Introduction
Cervical carcinoma (CC) is the only major
gynecological malignancy that is clinically staged (1,2). Pelvic
lymph node staging (N-staging) is an important prognostic factor in
early-stage CC, as patients with lymph node metastasis (LNM) have
significantly lower survival rates than those without detectable
nodal metastases (3–7).
In recent years, diagnostic modalities, including
18F-fludeoxyglucose positron emission
tomography/computed tomography ([18F]FDG-PET/CT) and
magnetic resonance imaging (MRI), have been used in the initial
assessment of nodal involvement (8).
However, neither method has been formally included as part of the
International Federation of Gynecology and Obstetrics staging of CC
(9). MRI and CT exhibit low
sensitivity and specificity and thus cannot be used to detect nodal
involvement (8). Functional imaging
modalities such as [18F]FDG-PET and
[18F]FDG-PET/CT have shown a better performance than MRI
and CT and, therefore, the National Comprehensive Cancer Network
clinical guidelines recommend the use of [18F]FDG-PET/CT
as a routine procedure in the assessment of patients with CC
(10).
Conclusions regarding the sensitivities and
specificities of these diagnostic modalities have been based on
studies with heterogeneous study designs that have only included a
small number of patients (8,11,12) which
demonstrates the difficulty of evaluating the pelvic N-staging of
CC patients by [18F]FDG-PET/CT and MRI. Identifying a
secure and objective method for the detection of LNM in CC patients
would improve the management of therapy and enable a realistic
evaluation of the prognosis of patients. Integrated
[18F]FDG-PET/MRI devices are currently commercially
available and thus may benefit patients in this context.
The aim of the present retrospective study was to
assess the specificity, sensitivity, positive predictive value
(PPV) and negative predictive value (NPV) of
[18F]FDG-PET/CT (PET/CT), MRI and
[18F]FDG-PET/MRI (PET/MRI), in order to determine the
optimum method for performing pelvic N-staging of patients with
untreated CC.
Patients and methods
Patients
Between January 2008 and July 2011, 152 women were
referred to the PET/CT center of the Vienna General Hospital
(Vienna, Austria) for initial staging of verified CC. From these
152 patients, 192 PET/CT images were acquired. Of the 152 patients,
27 patients with different stages [International Federation of
Gynecology and Obstetrics staging system (8); 12 with ≥IB2, 12 with <IB2 and 3 with
unknown stages], and with an average age of 46 years (age range,
22–68 years), fulfilled the inclusion criteria for the present
study. The inclusion criteria were as follows: The patient had
undergone PET/CT and MRI scans prior to treatment; diagnostic
imaging results had been obtained <45 days prior to adenectomies
to exclude further tumor progression; and there had been a period
of <45 days between the two diagnostic imaging sessions. The
gold standard for the current retrospective study was the
histological analysis of specimens obtained via lymphadenectomies.
PET/CT and MRI images were analyzed by two experts (one expert in
MRI/CT and one expert in PET). Increased uptake of FDG on the PET
images and lesions detected from CT and MRI were used to conduct
pelvic N-staging. Subsequently, the experts' findings were compared
with the histopathological results from the corresponding
lymphadenectomies. The present study was approved by the ethics
committee of the Medical University of Vienna, Austria and, as the
current study was a retrospective study, informed consent from the
patients was not required.
Pelvic N-staging
Pelvic N-staging was performed by looking at nine
anatomical localizations using CT and/or MRI for the presence of
LNMs, including the arteria iliaca communis dexter (A), the arteria
iliaca communis sinister (B), the arteria iliaca externa dexter
(C), the arteria iliaca externa sinister (D), the arteria iliaca
interna dexter (E), the arteria iliaca interna sinister (F), the
musculus obturatorius dexter (G), the musculus obturatorius
sinister (H) and the sacrum (I). Pelvic N-staging was confirmed by
standard histological analyses, including immunohistochemistry or
hematoxylin and eosin staining. PET/CT, MRI and PET/MRI were the
diagnostic modalities assessed in the current study.
