Introduction
Glioma is the most prevalent and lethal primary
brain tumor in adult humans and is characterized by excessive tumor
proliferation, diffused infiltration and immunosuppression
(1,2).
Although patients with malignant gliomas undergo aggressive
treatment, for example surgical resection combined with
radiotherapy and chemotherapy, high rates of morbidity and
mortality continue to prevail. For glioblastomas in particular, the
overall survival period is only ~15 months post-diagnosis (3). Therefore, there is an imperative
requirement to develop novel therapeutic strategies and identify
more specific biomarkers for the diagnosis and treatment of
gliomas. Immunotherapeutic strategies have garnered increasing
attention as a potential treatment approach for gliomas.
Carcinoembryonic antigen-related cell adhesion
molecule 1 (CEACAM1) is expressed on the surface of a wide variety
of epithelial cells and immune cells. CEACAM1 is considered to be a
specific biomarker, associated with tumor progression, metastasis
and poor prognosis (4–6). Additionally, previous studies have
demonstrated that CEACAM1 is an immune checkpoint regulator
expressed on the surface of effector immune cells, and is
considered to be a crucial molecule in the downregulation of immune
responses (7,8). CEACAM1 may inhibit cytotoxicity and
attenuate antitumor immunity in natural killer (NK) cells (9,10).
Coincidentally, CEACAM1-silenced tumor cells exhibit a greater
sensitivity to the cytotoxic effects of NK cells (11). Furthermore, it has been demonstrated
that antitumor effects may be enhanced in malignant melanoma using
monoclonal antibodies to block the inhibitory CEACAM1 pathway
(12). Notably, a combined treatment
of cytokine-induced killer cells (CIK) with CEACAM1-specific
antibodies has demonstrated an enhanced therapeutic efficacy in
patients with lung cancer compared with CIK therapy alone (13). Thus, CEACAM1 may be a promising target
candidate for tumor immunotherapies.
Homophilic interactions of CEACAM1, occurring
between CEACAM1+ tumor-infiltrating lymphocytes (TILs)
and CEACAM1+ tumor cells, may inhibit multiple effector
functions of TILs (8,14,15).
Therefore, tumors may adopt certain mechanisms that utilize key
immune checkpoint molecules in order to avoid immunological attacks
(16). In fact, gliomas may form an
immunosuppressive environment that inhibits the antitumor immune
response through the dysregulated expression of inhibitory immune
checkpoint molecules [including B7-H3 (17,18),
galectin-9 (19) and programmed death
ligand 1 (20)] on the cell surface.
These studies imply that the immune checkpoint regulation performed
by CEACAM1 homophilic interactions may serve a pivotal function in
the downregulation of immunological responses to tumors and may
influence the progression of tumors.
However, currently, to the best of our knowledge,
there have been no studies investigating the function of CEACAM1 as
an immune checkpoint molecule in the immune microenvironment of
gliomas. In the present study, the expression levels of CEACAM1 in
T cells and tumor tissues, and their association with various
clinicopathological characteristics in patients with gliomas, were
assessed.
Materials and methods
Characteristics of the study
population
A total of 51 glioma tissues and peripheral blood
samples were obtained from patients with glioma at the First
Hospital of Shanxi Medical University (Taiyuan, China) between May
2013 and March 2016. All the patients were newly diagnosed and
confirmed as patients with gliomas through histopathological
examination and had not received any tumor treatment prior to
surgical operation. The glioma tissues were pathologically graded
according to the World Health Organization (WHO) classification of
the central nervous system tumors (21). Patients with other diseases were
excluded, including those with severe infections and other types of
tumor. The clinical information of patients with gliomas was
obtained from their medical records. All the healthy control
subjects were matched with the patients with gliomas according to
sex and age. Additionally, 10 non-tumorous brain tissues, obtained
from patients with severe craniocerebral injuries who were
undergoing surgical treatment, were selected as control tissues.
All participants provided written informed consent, and the present
study was approved by the Clinical Research Ethics Committee of
Shanxi Medical University (Taiyuan, China).
Flow cytometry
TILs were separated from fresh glioma tissues, as
follows. Tumor tissues were minced with sterile tissue scissors,
and then digested with collagenase IV (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), hyaluronidase (Shanghai Seebio
Biological Technology Co., Ltd., Shanghai, China) and DNase I
(Zhejiang Ontores Biotechnologies Co., Ltd., Zhejiang, China) in
sterile PBS (Wuhan Boster Biological Technology, Ltd., Wuhan,
China), at 37°C for 45 min. The digested tissues were filtered
using a 100 µm stainless steel filter, and the resulting cells were
separated with Percoll solution (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 800 × g for 20 min at room temperature.
Mononuclear cells were isolated from venous blood using Ficoll
density gradient centrifugation (Tianjin Haoyang Biological
Products Technology Co., Ltd., Tianjin, China), according to the
manufacturer's instructions. Cells were incubated at 4°C for 30 min
in a dark room with the following monoclonal antibodies:
Phycoerythrin/cyanine-5-conjugated anti-human cluster of
differentiation 3 (CD3) (cat. no. 15-0038-42, 6 µg/ml−1;
eBioscience; Thermo Fisher Scientific, Inc.),
allophycocyanin-conjugated anti-human CD4 (cat. no. 17-0049-42, 50
µg/ml−1; eBioscience; Thermo Fisher Scientific, Inc.),
fluorescein isothiocyanate (FITC)-conjugated anti-human CEACAM1
(cat. no. sc-70450, 100 µg/ml−1; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), and FITC-conjugated mouse
immunoglobulin G1κ isotype control antibody (cat. no. 11-4714-41;
100 µg/ml−1; eBioscience; Thermo Fisher Scientific,
Inc.). In total, ≥10,000 cells were collected and analyzed using a
BD FACSCanto™ II flow cytometer (BD Biosciences, Franklin Lakes,
NJ, USA). The data were analyzed using FlowJo software (version
5.0; FlowJo, LLC, Ashland, OR, USA).
