Introduction
Head and neck squamous cell carcinoma (HNSCC), with
600,000 new cases/year, represents the sixth most common tumor
entity worldwide (1). According to
the Robert Koch Institute (Berlin, Germany), ~13,000 new cases were
diagnosed in Germany in 2012 (2).
With respect to the treatment of HNSCC, comprising surgery,
radiation and chemotherapy, the 5-year survival rate has not
markedly changed in recent decades (1,3).
Pembrolizumab and nivolumab, ‘checkpoint inhibitors’ that exhibit
antitumor efficacy in other tumor entities, were recently approved
by the US Food and Drug Administration as the only treatment
innovations in the past decade (4).
Apoptosis is of fundamental importance to normal and
abnormal cells. In numerous diseases, including HNSCC, programmed
cell death is impaired and cells manage to evade apoptosis
(5). There are two main apoptotic
pathways. The extrinsic pathway is activated from the direct
cellular environment by extracellular ligands that bind tumor
necrosis factor receptor-superfamily (TNFR) members, including Fas
or TNFR1. The intrinsic pathway of apoptosis is induced in response
to nuclear stress, resulting in the release of second
mitochondria-derived activator of caspase (SMAC)/direct IAP-binding
protein with low PI, among other factors from the mitochondria, and
the formation of the ‘apoptosome’ (6). Caspase cascades are activated via the
intrinsic and extrinsic pathways and result in apoptosis. The
so-called inhibitor of apoptosis proteins (IAPs) comprise X
chromosome-linked IAP (XIAP), cellular IAP1 (cIAP1) and cIAP2,
among others. These proteins counteract apoptosis by directly
binding and inhibiting caspases and activating the anti-apoptotic
nuclear factor (NF)-κB pathway (7).
Potent antagonists of IAPs, including SMAC, eliminate their
inhibitory effect by directly binding these proteins (8,9). ‘SMAC
mimetics’, synthetic SMAC analogs, including birinapant, which
degrades cIAP1/2 and inhibits XIAP, result in caspase activation by
interacting with IAPs and blocking their signaling pathways
(6). Overexpressed in solid tumors of
the head and neck, IAPs serve an important function as predictors
of the cisplatin response and patient prognosis (10,11). In a
preclinical setting, Matzinger et al (12) demonstrated the radiosensitizing
activity of the SMAC mimetic Debio 1143. Eytan et al
(13) described the antitumor
activity of birinapant alone or in combination with tumor necrosis
factor-α (TNF-α), TNF-related apoptosis inducing ligand and
docetaxel in preclinical models of HNSCC. Furthermore, Sun et
al (10) demonstrated the
chemosensitizing activity of SMAC in combination with different
anticancer agents in vitro. Recently, several early clinical
trials have attempted to evaluate the efficacy of SMAC mimetics
alone or in combination with other anticancer drugs (14).
Based on this evidence, the aim of the present study
was to assess the treatment efficacy of the SMAC mimetic,
birinapant, a bivalent SMAC mimetic targeting cIAP1/2 and XIAP with
Fas ligand (FasL), a Fas-binding ligand, resulting in its
trimerization and activation of the extrinsic pathway. To the best
of our knowledge, the present study is the first to evaluate this
combination treatment in HNSCC cell lines.
Materials and methods
Cell lines
As previously described, the cell lines were
cultured at 37°C in a humidified atmosphere of 5%
CO2/95% air, and the medium was changed 2–3 times/week
(15,16). Certain cell lines (PCI-1, PCI-9,
PCI-13, PCI-52 and PCI-68) were established at the Cancer Institute
of the University of Pittsburgh (Pittsburgh, PA, USA), while the
other cell lines (Detroit 562, FaDu, SCC9 and SCC25) were purchased
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). The cells were cultured at 37°C in Dulbecco's modified
Eagle's medium supplemented with 10% fetal calf serum, and 1%
penicillin/streptomycin (all from Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and 1% glutamine (Biochrom KG, Berlin,
Germany).
Drugs
Birinapant (Medivir AB, Huddinge, Sweden) was stored
according to the manufacturer's protocol. Affinity chromatography
with anti-FLAG M2 agarose beads (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was used to purify human recombinant
FLAG-tagged soluble Fc-Flag-FasL from the supernatants of 293 cells
transfected with the corresponding expression plasmid. FasL
(FasL-Fc is referred to as FasL throughout the paper)
concentrations (50, 25, 12, 6, 3 and 1 ng/µl) and birinapant
concentrations (200, 100, 50, 25, 12, 6, 3, 1 and 0.5 µM) were
derived from log2 dilutions. These findings were based
on multiple experiments, the results of which were consistent with
the literature (13,17). For experiments using combination
treatment with FasL and birinapant, the aforementioned
log2 dilution of FasL and a constant concentration
(IC10) of birinapant were used for each cell line
(Table I). Subsequently, the cultures
were incubated at 37°C for 72 h.
 | Table I.Name and origin of the ten cell lines
used in the present study. |
Table I.