Histological analysis
Serial paraffin sections (2-µm thickness) of
formalin-fixed lymph nodes were cut, deparaffinized using xylol,
rehydrated and stained with hematoxylin/eosin. Pretreatment of
deparaffinized and rehydrated sections and immunostaining was
performed using the BenchMark Ultra fully automated slide staining
system (Ventana Medical Systems, Inc., Tucson, AZ, USA). After
pretreatment for 60 min with Cell Conditioner 1 (pH 8, Ventana
Medical Systems, Inc.), the sections were stained for 32 min at
36°C with pancytokeratin antibody (clone AE1/AE3; cat. no. M351501;
Dako Österreich GmbH, Vienna, Austria) at a dilution of 1:100.
Chromogenic visualization was performed with the ultraView
Universal DAB Detection kit (Ventana Medical Systems, Inc.), after
which counterstaining with hematoxylin and bluing with Bluing
Reagent (Ventana Medical Systems, Inc.) was performed with the
appropriate Ventana Ancillary Reagents, according to the
manufacturer's protocol.
PET/CT
PET/CT was performed using a 64-row, multi-detector
PET/CT system (Biograph™ TruePoint™ 64; Siemens AG, Munich,
Germany). Prior to imaging, patients fasted for 5 h; the glucose
cut-off level was 150 mg/dl. PET was performed 50–60 min after the
intravenous administration of 300 MBq of 18F-FDG, with a
three-minute acquisition period per bed position. PET images were
reconstructed using the Siemens TrueX algorithm, with four
iterations per 21 subsets, a 5 mm slice thickness and a 168×168
matrix. Venous-phase contrast enhanced CT was performed by the
intravenous injection of 100 ml Iomeron 300 (Bracco, Milan Italy),
which is a tri-iodinated, non-ionic contrast medium, at a rate of 2
ml/sec, followed by a 50 ml saline flush and CT with the following
parameters: A tube voltage of 120 mA, a tube current of 230 kV,
collimation of 64×0.6 mm, a slice thickness of 3 mm with 2 mm
increments and a 512×512 matrix. On CT, lymph nodes with a
short-axis diameter ≥10 mm, in combination with a lack of a fatty
hilum or inhomogeneous density, were regarded as pathological. On
PET, an increased 18F-FDG uptake, as assessed visually,
with a maximal standardised uptake value (SUV) higher than the
mediastinal blood pool was regarded as pathological.
MRI
MRI was performed using a 3 Tesla MRI scanner, the
Magnetom Trio (Siemens AG), with a standard body array coil.
Sagittal, axial and coronal T2 Turbo Spin-Echo (TSE) sequences were
obtained, with a repetition time (TR) of 4,630 msec, an echo time
of 89 msec and a layer thickness of 3 mm. The axial sequence
included T1 TSE with a TR of 652 msec, an echo time (TE) of 12 msec
and a layer thickness of 4.5 mm. Additionally, there was an axial
and sagittal T1 TSE with fat saturation following the intravenous
application of 10 ml Dotarem, with a TR of 650 msec, a TE of 12
msec and a layer thickness of 4 mm. Lymph nodes with a short-axis
diameter ≥10 mm, in combination with a lack of a fatty hilum or
inhomogeneous signal on unenhanced or contrast-enhanced sequences,
were regarded as pathological.
(Virtual) PET/MRI
The PET data, which was extracted from the hybrid
PET/CT study, and MRI were combined to provide a virtual PET/MRI
study and were compared with the histology results. Virtual PET/MRI
results were calculated in two different ways. One group of results
was PET-guided, meaning that if LNM was considered positive on PET
but negative on MRI, the combined result was positive. The other
group of results was MRI-guided, meaning that if LNM was considered
positive on MRI but negative on PET, the combined result was
positive.
Statistical analysis
The current study was an open, single-site,
retrospective data analysis. The results obtained by the two
experts were compared with the histopathological results.
Specificity, sensitivity, likelihood ratios, PPV and NPV were
calculated for PET/CT, PET (extracted from PET/CT), MRI and
(virtual) PET/MRI on a per-patient basis. The (virtual) PET/MRI
results were obtained by combining the results from the extracted
PET and MRI surveys. If the results were concordant, the combined
PET/MRI result was considered either positive or negative. In case
of a discrepancy between the MRI and PET results, consent was
obtained from the experts (radiologists and nuclear physicians) in
terms of considering the diagnosis of PET and the diagnosis of MRI
together as the final outcome. The final evaluation was performed
in two different ways; as either MRI- or PET-guided. Statistical
analysis was performed using SPSS Statistics ver. 22.0 (IBM SPSS,
Armonk, NY, USA).