ELISA
Venous blood was centrifuged at 800 × g for 8 min at
room temperature. Plasma was collected and stored at −80°C in a
freezer. The concentrations of interferon (IFN)-γ (pg/ml) were
identified using an ELISA kit (cat. no. EK0373; Wuhan Boster
Biological Technology, Ltd.), according to the manufacturer's
instructions.
Immunohistochemistry (IHC)
A total of 51 glioma tissues and 10 non-tumorous
brain tissues were collected. The formalin-fixed and
paraffin-embedded tissues were cut into sections 3–4 µm thick.
CEACAM1 expression was determined using the streptavidin-biotin
complex IHC method using a diaminobenzidine kit (cat. no. AR1000;
Wuhan Boster Biological Technology, Ltd.), according to the
manufacturer's instructions. Tissue sections were incubated with
rabbit anti-human CEACAM1 monoclonal antibody at 37°C for 2 h.
(dilution 1:100, cat. no. EPR4048; Abcam, Cambridge, UK). Sections
were viewed in five random fields at ×400 magnification, using
light microscopy, and the positive rate and staining intensity of
CEACAM1 were analyzed using the Aperio Digital Pathology Slide
Scanner (Leica Microsystems, Inc., Buffalo Grove, IL, USA). CEACAM1
expression was scored using the immunoreactive scoring (IRS)
method, according to the distribution and staining intensity. IRS =
percentage of positive cells (PP) × staining intensity (SI). The PP
was scored as follows: 0%, 0; 0–25%, 1; 25–50%, 2; 50–75%, 3; and
75–100%, 4. The SI was determined as follows: Absent, 0; weak, 1;
moderate, 2; and strong, 3 (19).
Statistical analyses
Statistical analyses were performed using GraphPad
Prism (version 5.0; GraphPad Software Inc., La Jolla, CA, USA) and
SPSS (version 19.0; IBM Corp, Armonk, NY, USA). Data are presented
as the mean ± standard error of the mean. Analysis of variance with
Kruskal-Wallis and Nemenyi post hoc tests, and Spearman's
correlation analysis were used to analyze the differences and
correlations, respectively, between the different study groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characteristics of the study
population
In total, 51 patients with glioma and 30 healthy
control subjects were selected. Their clinical and pathological
characteristics are presented in Table
I. Patients with glioma and the control subjects did not
significantly differ in age or sex (P>0.05). According to the