Name and origin of the ten cell lines
used in the present study.
| Cell line | Description |
|---|
| PCI-1 | Laryngeal carcinoma
of the glottis of a male patient |
| PCI-9 | Primary carcinoma of
the tongue of a male patient |
| PCI-13 | Carcinoma of the
retromolar triangle of a male patient |
| PCI-52 | Primary carcinoma
of the aryepiglottic fold of a male patient |
| PCI-68 | Primary tongue
carcinoma of a male patient |
| FaDu | Pharyngeal
carcinoma of a female patient |
| Detroit 562 | Hypopharyngeal
carcinoma of a male patient |
| SCC9 | Carcinoma of the
tongue of a male patient |
| SCC25 | Carcinoma of the
tongue of a male patient |
| HaCaT | Keratinocytes |
FACS analysis and Annexin V assay
Cell surface Fas receptor expression was measured
via flow cytometry (FACS). Anti-cluster of differentiation 95
(CD95)-phycoerythrin (PE) mouse anti-human (BD Biosciences,
Franklin Lakes, NJ, USA) antibody was used to label Fas receptors.
Immunoglobulin G2B isotype control PE antibody (R&D
Systems, Inc., Minneapolis, MN, USA) was used as a negative
control. The reagents were stored and used according to the
manufacturer's instructions.
For the analysis of apoptosis, the cells were
double-stained with Annexin V and 7-AAD using the Annexin V PE
Apoptosis Detection kit (eBioscience Inc.; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
cells were analyzed using a BD FACSCalibur platform with CellQuest
Pro 5.1 software (BD Biosciences). The results are provided as dot
plots with the relative signal intensity of Annexin V vs. 7-AAD.
Unstained cells were used as a negative control, and the dot plots
were subdivided into four quadrants. The graphs were generated
using FlowJo software (version 10; FlowJo LLC, Ashland, OR, USA).
In these FACS plots, necrotic cells are represented in the upper
left quadrant (Q1). Late apoptotic and dead cells are shown in the
upper right quadrant (Q2). Early apoptotic cells are represented in
the lower right quadrant (Q3), and live cells are shown in the
lower left quadrant (Q4). The percentage of the total cell count is
expressed in each quadrant.
Crystal violet assay
Cells of each line (PCI-1, PCI-9, PCI-13, PCI-52,
PCI-68, Detroit 562, FaDu, SCC9, SCC25 and HaCaT) were seeded at
10,000 cells/well at 37°C in DMEM supplemented with 10% fetal calf
serum, 1% penicillin/streptomycin and 1% glutamine. On the
following day, for treatment with a single agent, concentrations of
log2 dilution were added. For combination treatment, the
IC10 of birinapant and a log2 dilution of
FasL were added, followed by incubation at 37°C for 72 h.
Subsequently, the medium was removed, and the remaining cells were
stained at room temperature for 12 min with 50 µl/well crystal
violet solution (1% crystal violet; Carl Roth GmbH, Karlsruhe,
Germany) in 20% methanol/double-distilled water and subsequently
washed several times with distilled water. The plates were then
dried for 24 h. The absorbance was measured at 595 nm using a
microplate reader (Tecan Spectra Rainbow microplate reader; Tecan
Deutschland GmbH, Crailsheim, Germany). All experiments were
performed in triplicate, and the mean was calculated from at least
three independent experiments.
Statistical analysis
Statistical analysis was performed using Prism
(version 6.04; GraphPad Software, Inc., La Jolla, CA, USA) and
Microsoft Excel 2016 software (Microsoft Corporation, Redmond, WA,
USA). P<0.05 was considered to indicate a statistically
significant difference. The Mann-Whitney test was used to compare
mono (FasL) and combination (FasL + birinapant) treatments at
different concentrations in each cell line. Using a nonlinear
regression analysis, the IC10 was calculated for each
cell line. For cell lines for which no IC10 value could
be calculated on the basis of their dose-response curves, the
concentration that resulted in the first significant (P<0.05)
cell count decrease was defined as the ICscr (µM) and
used for combination treatment. Additive and synergistic effects
were distinguished by multiplying the percentage cell number of the
mono treatments (FasL and birinapant). If the result was greater
than the actual percentage cell count of the combination treatment,
the effect was assumed synergistic, if the result was smaller and
the combination treatment revealed increased cell count reductions
compared with both mono treatments, the effects were assumed
additive.
Results
Fas receptor expression in each cell
line
Fas receptor expression was detected in each cell
line (PCI-1, PCI-9, PCI-13, PCI-52, PCI-68, Detroit 562, FaDu,
SCC9, SCC25 and HaCaT) via flow cytometry (Fig. 1). A dot plot for each cell line was
generated to visualize the viable cell fraction. In the histograms,
the light gray peak indicates the isotype control, while the dark
gray indicates the positive control for Fas receptor
expression.