Results
PET/CT
In total, 14 patients (52%) had no positive LNM
detected by PET/CT (no pathological FDG uptake, no suspicious lymph
nodes. A further 12 patients had positive FDG-uptake in different
pelvic lymph node regions and different numbers of positive LNM. In
one patient, positive FDG-uptake was not identified by FDG-PET;
however, a pathological lymph node was detected by CT. Therefore,
13 patients (48%) had positive LNM detected by PET/CT. In total, 27
pelvic lymph node regions with LNM were detected in eight different
locations. The average SUVmax was 7.7 (range, 3.7–12.7). The most
common areas LNM was observed in were B (12/17, 44%), A (6/27, 22%)
and D (3/27, 11%). The average LNM detected per lymph node region
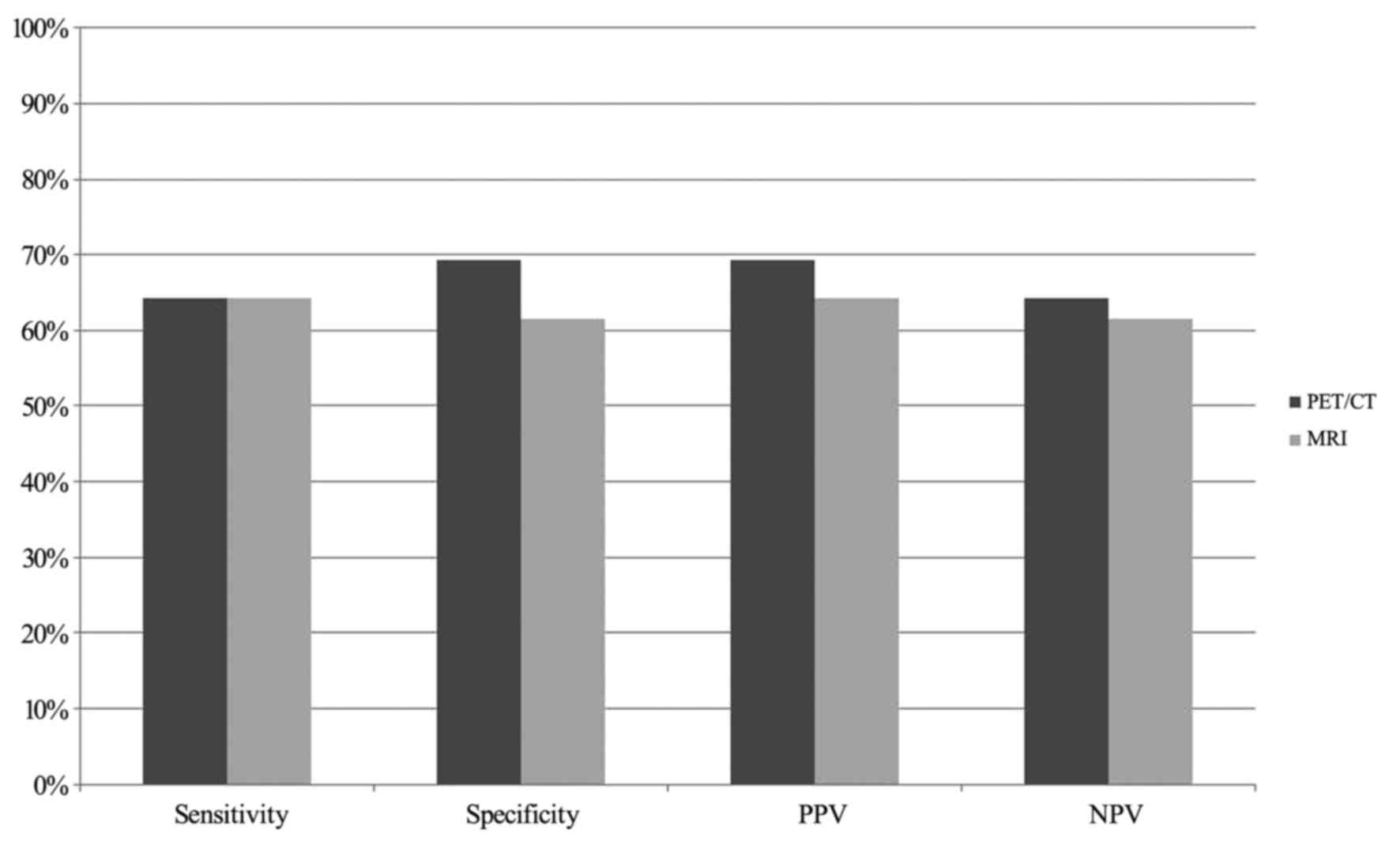
was 2.1 metastases (range, 1–6 metastases). The sensitivity and
specificity of PET/CT in detecting pelvic LNM were 64 and 69%,
respectively. The PPV and NPV for PET/CT was 69 and 54%,
respectively (Fig. 1). The positive
likelihood ratio was 2.06 and the negative likelihood ratio was
0.52. In one patient, a singular osseous metastasis was detected.
However, the other patients had no distant metastases.