WHO classification of glioma grades, patients with glioma were
classified as follows: Four grade I patients; 11 grade II patients;
26 grade III patients; and 10 grade IV patients. According to the
clinical records, the Karnofsky scores (22) of patients with glioma were calculated
as follows: ≥80, 19 individuals; 60–80, 25 individuals; and <60,
7 individuals.
 | Table I.Study populations. |
Table I.
Study populations.
| Variable | Patients with
glioma (%) (n=51) | Healthy controls
(%) (n=30) | P-value |
|---|
| Age, years |
|
| N.S. |
|
≥60 | 28 (54.9) | 16 (53.3) |
|
|
<60 | 23 (45.1) | 14 (46.7) |
|
| Sex |
|
| N.S. |
|
Male | 30 (58.8) | 17 (56.7) |
|
|
Female | 21 (41.2) | 13 (43.3) |
|
| WHO grade |
|
|
|
| I | 4 (7.8) |
|
|
| II | 11 (21.6) |
|
|
|
III | 26 (51.0) |
|
|
| IV | 10 (19.6) |
|
|
| Karnofsky
score |
|
|
|
|
≥80 | 19 (37.3) |
|
|
|
60–80 | 25 (49.0) |
|
|
|
<60 | 7 (13.7) |
|
|
Increased expression of CEACAM1 on
peripheral and tumor-infiltrating T cells in patients with
glioma
To determine the function of CEACAM1 in gliomas, the
expression of CEACAM1 on PBMCs and TILs obtained from patients with
gliomas and PBMCs obtained from control subjects were analyzed
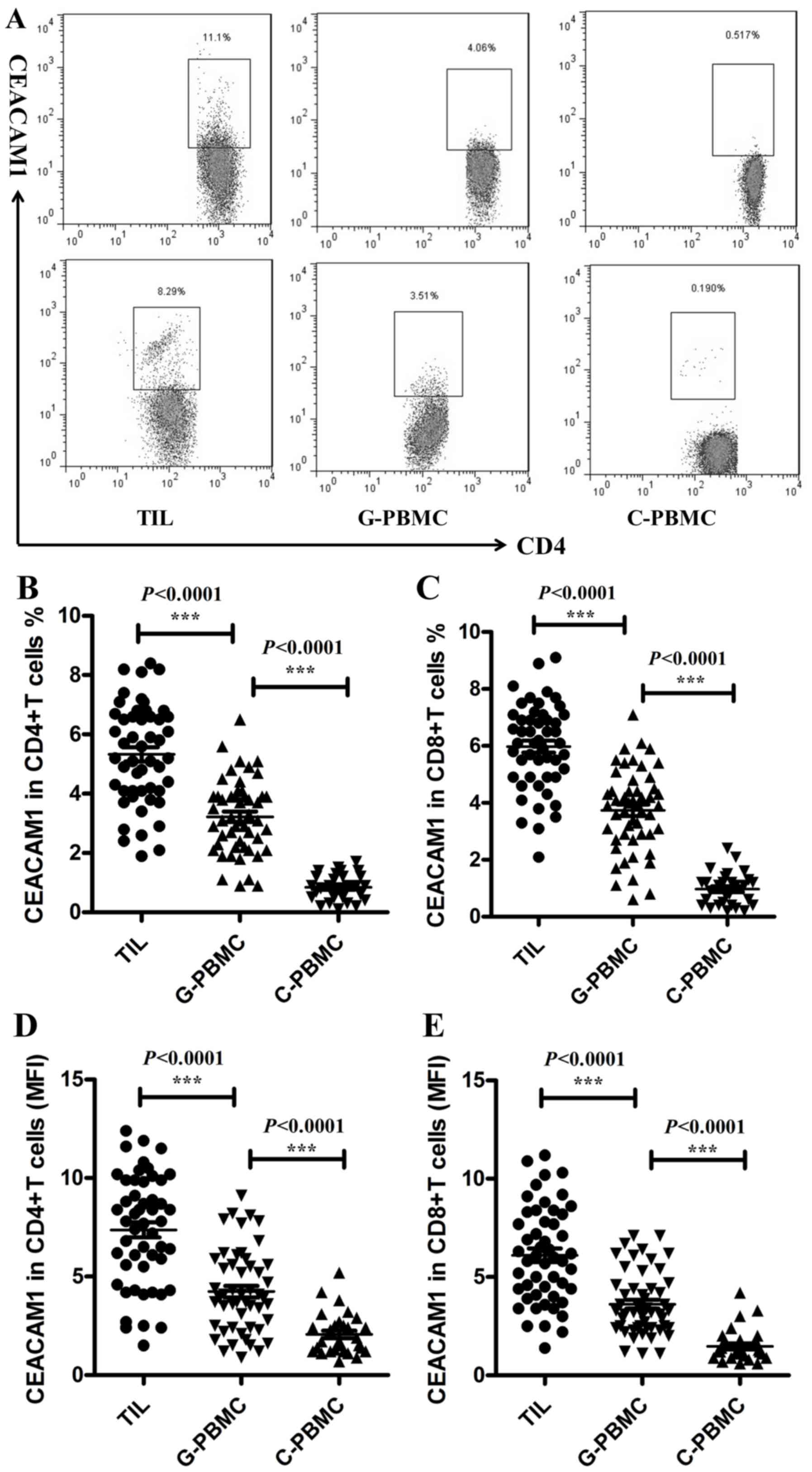
using flow cytometry. As presented in Fig. 1A and B, significant increases in the
proportion of CEACAM1+ cells were observed among
CD4+ T cells (TILs, 5.33±0.24%, n=51; patient PBMCs,
3.22±0.17%, n=51; control PBMCs, 0.84±0.08%, n=30; P<0.0001) and
among CD8+ T cells (Fig.
1C; TILs, 5.98±0.21%, n=51; patient PBMCs, 3.74±0.20%, n=51;
control PBMCs, 0.97±0.10%, n=30; P<0.0001) in PBMCs of patients
with glioma, and this percentage was increased further in TILs of
patients with glioma. Similar results were observed when the mean
fluorescence intensity (MFI) of CEACAM1 on CD4+T cells
(Fig. 1D; TILs, 7.37±0.39%, n=51;
patient PBMCs, 4.24±0.29%, n=51; control PBMCs, 2.07±0.41%, n=30;
P<0.0001) and CD8+ T cells (Fig. 1E; TILs, 6.11±0.34%, n=51; glioma
patient PBMCs, 3.62±0.23%, n=51; control PBMCs, 1.47±0.15%, n=30;
P<0.0001) was analyzed. The increased expression of CEACAM1 on T
cells may indicate that CEACAM1 participates in the tumorigenesis
of gliomas.
Expression of CEACAM1 on T cells is
associated with different glioma grades
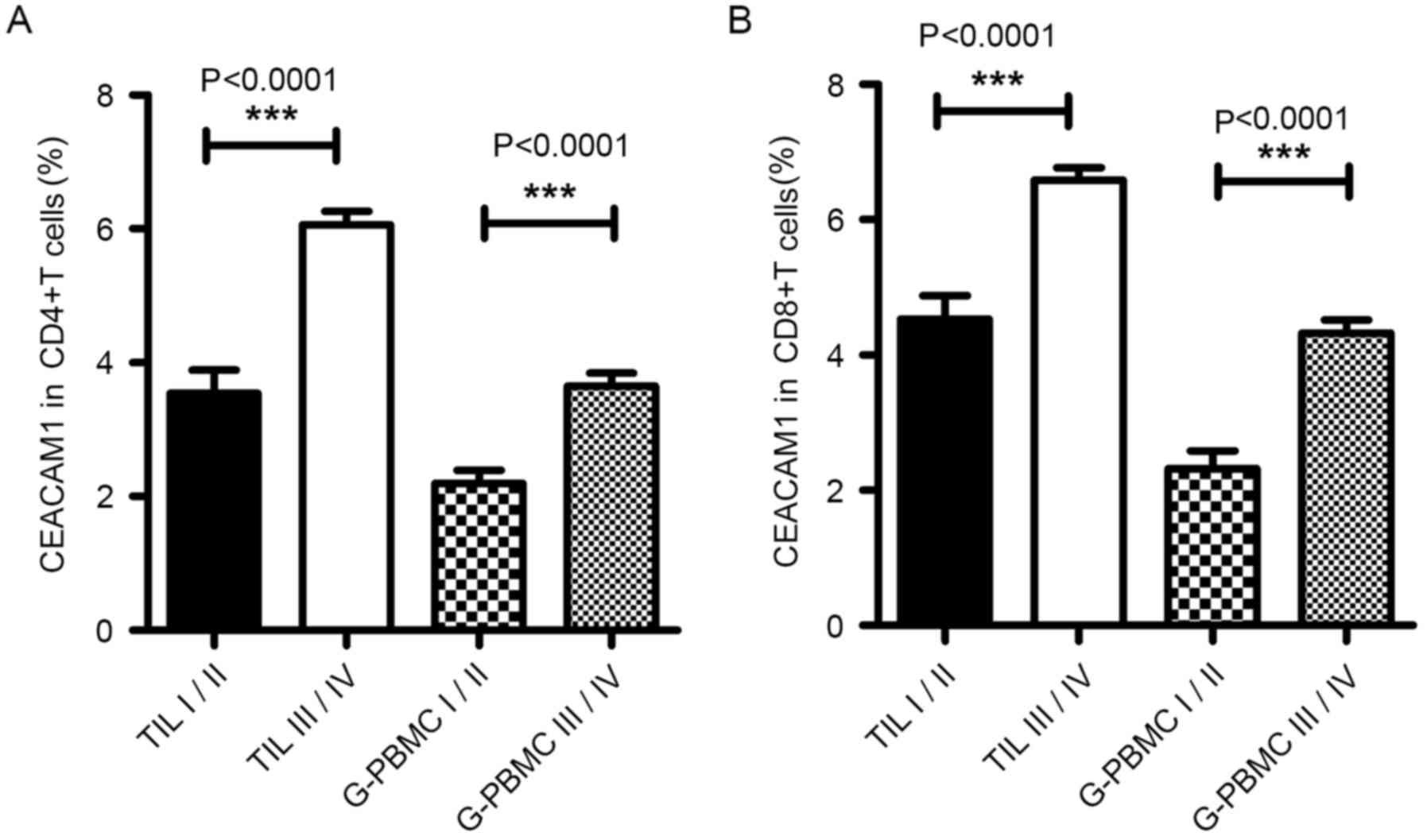