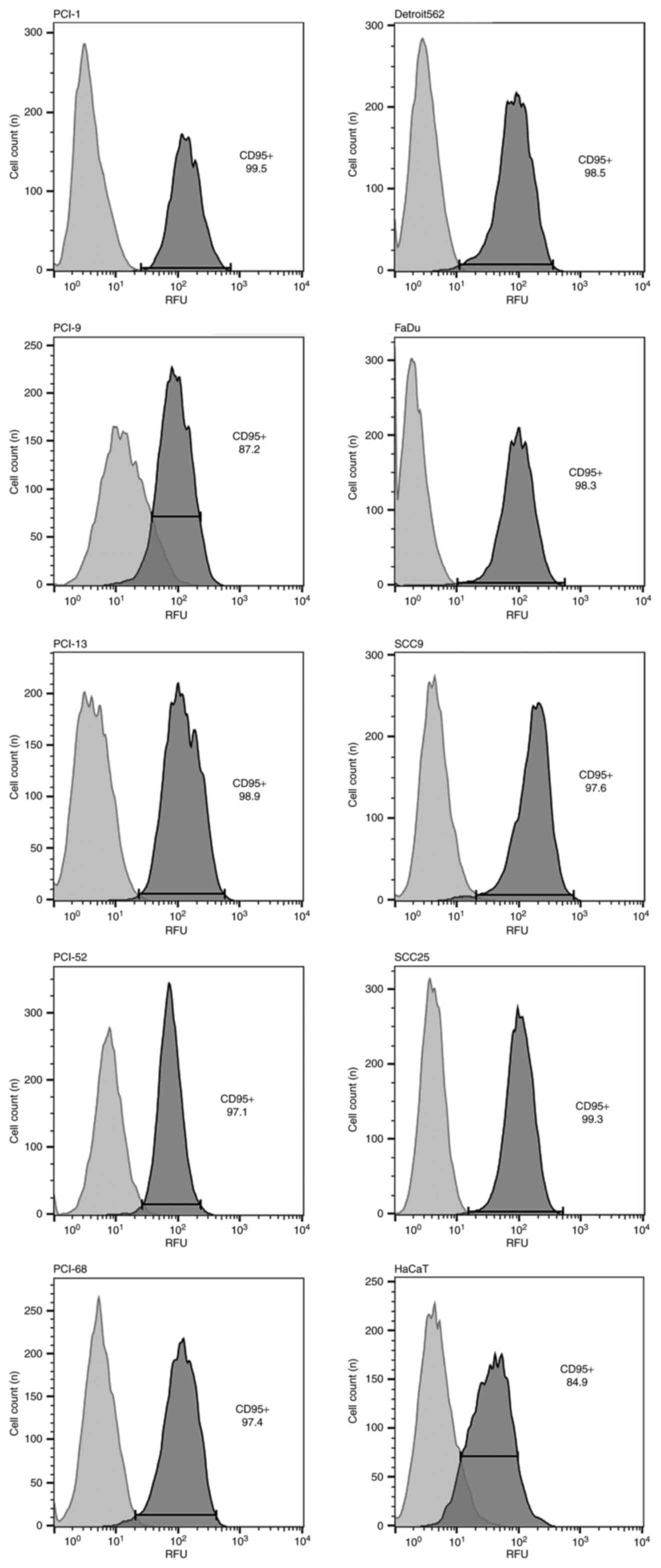 | Figure 1.Results of flow cytometry analysis.
CD95 expression was detected in all cell lines (PCI-1, PCI-9,
PCI-13, PCI52, PCI-68, Detroit 562, FaDu, SCC9, SCC25 and HaCaT).
In the histograms, the light gray peak represents the negative
isotype control, and the dark gray peak represents the positive
control for CD95. CD95, Fas cell surface death receptor. |
Efficacy of FasL in the cell
lines
The majority of the cell lines used in the present
study did not exhibit significant reductions in cell count
following mono treatment after incubation for 72 h with FasL
(Fig. 2). The cell line PCI-1
responded with a 16.81% reduction in initial cell number at a FasL
concentration of 50 ng/µl. The cell lines PCI-9 and PCI-13
exhibited 61.09 and 53.46% reductions in cell count, respectively
at a FasL concentration of 50 ng/µl, while the other cell lines
(PCI-52, PCI-68, Detroit 562, FaDu, SCC9 and SCC25) exhibited only
minimal responses toward the applied concentrations, with cell
count reductions ranging from 82.65–100% of the initial cell
number. In addition, the control cell line HaCaT exhibited only
minor effects when treated with FasL (50 ng/µl), with a cell count
decrease of 69.98%.