MRI
Of the 27 patients, 13 had no positive LNM detected
by MRI and 14 had positive LNM detected by MRI. In total, 27 pelvic
lymph node regions with LNM were detected in nine different
locations. The most common areas were B (9/27, 33%), A (8/17, 30%)
and G (3/27, 11%). The average number of detected LNM was 2.1
(range, 1–4 metastases).
The sensitivity of MRI was 64%, the specificity was
62%, the NPV was 64% and the PPV was 64% (Fig. 1). The positive likelihood ratio was
1.68 and the negative likelihood ratio was 0.58.
Virtual PET/MRI
Of the 27 patients, 15 (56%) were considered
negative on the PET-guided PET/MRI. The remaining 12 patients (44%)
were considered positive. In the MRI-guided PET/MRI, 13 of the 27
patients (48%) were classified as negative and the remaining 14
(52%) were considered positive. The sensitivity of both the
PET-guided PET/MRI and the MRI-guided PET/MRI was 64% and the
specificity was 77 and 62%, respectively. The PPV was 75% for
PET-guided PET/MRI and 64% for MRI-guided PET/MRI, and the NPV was
67 and 62%, respectively (Fig. 2).
The positive likelihood ratio was 2.29 for the PET-guided PET/MRI
and 1.68 for the MRI-guided PET/MRI. The negative likelihood ratios
were 0.5 and 1.68 for the PET-guided PET/MRI and the MRI-guided
PET/MRI, respectively. The results of all diagnostic modalities
(PET/CT, MRI and the virtually combined PET/MRI), according to our
criteria for pathological/non-pathological status, were compared to
the results from the histological analysis (affected/non-affected)
in terms of the sensitivity, specificity, PPV, NPV and likelihood
ratio.
PET/CT vs. virtual PET/MRI
PET/CT and the virtual PET/MRI exhibited the same
low sensitivity (64%). PET/MRI exhibited slightly better results
than PET/CT regarding specificity (77 vs. 69%, respectively), PPV
(75 vs. 69%, respectively) and NPV (67 vs. 64%, respectively)
(Fig. 2).
Histology
Of the 27 patients included in the present study,
>329 pelvic lymph nodes were removed (average, 12 lymph
nodes/patient). Histological reports indicated that there were 13
patients (48%) with no pelvic LNM. In the remaining 14 patients
(52%), positive LNM was detected and verified by histology. In
total, 28 pelvic LNM were detected in seven different locations.
The most common areas were B (7/28, 25%), A and D, (both 6/28, 21%)
and H (3/28, 11%). The average number of detected LNM was 2 per
patient (range, 1–5 metastases).
There were 10 patients with squamous cell carcinoma
and 5 patients with adenocarcinoma; 13 histological reports did not
describe the type of CC. Of the 12 patients with CC <IB2, 4
exhibited histologically verified LNM, while, of the 12 patients
with ≥IB2, LNM was detected in 8 patients.
Discussion
N-staging is one of the most important factors in
predicting the prognosis and survival of CC patients (13). CC first spreads to the pelvic area
along the external and internal iliac vascular system, as well as
to the presacral space (14). The
incidence of pelvic LNM in the early stages of CC ranges from
10.9–44.7% (15,16). In the current study, a third of
patients with CC <IB2 had histologically verified LNM, whereas
66% of patients with higher-stage CC had LNM. For patients with
higher-stage CC, treatment is usually a combination of radiation
therapy and chemotherapy (8).
Information regarding pelvic lymph node status may not be
important, since such patients usually receive pelvic external beam
radiation therapy, which improves survival rates (15,16). The
region within the pelvic area where LNM is most commonly detected
obturatural [57.5–76.4% (15,16)]. Of the 27 patients included in the
present study, 14 (52%) had histologically verified pelvic LNM.