The WHO classification of glioma grades is based on
the malignancy of the tumors and is broadly used as a prognostic
factor for gliomas. High-grade gliomas (WHO grade III–IV) are
poorly differentiated or undifferentiated, progress rapidly and are
associated with a poorer overall survival rate, compared with
low-grade gliomas. The association between the expression of
CEACAM1 on T cells and different glioma grades among patients was
analyzed. As presented in Fig. 2A and
B, expression of CEACAM1 on the CD4+ T cells (glioma
grade I–II, 2.20±0.20%, n=15; glioma grade III–IV, 3.65±0.19%,
n=36; P<0.0001) and CD8+ T cells (glioma grade I–II,
2.32±0.27%, n=15; and glioma grade III–IV, 4.33±0.19%, n=36;
P<0.0001) in PBMCs was significantly increased in high-grade
gliomas compared with low-grade gliomas; the expression of CEACAM1
on CD4+ T cells (glioma grade I–II, 3.55±0.34%, n=15;
glioma grade III–IV, 6.07±0.20%, n=36; P<0.0001) and
CD8+ T cells (glioma grade I–II, 4.53±0.34%, n=15;
glioma grade III–IV, 6.59±0.18%, n=36; P<0.0001) was further
increased in TILs, suggesting that the proportion of
CEACAM1+ T cells in patients with glioma was associated
with the WHO grade of the glioma. These results indicated that the
expression levels of CEACAM1 in T cells were associated with the
glioma grade and that CEACAM1 may be used as a specific biomarker
for the prognosis of glioma.
Expression of CEACAM1 on T cells is
negatively correlated with different Karnofsky scores in patients
with glioma
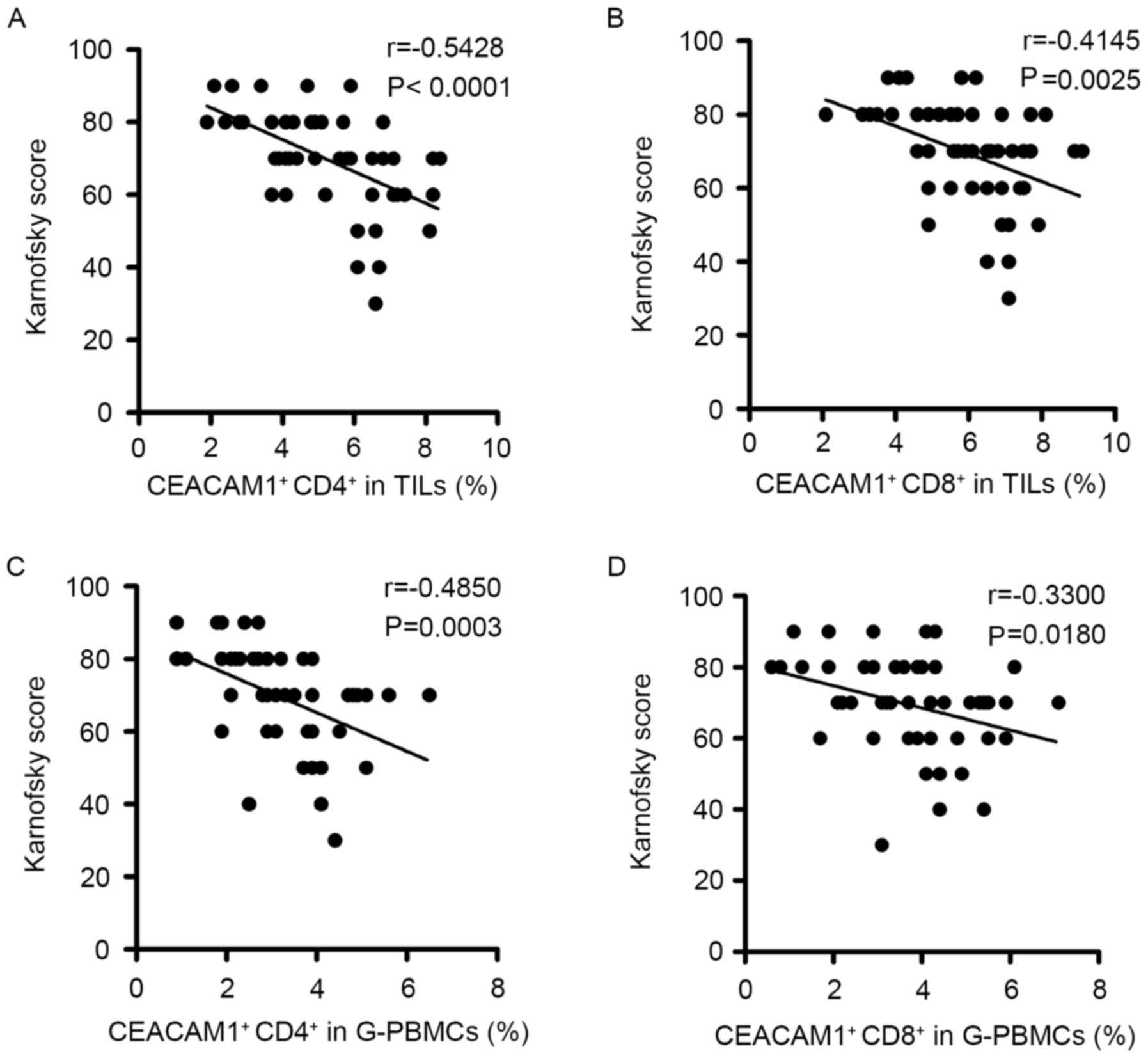
The Karnofsky score is widely used to assess the
severity of the cancer in patients; a lower Karnofsky score
indicates poorer prognosis (23). The
correlation between the expression of CEACAM1 on T cells in
patients with glioma and their Karnofsky scores was analyzed
(Fig. 3). The results revealed that
the frequencies of CEACAM1 expressed on CD4+ T cells
(TILs, r=−0.5428, P<0.0001; PBMCs, r=−0.4850, P=0.0003; Fig. 3A and C, respectively) and
CD8+ T cells (TILs, r=−0.4145, P=0.0025; PBMCs,
r=−0.3300, P=0.0180; Fig. 3B and D,
respectively) in TILs and PBMCs were negatively correlated with the
Karnofsky scores of patients with glioma. These data suggested that
the expression levels of CEACAM1 on T cells may be significantly
associated with the severity of the glioma.