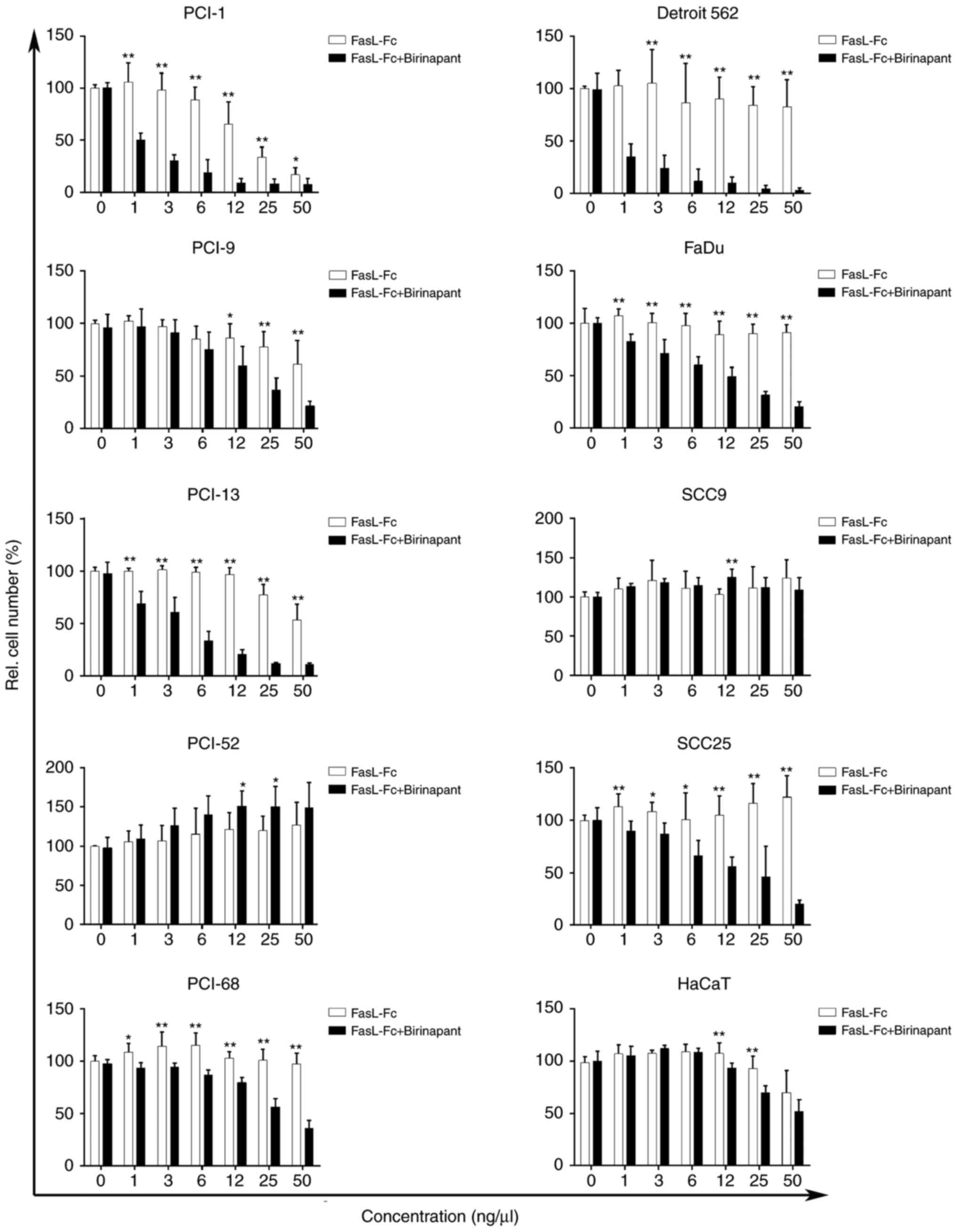 | Figure 2.Relative cell number (%), as measured
using crystal violet assay. As indicated by the white columns, mono
treatment with FasL resulted in a concentration-dependent decrease
in relative cell number in PCI-1, PCI-9 and PCI-13 cell lines. The
PCI-52, PCI-68, Detroit 562, FaDu, SCC9 and SCC25 cell lines
exhibited little to no response toward FasL mono treatment. As
indicated by the black columns, treatment with FasL (log2) +
birinapant (IC10/ICscr) resulted in a
concentration-dependent decrease of the viable cell fraction in
eight cell lines (PCI-1, PCI-9, PCI-13, PCI-68, Detroit 562, FaDu,
SCC25 and HaCaT) with additive and synergistic effects, while two
cell lines (PCI-52 and SCC9) did not respond to exposure to FasL
and birinapant. *P≤0.05; **P≤0.01 (corresponding concentrations of
mono and combination treatment were compared). FasL, Fas ligand-Fc;
rel., relative; IC10, inhibitory concentration of 10%;
ICscr, inhibitory concentration of the first significant
cell number reduction. |
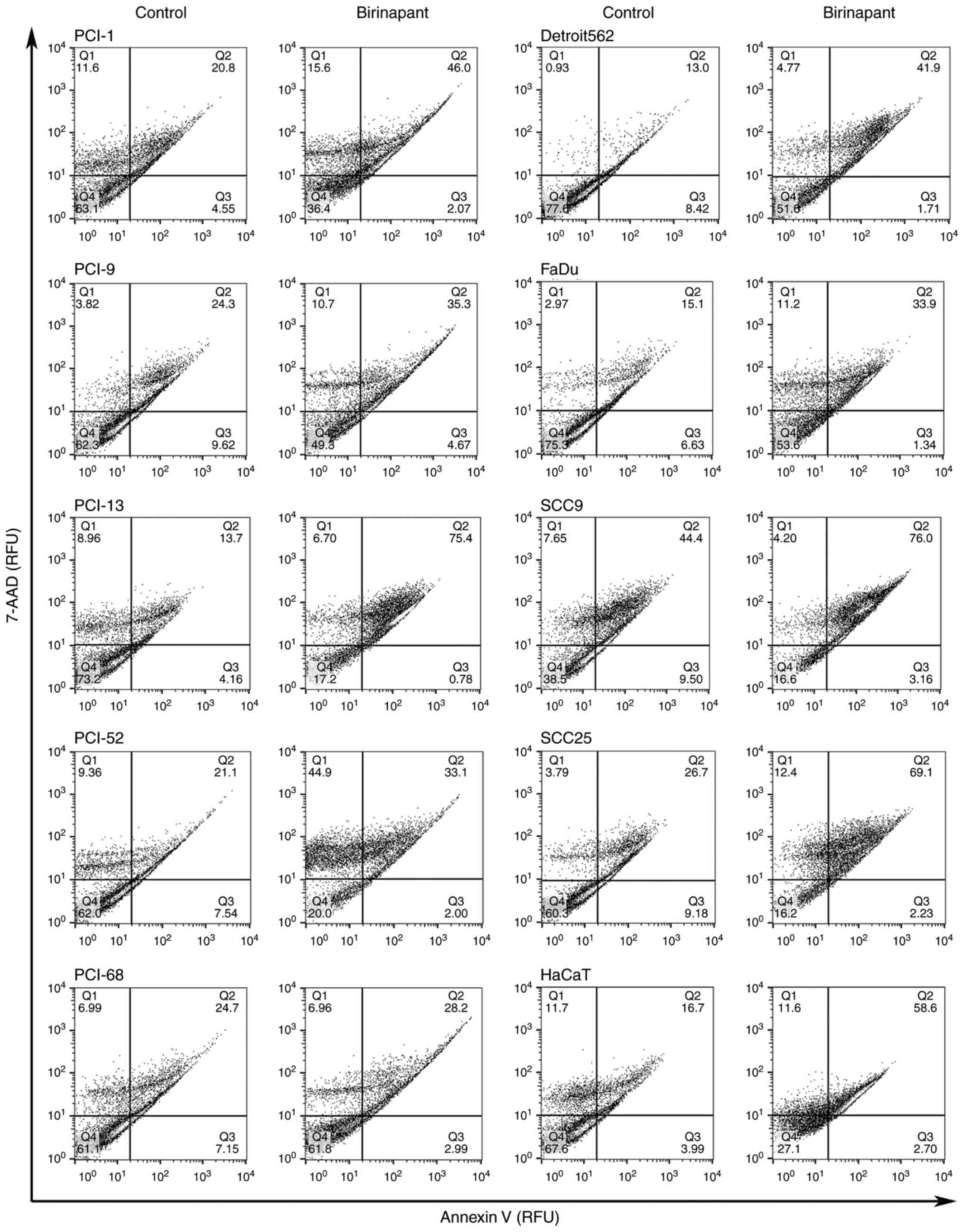
Efficacy of birinapant in the cell
lines
The present study also assessed the efficacy of
birinapant on the cell lines. A crystal violet assay was used to
determine the dose-response curves for mono treatment with
birinapant and to establish the respective inhibitory
concentrations (IC10/ICscr) for each cell
line for use in combination treatment. The calculated
IC10 values were: 18.2 µM for PCI-1, 1.3 µM for PCI-9