Data analysis verified that the area where LNM was detected most
frequently was region B, with 44% detected by PET/CT, 33% by MRI
and 25% by histological analysis. Lymph nodes showing an increased
uptake of 18F-FDG, or enlarged lymph nodes (short-axis
diameter ≥10 mm) with no fatty hilum and an inhomogeneous density
(on CT) or signal (on MRI), were considered to be pathological. In
all other cases, the lymph nodes were considered to be normal.
Wright et al (17) and Sironi et al (18) analyzed the preoperative lymph nodes of
early-stage CC patients by PET/CT, and compared the results to
histological outcomes. The results suggested a specificity of
90–97%, a sensitivity of 53–73% and a PPV of 71% for the detection
of LNM in patients with CC. Williams et al (19) evaluated the accuracy of CT, MRI and
FDG-PET in detecting pelvic LNM and verified the outcomes with
histopathological results. The authors evaluated 8 cases and
determined that CT was the most specific method, with a 97%
accuracy rate, followed by MRI and PET, with 90 and 77% accuracy
rates, respectively (19). However,
the sensitivity of all diagnostic modalities assessed was low: 48%
for CT, 54% for MRI and 25% for PET (19). With regard to the results of the
Second International Conference on Cervical Cancer, PET seems to be
the best diagnostic modality, as well as the best non-invasive
method, for the N-staging of CC (9).
Each diagnostic modality has its own individual
strengths and weaknesses. In existing guidelines for the N-staging
of CC, different studies have made various suggestions regarding
the use of PET, PET/CT and MRI (20,21). The
Information Centre for Standards in Oncology of the German Cancer
Society recommended neither PET/CT nor MRI for N-staging in CC
patients, although they recommend the use of PET or ultrasound
scanning for N-staging in specific cases (20). The European Society of Urogenital
Radiology suggested that lymph node detection should be performed
with axial T1-weighted sequences (MRI) to assess suspicious pelvic
and abdominal lymph nodes, as well as the intravenous
administration of gadolinium-chelate for lesions <2 cm (21). The variety of different guidelines
reflects the discussions and controversies regarding the optimum
method for the N-staging of CC.
The current study aimed to critically assess the
usefulness of PET/CT and MRI in the N-staging of diagnosed but
untreated CC patients. Relatively low sensitivity and moderate
specificity, PPV and NPV was observed for PET/CT and MRI in the
pelvic N-staging of CC. However, it is well known that
physiologically enhanced FDG activity in the gut may lead to
false-positive or false-negative results (22). Furthermore, in the present study, MRI
and PET/CT scans were performed at different time-points;
therefore, the anatomic conditions of the urinary bladder and gut
may have been different. Compared with the published data on this
topic, the current study demonstrated that these diagnostic
modalities did not achieve satisfactory results in the N-staging of
CC.
Further medical progress and future technical
developments are necessary to enable the generation of accurate
guidelines for the pelvic N-staging of CC. MRI is already a
valuable diagnostic modality in staging CC and its use has been
suggested in staging guidelines for CC (23). PET/CT is currently effective at
detecting early recurrences in CC patients (24).
In 2006, combined PET/MR imaging was proposed for
imaging patients and the first prototype designs became available
(25,26). Since then, huge progress has been made
regarding methodological approaches and technical versatility
(27–29). At present, three major companies
specializing in imaging hardware offer PET/MRI with various system
designs (30). General expectations
for PET/MRI are high. A recent study assessed the efficacy of
integrated PET/MRI for the whole body staging of patients with
primary CC (31). In their
preliminary results, the authors concluded that integrated PET/MRI
had a high potential to accurately assess primary tumors and detect
LNM in patients with CC.
In the present study, the potential usefulness of
PET/MRI in the N-staging of CC was evaluated in a virtual setting.
This virtual setting comprised the superior and inferior results of
the combined modalities. PET/MRI exhibited a low sensitivity and
moderate specificity, PPV and NPV and, therefore, was not clearly
superior to PET/CT or MRI in the pelvic N-staging of CC. Improved
and optimized protocols for PET/MRI (including contrast-enhanced
MRI, combining FDG uptake with diffusion weighted imaging and
simultaneous data acquisition) may improve the interpretation of
images. However, with regards to N-staging for CC patients, PET/CT
will remain the preferred diagnostic modality for the foreseeable
future, due to its high availability and shorter image acquisition
time.