Expression of CEACAM1 is negatively
correlated with the plasma IFN-γ level in patients with glioma
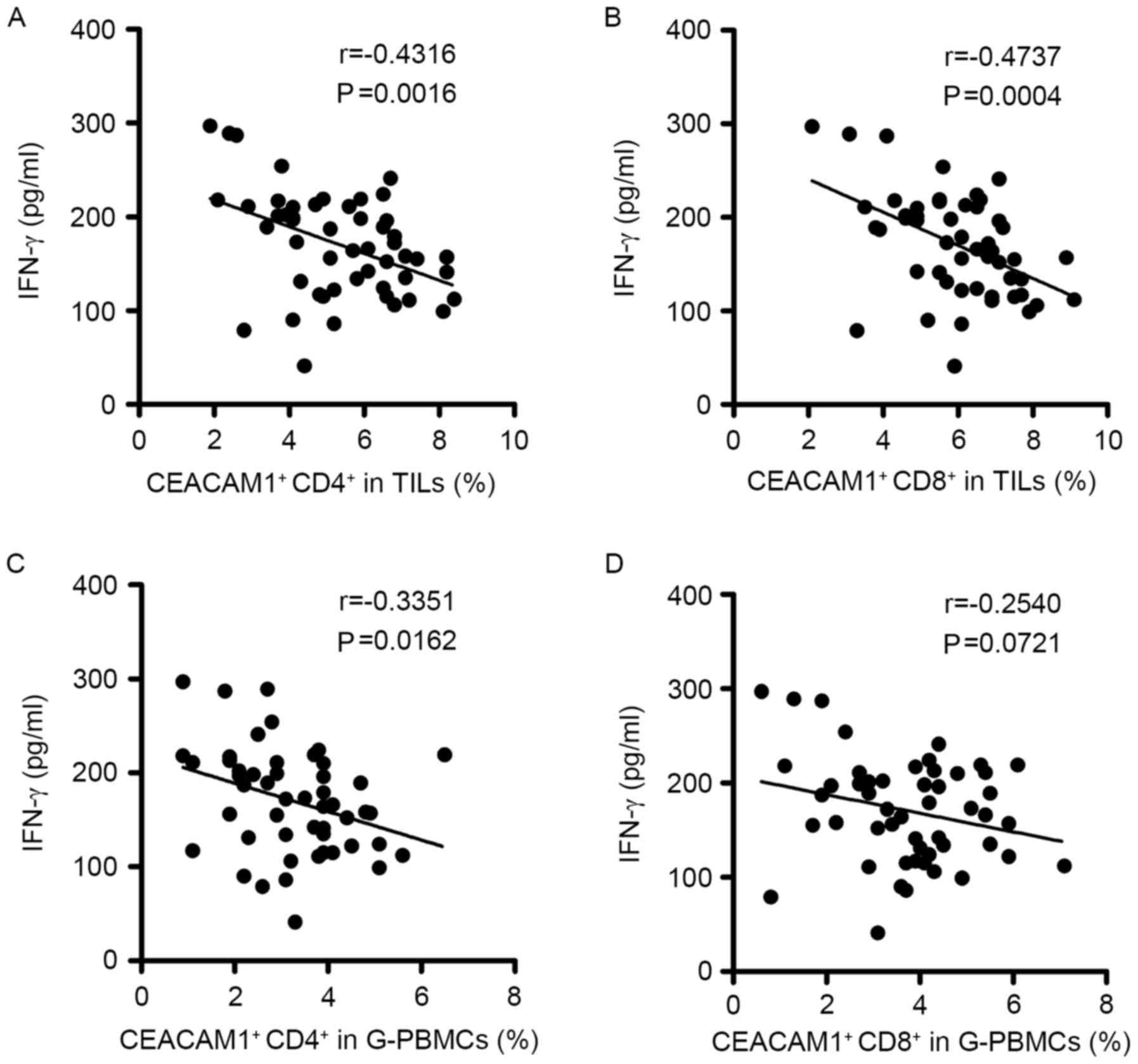
IFN-γ is secreted by activated immune cells and
serves a vital function in antitumor immunity (24). Therefore, the plasma IFN-γ levels of
patients with glioma was analyzed using an ELISA (Fig. 4). The data indicated that plasma IFN-γ
levels were negatively correlated with the expression of CEACAM1 on
CD4+ T cells in TILs (r=−0.4316, P=0.0016; Fig. 4A) and PBMCs (r=−0.3351, P=0.0162;
Fig. 4C). CEACAM1+
CD8+ T cells in TILs were also negatively correlated
with the plasma IFN-γ levels of patients with glioma (r=−0.4737,
P=0.0004; Fig. 4B), whereas those in
PBMCs did not demonstrate any significant correlation with IFN-γ
levels in serum (r=−0.2540, P=0.0721; Fig. 4D). In general, these data demonstrated
that the expression levels of CEACAM1 in the T cells of patients
with glioma were associated with the antitumor factor IFN-γ, and
indicated that increased CEACAM1 expression may have a negative
regulatory effect on T cells by suppressing the IFN-γ-secreting
capability of T cells.