and 11.5 µM for PCI-13. The first concentrations generating a
significant cell count reduction, which were subsequently used for
combination treatment, were 100 µM for PCI-52, 25 µM for PCI-68, 50
µM for Detroit 562, 25 µM for FaDu, 1.6 µM for SCC9, 1.6 µM for
SCC25 and 1.6 µM for HaCaT. To confirm the apoptosis-sensitizing
activity of birinapant in these cell lines, flow cytometry analysis
was performed using an Annexin V assay. The apoptosis assay
indicated that the cell lines exhibited mixed responses when
exposed to IC10-/ICscr concentrations
(Fig. 3). While the first image
depicts the control, the second image represents the results of the
apoptosis assay following incubation for 48 h with birinapant at
the cell line-specific concentrations. Out of ten cell lines, four
cell lines (PCI-9, PCI-52, PCI-68 and SCC9) revealed only a slight
effect, with an increase in the specific apoptotic rate at a factor
of 1.14–1.71 following incubation with
IC10/ICscr birinapant. A stronger effect was
achieved following the incubation of the cell lines PCI-1, Detroit
562, FaDu and SCC25 with birinapant, where the apoptotic rate was
increased by a factor of 2.21–3.22. In addition, the control cell
line HaCaT exhibited a 3.5-fold increase in apoptotic rate
following incubation with IC10/ICscr
birinapant. The largest increase in apoptotic rate following
birinapant incubation was observed in the cell line PCI-13 (factor
of 5.5). All cell lines exhibited a specific increase in apoptotic
rate following birinapant treatment.