The current study was limited in a number of ways.
The most significant limitations were the small population, the
retrospective study design and the potential discrepancy between
the results of the diagnostic modalities and histological analysis
(lymph node mapping). With regards to histology, all calculations
were based on a per-patient analysis. PET/MRI was evaluated
virtually; the results were not obtained on an actual PET/MRI
device.
In conclusion, pelvic N-staging in CC remains an
unresolved problem in the clinical setting. Based on the data
analysis performed in the current study, PET/CT and MRI are
suboptimal diagnostic modalities for the pelvic N-staging of CC.
However, they are recommended because of the lack of superior
non-invasive imaging modalities. PET/MRI does not necessarily lead
to better results than PET/CT, and expectations regarding the use
of PET/MRI in this context may be too optimistic.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hiddemann W and Bartram CR: Die Onkologie.
2nd edition. Springer Berlin Heidelberg; Berlin: 2010, View Article : Google Scholar
|
|
3
|
Piver MS, Rutledge F and Smith JP: Five
classes of extended hysterectomy for women with cervical cancer.
Obstet Gynecol. 44:265–272. 1974.PubMed/NCBI
|
|
4
|
Ishikawa H, Nakanishi T, Inoue T and
Kuzuya K: Prognostic factors of adenocarcinoma of the uterine
cervix. Gynecol Oncol. 73:42–46. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kjorstad KE, Kjolvenstvedt A and Strickert
T: The value of complete lymphadenectomy in radical treatment of
cancer of the cervix, stage IB. Cancer. 54:2215–2219. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Noguchi H, Shiozawa I, Sakai Y, Yamazaki T
and Fukuta T: Pelvic lymph node metastasis of uterine cervical
cancer. Gynecol Oncol. 27:150–158. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inoue T and Morita K: The prognostic
significance of number of positive nodes in cervical carcinoma
stages IB, IIA, and IIB. Cancer. 65:1923–1927. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Selman TJ, Mann C, Zamora J, Appleyard TL
and Khan K: Diagnostic accuracy of tests for lymph node status in
primary cervical cancer: A systematic review and meta-analysis.
CMAJ. 178:855–862. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Follen M, Levenback CF, Iyer RB, Grigsby
PW, Boss EA, Delpassand ES, Fornage BD and Fishman EK: Imaging in
cervical cancer. Cancer. 98 Suppl 9:S2028–S2038. 2003. View Article : Google Scholar
|
|
10
|
National Comprehensive Cancer Network
(NCCN): NCCN Clinical practice guidelines in oncology: Cervical
cancer, 10/25/12 update. National Comprehensive Cancer Network.
2013.
|
|
11
|
Park W, Park YJ, Huh SJ, Kim BG, Bae DS,
Lee J, Kim BH, Choi JY, Ahn YC and Lim DH: The usefulness of MRI
and PET imaging for the detection of parametrial involvement and
lymph node metastasis in patients with cervical cancer. Jpn J Clin
Oncol. 35:260–264. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi HJ, Roh JW, Seo SS, Lee S, Kim JY,
Kim SK, Kang KW, Lee JS, Jeong JY and Park SY: Comparison of the
accuracy of magnetic resonance imaging and positron emission
tomography/computed tomography in the presurgical detection of
lymph node metastases in patients with uterine cervical carcinoma:
A prospective study. Cancer. 106:914–922. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eifel PJBJ and Thigpen JT: Cancer of the
cervix, vagina, and vulva, Cancer: principles and practice of
oncology 1433–75. 1997.
|
|
14
|
Son H, Kositwattanarerk A, Hayes MP,
Chuang L, Rahaman J, Heiba S, Machac J, Zakashansky K and
Kostakoglu L: PET/CT evaluation of cervical cancer: Spectrum of
disease. Radiographics. 30:1251–1268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang H, Xie KY and Cao BR: Clinical
analysis of lymph node metastasis in 695 cases of early invasive
cervical carcinoma. Zhonghua Yi Xue Za Zhi. 91:616–618. 2011.(In
Chinese). PubMed/NCBI
|
|
16
|
Winter R, Petru E and Haas J: Pelvic and
para-aortic lymphadenectomy in cervical cancer. Baillieres Clin
Obstet Gynaecol. 2:857–866. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wright JD, Dehdashti F, Herzog TJ, Mutch
DG, Huettner PC, Rader JS, Gibb RK, Powell MA, Gao F, Siegel BA and
Grigsby PW: Preoperative lymph node staging of early-stage cervical
carcinoma by [18F]-fluoro-2-deoxy-D-glucose-positron emission
tomography. Cancer. 104:2484–2491. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sironi S, Buda A, Picchio M, Perego P,
Moreni R, Pellegrino A, Colombo M, Mangioni C, Messa C and Fazio F:
Lymph node metastasis in patients with clinical early-stage
cervical cancer: Detection with integrated FDG PET/CT. Radiology.