CEACAM1 expression in glioma tissues
is positively associated with the proportion of CEACAM1+
T cells in TILs and the glioma grade
Homophilic interactions of CEACAM1 may occur between
CEACAM1+ TILs and CEACAM1+ tumor cells, which
leads to the apoptosis of CEACAM1+ T cells (8,14). Thus,
the homophilic interactions of CEACAM1 may serve a pivotal function
in immunosuppression. To assess the importance of CEACAM1
homophilic interactions in gliomas, further investigations were
made into the expression of CEACAM1 in glioma tissues and the
control brain tissues using IHC analysis. Negligible or no staining
of CEACAM1 in the control brain tissues was observed; however, an
increased positive expression of CEACAM1 was observed in the tumor
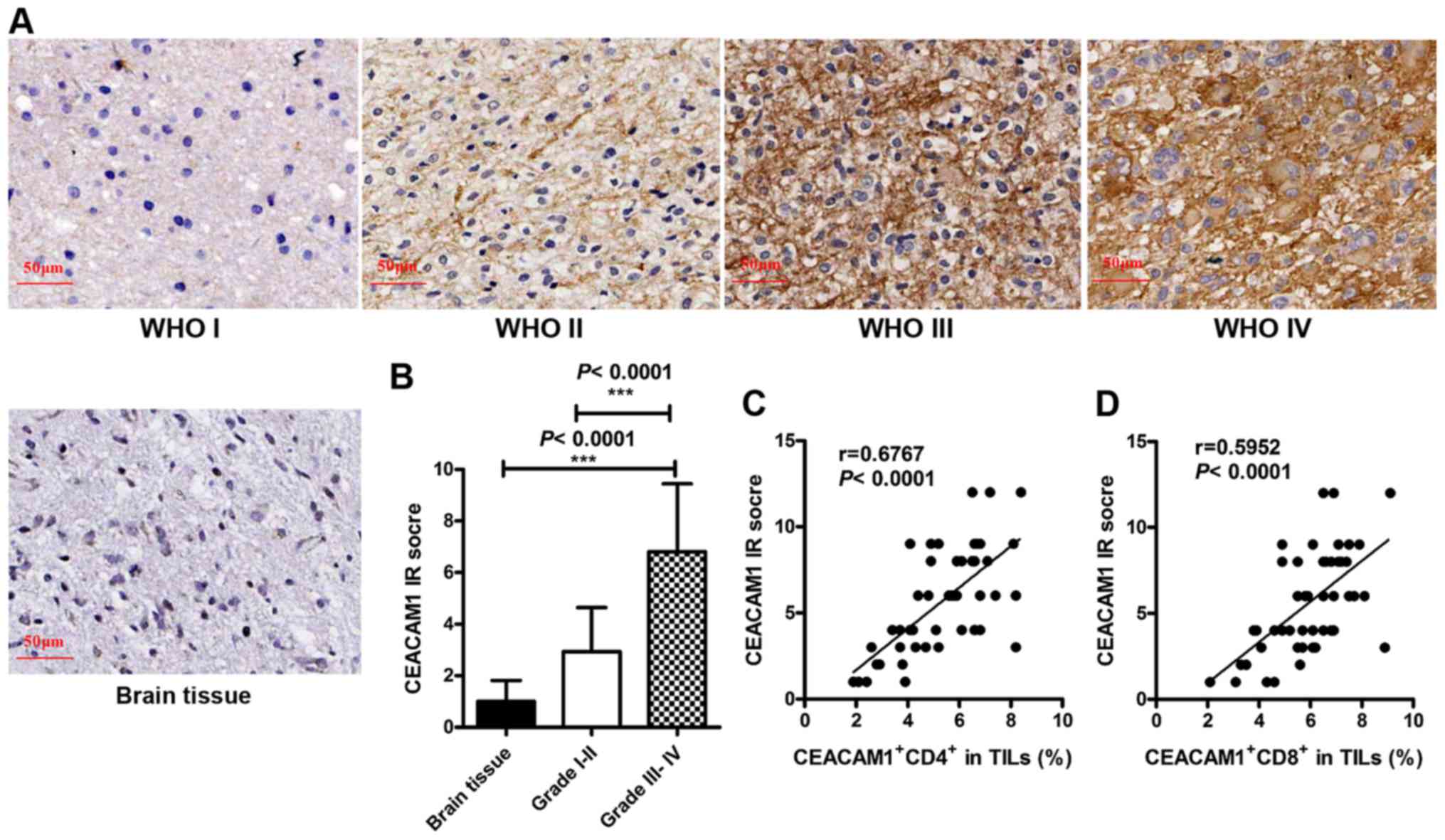
tissues of patients with glioma following IHC staining (Fig. 5A). Additionally, IRS was used (based
on the distribution and intensity of CEACAM1 staining) to score the
expression levels of CEACAM1. The data revealed that in patients
with glioma, the expression levels of CEACAM1 in tumor tissues were
associated with the grade of the glioma. Compared with control
brain tissues, CEACAM1 expression in grade III–IV glioma was
increased; however, the difference was not statistically
significant in grade I–II glioma (brain tissue, 1.000±0.25822,
n=30; glioma grade I–II, 2.933±0.4415, n=15; P=0.003; glioma grade
III–IV, 6.806±0.4397, n=36; P<0.0001; Fig. 5B). Furthermore, CEACAM1 expression in
tumor tissues was positively correlated with the proportion of
CEACAM1+ CD4+ T cells (r=0.6767, P<0.0001;
Fig. 5C) and CEACAM1+
CD8+ T cells in TILs (r=0.5952, P<0.0001; Fig. 5D). These observations suggested that
the expression of CEACAM1 on T cells and glioma cells was
increased, indicating that the homophilic interactions of CEACAM1
may be crucial in the development and progression of gliomas.
Discussion
CEACAM1 serves a crucial function in immune
regulation and the evasion of immune surveillance. However, the
effects of CEACAM1 on the immunoenvironment of gliomas had not
previously been investigated. In the present study, the expression
levels of CEACAM1 in glioma were determined and the association
between CEACAM1 expression and multiple clinicopathological
parameters in patients with glioma were analyzed. To the best of
our knowledge, the results of the present study provide the first
evidence indicating that CEACAM1 homophilic interactions
participate in the development and progression of gliomas,
potentially by regulating CD4+ and CD8+ T
cell subsets.