Apoptosis-sensitizing activity of
combination treatment with FasL and birinapant
The crystal violet assay was used to determine the
dose-response curves for combination treatment with FasL with an
initial concentration of 50 ng/µl log2 dilution and a
constant concentration of birinapant
(IC10/ICscr; Fig.
2). Mono treatment and combination treatment were plotted
against each other. The cell lines PCI-52 and SCC9 did not exhibit
significant reductions in cell number following mono or combination
treatment. In addition, the use of combination treatment did not
reveal any additive or synergistic effects compared with mono
treatment with FasL, which was supported by the finding in the
Annexin V assay with only a slight increase in apoptotic rate
(Fig. 3). However, the cell line
PCI-9 exhibited a positive effect in response to combination
treatment through direct comparison of the different treatment
regimens. Additive effects in PCI-9 were observed at concentrations
of FasL ranging from 3–50 ng/µl and
IC10/ICscr birinapant, and significant
(P<0.05) reductions in cell number were achieved at a
concentration of 12 ng/µl FasL and IC10/ICscr
birinapant in comparison to mono treatment with FasL (12 ng/µl;
Fig. 2). The cell line SCC25
exhibited significant reductions in cell number even when exposed
to the lowest concentration (1 ng/µl) of FasL and
IC10/ICscr birinapant with additive effects,
while synergistic effects were achieved at a concentration of 25
ng/µl FasL and IC10/ICscr birinapant, which
is also consistent with the findings in the apoptosis assay. The
remaining five cell lines (PCI-1, PCI-13, PCI-68, Detroit 562 and
FaDu) responded to combination treatment with significant
(P<0.01 for PCI-1, PCI-13, Detroit 562 and FaDu, P<0.05 for
PCI-68) reductions in cell count when exposed to the lowest
concentration (1 ng/µl) of FasL and
IC10/ICscr birinapant in comparison to mono
treatment with FasL (1 ng/µl), and demonstrated synergistic effects
when exposed to all concentrations (1–50 ng/µl) of FasL and
IC10/ICscr birinapant, which was also
consistent with the results in the Annexin V assay (Fig. 3). Only PCI-1 and PCI-13 cells
exhibited additive effects at the highest combination concentration
(50 ng/µl FasL and IC10/ICscr birinapant).
The HaCaT keratinocyte cell line exhibited slight effects in
response to FasL mono treatment, and additive effects were observed
in response to combination treatment. Significant (p<0.01)
differences in cell number were observed in HaCaT at the
concentrations of 12 and 25 ng/µl FasL and
IC10/ICscr birinapant in comparison to mono
treatment with FasL (12 and 25 ng/µl).
Discussion
The present study suggested an important role for
apoptosis sensitization with SMAC mimetics in the treatment of
HNSCC cell lines. One of the main aims of HNSCC treatment is to
induce apoptosis with an adjuvant concurrent high-dose
platinum-based chemotherapy with single-fraction radiation
(18). A characteristic of all cancer
cells is their ability to evade apoptotic death pathways (5). The most common evasion mechanism is
through the loss of the apoptosis control protein, p53, which is
mutated or absent in half of all types of cancer (19). Similarly, proteins produced by the
human papilloma virus (e.g., E6) bind to p53 to suppress its
function (19). Cancer cells also
overexpress anti-apoptotic proteins, including members of the IAP
family, which are often deregulated or overexpressed in HNSCC
(20,21). Activation of the apoptotic pathway
induces the mitochondrial outer membrane to release SMAC into the
cytosol. Following binding to the corresponding IAPs, a
pro-apoptotic effect is caused by the degradation of proteins by
proteasomes (22,23). Thus far, several monovalent and
bivalent SMAC mimetics have entered clinical testing for dose
escalation, mono therapy or combination therapy with chemotherapy
and/or radiation (12,24–26).