238:272–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Williams AD, Cousins C, Soutter WP,
Mubashar M, Peters AM, Dina R, Fuchsel F, McIndoe GA and deSouza
NM: Detection of pelvic lymph node metastases in gynecologic
malignancy: A comparison of CT, MR imaging, and positron emission
tomography. Am J Roentgenol. 177:343–348. 2001. View Article : Google Scholar
|
|
20
|
Beckmann MW: Interdisziplinäre S
2-Leitlinie für die Diagnostik und Therapie des Zervixkarzinoms.
Informationszentrum für Standards in der Onkologie (ISTO). Deutsche
Krebsgesellschaft. 2008.(In German).
|
|
21
|
Balleyguier C, Sala E, Da Cunha T, Bergman
A, Brkljacic B, Danza F, Forstner R, Hamm B, Kubik-Huch R, Lopez C,
et al: Staging of uterine cervical cancer with MRI: Guidelines of
the European Society of Urogenital Radiology. Eur Radiol.
21:1102–1110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shreve PD, Anzai Y and Wahl RL: Pitfalls
in oncologic diagnosis with FDG PET imaging: Physiologic and benign
variants. Radiographics. 19:61–77. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zaspel U and Hamm B: Aktueller Stellenwert
von MRT, CT und PET in der Diagnostik des Zervixkarzinoms. Der
Onkologe. 12:854–868. 2006. View Article : Google Scholar
|
|
24
|
Ryu SY, Kim MH, Choi SC, Choi CW and Lee
KH: Detection of early recurrence with 18F-FDG PET in patients with
cervical cancer. J Nucl Med. 44:347–352. 2003.PubMed/NCBI
|
|
25
|
Schlemmer HP, Pichler BJ, Schmand M,
Burbar Z, Michel C, Ladebeck R, Jattke K, Townsend D, Nahmias C,
Jacob PK, et al: Simultaneous MR/PET imaging of the human brain:
Feasibility study. Radiology. 248:1028–1035. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schmand M, Burbar Z, Corbeil J, Zhang N,
Michael C, Byars L, Eriksson L, Grazioso R, Martin M, Moor A, et
al: BrainPET: First human tomograph for simultaneous (functional)
PET and MR imaging. J Nucl Med Meet. 48 Suppl:45P2007.
|
|
27
|
Lee SI, Catalano O and Dehdashti F:
Evaluation of gynecologic cancer with MR imaging, 18F-FDG PET/CT
and PET/MR imaging. J Nucl Med. 56:436–443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barnwell J, Raptis CA, Mcconathy JE,
Laforest R, Siegel BA, Woodard PK and Fowler K: Beyond whole-body
imaging: Advanced imaging techniques of PET/MRI. Clin Nucl Med.
40:e88–e95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chopra S, Dora T, Dhanda S, Rangrajan V
and Shrivastava Kishore S: PET-MRI based molecular imaging as a
response marker in cervical cancer: A systematic review. Curr Mol
Imaging. 2:66–76. 2013. View Article : Google Scholar
|
|
30
|
Bailey DL, Antoch G, Bartenstein P,
Barthel H, Beer J, Bisdas S, Bluemke D, Boellaard R, Claussen CD,
Franzius C, et al: Combined PET/MR: The real work has just started.
summary report of the third international workshop on PET/MR
Imaging; February 17–21, 2014, Tübingen, Germany. Mol Imaging Biol.
17:297–312. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grueneisen J, Schaarschmidt BM, Heubner M,
Aktas B, Kinner S, Forsting M, Lauenstein T, Ruhlmann V and Umutlu
L: Integrated PET/MRI for whole-body staging of patients with
primary cervical cancer: Preliminary results. Eur J Nucl Med Mol
Imaging. 42:1814–1824. 2015. View Article : Google Scholar : PubMed/NCBI
|