Glioma cells may evade immune surveillance and
escape immune attacks by creating immunosuppressive environments
(25). One of the key mechanisms for
immunosuppression involves the upregulated expression of immune
checkpoint molecules [including programmed death ligand 1,
cytotoxic T lymphocyte-associated antigen-4, and CEACAM1 (26–28)] on
activated TILs. The dysregulated expression of CEACAM1 in tumors is
a key factor in the immune interaction between tumor cells and
lymphocytes. This interaction inhibits T cell-mediated
immunological cytotoxicity, which protects tumor cells from immune
attacks (8). Additionally, blocking
the CEACAM1 signaling pathway using specific monoclonal antibodies
has been demonstrated to be a promising method for reversing the
immune resistance of tumors (12).
The results of the present study identified that the expression
levels of CEACAM1 were increased in circulating CD4+ and
CD8+ T cells compared with those in control subjects,
and further increased in the CD4+ and CD8+ T
cells in tumor tissues of patients with glioma. Additional results
include that the expression of CEACAM1 on T cells in patients with
high-grade glioma was increased further compared with that in
patients with low-grade glioma. IHC analysis revealed that CEACAM1
expression was increased in glioma tissues compared with that in
brain tissues of control subjects, and the expression further
increased in high-grade gliomas. Taken together, these results
suggested that CEACAM1 may be a potential biomarker for the
prognosis of glioma, and the homophilic interactions of CEACAM1 may
be involved in the development of gliomas.
The Karnofsky score is a standard and practical
method for evaluating the severity of the tumors in patients with
glioma, and a lower Karnofsky score indicates a shorter survival
time for the patients (23). The
present study analyzed the association between the expression of
CEACAM1 on T cells and the Karnofsky scores of the patients with
glioma. Notably, a significantly negative correlation was observed
between Karnofsky scores and the expression of CEACAM1 on T cells.
Thus, the results of the present study indicated that CEACAM1 may
be associated with the severity of the glioma. IFN-γ is a major
pro-inflammatory cytokine in antitumor immune responses that may be
secreted by activated T lymphocytes. It has been reported that
IFN-γ may enhance antitumor effects and be used in therapeutic
approaches to treat tumors (24,29). The
results of the present study revealed that the plasma level of
IFN-γ in the venous blood was negatively correlated with the
expression of CEACAM1 on CD4+ and CD8+ T
cells in TILs and peripheral CD4+ T cells in patients
with glioma, indicating that CEACAM1 may inhibit antitumor immune
responses by downregulating IFN-γ.
The results of the present study were similar to the
results of previous studies on another important immune checkpoint
protein, T-cell immunoglobulin and mucin-domain containing-3
(Tim-3) in glioma (19,30). Notably, CEACAM1 has been identified as
a heterophilic ligand for Tim-3, and may regulate Tim-3-mediated
immune tolerance and exhaustion of T cells. The co-blockade of
CEACAM1 and Tim-3 resulted in a synergistic therapeutic effect in
mouse colorectal cancer models (31).
Collectively, these results, together with those of the present
study, suggest that homophilic or heterophilic interactions in
CEACAM1 and Tim-3 may serve an important function in gliomas via
numerous different immunological mechanisms.
Immunotherapy has emerged as a promising approach
for the treatment of gliomas. Nevertheless, the mechanisms by which
gliomas evade immunological surveillance and escape immunological
attacks remain unknown. In the present study, the expression of
CEACAM1 on CD4+ and CD8+ T cells in PBMCs and
TILs, and in glioma tissue were assessed. Furthermore, the
associations between the CEACAM1 homophilic interactions, tumor
progression and multiple clinicopathological parameters in patients
with glioma were analyzed. To the best of our knowledge, the
results of the present study provide direct evidence for the first
time that CEACAM1 participates in the progression of glioma by
regulating CD4+ and CD8+ T cells, and that it
may be a promising diagnostic biomarker and a therapeutic target
for glioma immunotherapies.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81470115), the
Science and Technology Research Program of Shanxi Province (grant
no. 2015025), the Basic Research Program of Shanxi Province (grant
no. 201601D011102) and the Graduate Student Education Innovation
Program of Shanxi Province (grant no. 2016BY081).
Glossary
Abbreviations
Abbreviations:
|
CEACAM1
|
carcinoembryonic antigen-related cell
adhesion molecule 1
|
|
TILs
|
tumor-infiltrating lymphocytes
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
WHO
|
World Health Organization
|
References
|
1
|
Porter KR, Mccarthy BJ, Freels S, Kim Y
and Davis FG: Prevalence estimates for primary brain tumors in the
United States by age, gender, behavior, and histology.
Neuro-Oncology. 12:520–527. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pan J, Zhao X, Lin C, Xu H, Yin Z, Liu T
and Zhang S: Immune responsive gene 1, a novel oncogene, increases
the growth and tumorigenicity of glioma. Oncol Rep. 32:1957–1966.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang N, Feng Y, Wang Q, Liu S, Xiang L,
Sun M, Zhang X, Liu G, Qu X and Wei F: Neutrophils infiltration in
the tongue squamous cell carcinoma and its correlation with CEACAM1
expression on tumor cells. PLoS One. 9:e899912014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kiriyama S, Yokoyama S, Ueno M, Hayami S,
Ieda J, Yamamoto N, Yamaguchi S, Mitani Y, Nakamura Y, Tani M, et
al: CEACAM1 long cytoplasmic domain isoform is associated with
invasion and recurrence of hepatocellular carcinoma. Ann Surg
Oncol. 21 Suppl 4:S505–S514. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dupuis ML, Fiori V, Soriani A, Ricci B,
Dominici S, Moricoli D, Ascione A, Santoni A, Magnani M and
Cianfriglia M: The human antibody fragment DIATHIS1 specific for
CEACAM1 enhances natural killer cell cytotoxicity against melanoma
cell lines in vitro. J Immunother. 38:357–370. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Markel G, Sapir Y, Mandel I, Hakim M,
Shaked R, Meilin E, McClanahan T, Loboda A, Hashmueli S and Ben
Moshe T: Inhibition of the novel immune checkpoint CEACAM1 to
enhance anti-tumor immunological activity. J Clin Oncol.