To induce apoptosis via the extrinsic pathway, in
the present study, it was first ensured that the corresponding
receptor was expressed in each cell line examined. Following the
activation of the extrinsic pathway using FasL, a majority of the
cell lines (PCI-52, PCI-68, Detroit 562, FaDu, SCC9 and SCC25) were
not responsive to this treatment. After incubating the cell lines
with birinapant as a single agent, each cell line exhibited an
increase in cell death in the Annexin V assay. Notably, when
exposed to combination treatment (FasL and birinapant
IC10/ICscr), four of the former six
FasL-resistant cell lines (PCI-68, Detroit 562, FaDu and SCC25)
were sensitive to treatment and exhibited synergistic effects,
while the effect of combination treatment was additive and
synergistic with respect to the different concentrations for four
(PCI-1, PCI-9, PCI-13 and HaCaT) of the previously sensitive cell
lines. However, the concentrations of birinapant used could be
limiting with regards to a potential prospective application in
vivo. Only two cell lines (PCI-52 and SCC9) did not respond to
combination treatment with FasL and birinapant. For SCC9, it is
likely that cIAP1 or cIAP2 was not affected by birinapant,
resulting in further inhibition of apoptosis. For the cell line
PCI-52, a previous study by the authors demonstrated the
degradation of cIAP1 through western blot analysis (15). However, a high concentration of
birinapant (Table II) was used to
observe minor effects of mono treatment on the cell line PCI-52 in
the Annexin V assay. Therefore, other anti-apoptotic proteins may
serve important roles in resisting cell death in these cell lines.
According to Khan et al (27),
survivin, another member of the IAP family, which contributes to
radioresistance in HNSCC, is associated with poor outcomes in
multiple types of cancer (28) and
this protein may affect the ongoing chemoresistance in cancer cell
lines. In support of this hypothesis, data from the same research
group revealed the downregulation of survivin by oxaliplatin,
resulting in enhanced radiosensitivity in HNSCC (27). Furthermore, silencing survivin
suppressed cell proliferation and induced caspase-dependent
apoptosis in HNSCC cells (29).
Similarly, the previously identified endogenous inhibitor of
apoptosis cellular FLICE-like inhibitory protein was implicated in
caspase-dependent apoptosis in HNSCC cells (30) by promoting activation of the NF-κB and
extracellular signal-regulated kinase signaling pathways (31).
 | Table II.IC10-/ICscr
concentrations of birinapant. |
Table II.
IC10-/ICscr
concentrations of birinapant.
| Cell line | IC10
(µM) | ICscr
(µM)a |
|---|
| PCI-1 | 18.2 |
|
| PCI-9 | 1.3 |
|
| PCI-13 | 11.5 |
|
| PCI-52 | – | 100 |
| PCI-68 | – | 25 |
| Detroit 562 | – | 50 |
| FaDu | – | 25 |
| SCC9 | – | 1.6 |
| SCC25 | – | 1.6 |
| HaCaT | – | 1.6 |
To the best of our knowledge, the present study is
the first to demonstrate the additive and synergistic effects of
combination therapy with FasL and birinapant for the treatment of
HNSCC. Notably, seven of the nine HNSCC cell lines exhibited
additive and/or synergistic effects when exposed to combination
treatment, indicating a potentially major impact on HNSCC
therapy.
Acknowledgements
The present study was supported by the Comprehensive
Cancer Center Mainfranken and the Interdisciplinary Center for
Clinical Research.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta S, Kong W, Peng Y, Miao Q and
Mackillop WJ: Temporal trends in the incidence and survival of
cancers of the upper aerodigestive tract in Ontario and the United
States. Int J Cancer. 125:2159–2165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moreira J, Tobias A, O'Brien MP and
Agulnik M: Targeted therapy in head and neck cancer: An update on
current clinical developments in epidermal growth factor
receptor-targeted therapy and immunotherapies. Drugs. 77:843–857.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2017. View Article : Google Scholar
|
|
6
|
Fulda S: Promises and challenges of smac
mimetics as cancer therapeutics. Clin Cancer Res. 21:5030–5036.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Derakhshan A, Chen Z and Van Waes C:
Therapeutic small molecules target inhibitor of apoptosis proteins
in cancers with deregulation of extrinsic and intrinsic cell death
pathways. Clin Cancer Res. 23:1379–1387. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: A link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu J and Zhang L: Apoptosis in human
cancer cells. Curr Opin Oncol. 16:19–24. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun Q, Zheng X, Zhang L and Yu J: Smac
modulates chemosensitivity in head and neck cancer cells through
the mitochondrial apoptotic pathway. Clin Cancer Res. 17:2361–2372.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang XH, Feng ZE, Yan M, Hanada S, Zuo H,
Yang CZ, Han ZG, Guo W, Chen WT and Zhang P: XIAP is a predictor of
cisplatin-based chemotherapy response and prognosis for patients
with advanced head and neck cancer. PLoS One. 7:e316012012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matzinger O, Viertl D, Tsoutsou P, Kadi L,
Rigotti S, Zanna C, Wiedemann N, Vozenin MC, Vuagniaux G and
Bourhis J: The radiosensitizing activity of the SMAC-mimetic, Debio
1143, is TNFα-mediated in head and neck squamous cell carcinoma.
Radiother Oncol. 116:495–503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eytan DF, Snow GE, Carlson SG, Schiltz S,
Chen Z and Van Waes C: Combination effects of SMAC mimetic
birinapant with TNFα, TRAIL, and docetaxel in preclinical models of
HNSCC. Laryngoscope. 125:E118–E124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Medicine USNLo: ClinicalTrials.gov, . U.S.
