Causes Control.
26:1163–1172. 2015. View Article : Google Scholar : PubMed/NCBI
|