34:2016.
|
|
8
|
Markel G, Seidman R, Stern N, Cohen-Sinai
T, Izhaki O, Katz G, Besser M, Treves AJ, Blumberg RS, Loewenthal
R, et al: Inhibition of human tumor-infiltrating lymphocyte
effector functions by the homophilic carcinoembryonic cell adhesion
molecule 1 interactions. J Immunol. 177:6062–6071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Markel G, Lieberman N, Katz G, Arnon TI,
Lotem M, Drize O, Blumberg RS, Bar-Haim E, Mader R, Eisenbach L and
Mandelboim O: CD66a interactions between human melanoma and NK
cells: A novel class I MHC-independent inhibitory mechanism of
cytotoxicity. J Immunol. 168:2803–2810. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hosomi S, Chen Z, Baker K, Chen L, Huang
YH, Olszak T, Zeissig S, Wang JH, Mandelboim O, Beauchemin N, et
al: CEACAM1 on activated NK cells inhibits NKG2D-mediated cytolytic
function and signaling. Eur J Immunol. 43:2473–2483. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Z, Chen L, Baker K, Olszak T, Zeissig
S, Huang YH, Kuo TT, Mandelboim O, Beauchemin N, Lanier LL and
Blumberg RS: CEACAM1 dampens antitumor immunity by down-regulating
NKG2D ligand expression on tumor cells. J Exp Med. 208:2633–2640.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ortenberg R, Sapir Y, Raz L, Hershkovitz
L, Ben Arav A, Sapoznik S, Barshack I, Avivi C, Berkun Y, Besser
MJ, et al: Novel immunotherapy for malignant melanoma with a
monoclonal antibody that blocks CEACAM1 homophilic interactions.
Mol Cancer Ther. 11:1300–1310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L, Wang J, Wei F, Wang K, Sun Q,
Yang F, Jin H, Zheng Y, Zhao H, Wang L, et al: Profiling the
dynamic expression of checkpoint molecules on cytokine-induced
killer cells from non-small-cell lung cancer patients. Oncotarget.
7:43604–43615. 2016.PubMed/NCBI
|
|
14
|
Li Y and Shively JE: CEACAM1 regulates
Fas-mediated apoptosis in Jurkat T-cells via its interaction with
β-catenin. Exp Cell Res. 319:1061–1072. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grayowen SD and Blumberg RS: CEACAM1:
Contact-dependent control of immunity. Nat Rev Immunol. 6:433–446.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ashkenazi S, Ortenberg R, Besser M,
Schachter J and Markel G: SOX9 indirectly regulates CEACAM1
expression and immune resistance in melanoma cells. Oncotarget.
7:30166–30177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou Z, Luther N, Ibrahim GM, Hawkins C,
Vibhakar R, Handler MH and Souweidane MM: B7-H3, a potential
therapeutic target, is expressed in diffuse intrinsic pontine
glioma. J Neurooncol. 111:257–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lemke D, Pfenning PN, Sahm F, Klein AC,
Kempf T, Warnken U, Schnölzer M, Tudoran R, Weller M, Platten M and
Wick W: Costimulatory protein 4IgB7H3 drives the malignant
phenotype of glioblastoma by mediating immune escape and
invasiveness. Clin Cancer Res. 18:105–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Z, Han H, He X, Li S, Wu C, Yu C and
Wang S: Expression of the galectin-9-Tim-3 pathway in glioma
tissues is associated with the clinical manifestations of glioma.
Oncol Lett. 11:1829–1834. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wainwright DA, Chang AL, Dey M,
Balyasnikova IV, Kim CK, Tobias A, Cheng Y, Kim JW, Qiao J, Zhang
L, et al: Durable therapeutic efficacy utilizing combinatorial
blockade against IDO, CTLA-4 and PD-L1 in mice with brain tumors.
Clin Cancer Res. 20:5290–5301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Johnson MJ, Bland JM, Davidson PM, Newton
PJ, Oxberry SG, Abernethy AP and Currow DC: The relationship
between two performance scales: New York heart association
classification and karnofsky performance status scale. J Pain
Symptom Manage. 47:652–658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng F, Kuai D, Wang H, Li T, Miao W, Liu
Y and Fan Y: Reduced expression of microRNA-497 is associated with
greater angiogenesis and poor prognosis in human gliomas. Hum
Pathol. 58:47–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hong B, Li H, Lu Y, Zhang M, Zheng Y, Qian
J and Yi Q: USP18 is crucial for IFN-γ-mediated inhibition of B16
melanoma tumorigenesis and antitumor immunity. Mol Cancer.
13:1322014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Platten M, Weller M and Wick W: Shaping
the glioma immune microenvironment through tryptophan metabolism.
CNS Oncol. 1:99–106. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim ES, Kim JE, Patel MA, Mangraviti A,
Ruzevick J and Lim M: Immune checkpoint modulators: An emerging
antiglioma armamentarium. J Immunol Res. 2016:46836072016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahn BJ, Pollack IF and Okada H:
Immune-Checkpoint blockade and active immunotherapy for glioma.
Cancers. 5:1379–1412. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng W, Liu C, Xu C, Lou Y, Chen J, Yang
Y, Yagita H, Overwijk WW, Lizée G, Radvanyi L and Hwu P: PD-1
blockade enhances T-cell migration to tumors by elevating IFN-γ
inducible chemokines. Cancer Res. 72:5209–5218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han S, Feng S, Xu L, Shi W, Wang X, Wang
H, Yu C, Dong T, Xu M and Liang G: Tim-3 on peripheral
CD4+ and CD8+ T cells is involved in the
development of glioma. DNA Cell Biol. 33:245–250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang YH, Zhu C, Kondo Y, Anderson AC,
Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T, et
al: CEACAM1 regulates TIM-3-mediated tolerance and exhaustion.
Nature. 517:386–390. 2015. View Article : Google Scholar : PubMed/NCBI
|