National Library of Medicine. Rockville Pike, Bethesda: 2017,
https://clinicaltrials.gov/ct2/results?cond=solid&term=smac&cntry1=&state1=&Search=SearchAugust
7–2017
|
|
15
|
Brands RC, Herbst F, Hartmann S, Seher A,
Linz C, Kübler AC and Müller-Richter UDA: Cytotoxic effects of
SMAC-mimetic compound LCL161 in head and neck cancer cell lines.
Clin Oral Investig. 20:2325–2332. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brands RC, Muller-Richter UD, De Donno F,
Seher A, Mutzbauer G, Linz C, Kübler AC and Hartmann S:
Co-treatment of wild-type EGFR head and neck cancer cell lines with
afatinib and cisplatin. Mol Med Rep. 13:2338–2344. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eytan DF, Snow GE, Carlson S, Derakhshan
A, Saleh A, Schiltz S, Cheng H, Mohan S, Cornelius S, Coupar J, et
al: SMAC mimetic birinapant plus radiation eradicates human head
and neck cancers with genomic amplifications of cell death genes
FADD and BIRC2. Cancer Res. 76:5442–5454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Adelstein DJ, Li Y, Adams GL, Wagner H Jr,
Kish JA, Ensley JF, Schuller DE and Forastiere AA: An intergroup
phase III comparison of standard radiation therapy and two
schedules of concurrent chemoradiotherapy in patients with
unresectable squamous cell head and neck cancer. J Clin Oncol.
21:92–98. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Samarasinghe B: Hallmarks of Cancer 3:
Evading Apoptosis. Scientific American. 2013.https://blogs.scientificamerican.com/guest-blog/hallmarks-of-cancer-3-evading-apoptosis/August
7–2017
|
|
20
|
De Maria S, Pannone G, Bufo P, Santoro A,
Serpico R, Metafora S, Rubini C, Pasquali D, Papagerakis SM,
Staibano S, et al: Survivin gene-expression and splicing isoforms
in oral squamous cell carcinoma. J Cancer Res Clin Oncol.
135:107–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khan Z and Bisen PS: Oncoapoptotic
signaling and deregulated target genes in cancers: Special
reference to oral cancer. Biochim Biophys Acta. 1836:123–145.
2013.PubMed/NCBI
|
|
22
|
Chen DJ and Huerta S: Smac mimetics as new
cancer therapeutics. Anticancer Drugs. 20:646–658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koff JL, Ramachandiran S and
Bernal-Mizrachi L: A time to kill: Targeting apoptosis in cancer.
Int J Mol Sci. 16:2942–2955. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amaravadi RK, Schilder RJ, Martin LP,
Levin M, Graham MA, Weng DE and Adjei AA: A phase I study of the
SMAC-mimetic birinapant in adults with refractory solid tumors or
lymphoma. Mol Cancer Ther. 14:2569–2675. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Infante JR, Dees EC, Olszanski AJ, Dhuria
SV, Sen S, Cameron S and Cohen RB: Phase I dose-escalation study of
LCL161, an oral inhibitor of apoptosis proteins inhibitor, in
patients with advanced solid tumors. J Clin Oncol. 32:3103–3110.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tolcher AW, Bendell JC, Papadopoulos KP,
Burris HA, Patnaik A, Fairbrother WJ, Wong H, Budha N, Darbonne WC,
Peale F, et al: A phase I dose-escalation study evaluating the
safety tolerability and pharmacokinetics of CUDC-427, a potent,
oral, monovalent IAP antagonist, in patients with refractory solid
tumors. Clin Cancer Res. 22:4567–4573. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Khan Z, Khan N, Tiwari RP, Patro IK,
Prasad GB and Bisen PS: Down-regulation of survivin by oxaliplatin
diminishes radioresistance of head and neck squamous carcinoma
cells. Radiother Oncol. 96:267–273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qi G, Kudo Y, Ando T, Tsunematsu T,
Shimizu N, Siriwardena SB, Yoshida M, Keikhaee MR, Ogawa I and
Takata T: Nuclear Survivin expression is correlated with malignant
behaviors of head and neck cancer together with Aurora-B. Oral
Oncol. 46:263–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khan Z, Tiwari RP, Khan N, Prasad GB and
Bisen PS: Induction of apoptosis and sensitization of head and neck
squamous carcinoma cells to cisplatin by targeting survivin gene
expression. Curr Gene Ther. 12:444–53. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Irmler M, Thome M, Hahne M, Schneider P,
Hofmann K, Steiner V, Bodmer JL, Schröter M, Burns K, Mattmann C,
et al: Inhibition of death receptor signals by cellular FLIP.
Nature. 388:190–195. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kataoka T, Budd RC, Holler N, Thome M,
Martinon F, Irmler M, Burns K, Hahne M, Kennedy N, Kovacsovics M
and Tschopp J: The caspase-8 inhibitor FLIP promotes activation of
NF-kappaB and Erk signaling pathways. Curr Biol. 10:640–648. 2000.
View Article : Google Scholar : PubMed/NCBI
|