Introduction
Cervical cancer is a frequent cause of cancer death
among women worldwide (1). The
standard treatment for locally advanced uterine cervical cancer
including International Federation of Gynecology and Obstetrics
(FIGO) stage IIIA, IIIB, and IVA cancer consists Platinum-based
concurrent chemoradiotherapy (CCRT) (2–4). However,
the prognosis of these patients is poor, and the 5-year survival is
<60% (5,6).
Successful neoadjuvant chemotherapy (NAC) can reduce
tumor size in patients with locally advanced cervical cancer,
thereby facilitating hysterectomy and improving the prognosis
(7). However, the prognosis worsens
if NAC is unsuccessful, because hysterectomy may no longer be
practicable and the switch to radiotherapy may delay the initiation
of the core treatment (8–10). Therefore, there is an urgent need to
identify predictive biomarkers of NAC efficacy for patients with
locally advanced uterine cervical cancer (10–14).
The antineoplastic activity of cisplatin is
primarily due to its ability to induce DNA damage, particularly
intrastrand DNA crosslinks, which leads to apoptosis (15). Nucleotide excision repair (NER) is a
pathway that identifies and repairs intrastrand DNA crosslinks.
Tumor sensitivity to cisplatin has been associated with a decrease
in the induction of DNA repair (16).
Consistent with this, overexpression of NER proteins confers
resistance to platinum-based drugs (17,18). The
xeroderma pigmentosum complementation group A (XPA) protein is an
indispensable factor for NER. Several reports have shown that XPA
recognizes and verifies DNA damage sites, stabilizes repair
intermediates, and contributes to the induction of other
NER-associated factors (19–24). XPA expression has been reported to
correlate with the resistance of nasopharyngeal carcinoma and lung
cancer cell lines to platinum drugs (17–25).
However, the role of XPA in the response of uterine cervical cancer
cell lines and uterine cervical cancer patients to cisplatin is not
clear.
Here, we sought to determine the utility of XPA
expression as a predictive biomarker by investigating the
relationship between tumor expression of XPA and the efficacy of
NAC for locally advanced uterine cervical cancer.
Materials and methods
Patients and tissue samples
We evaluated 56 patients with locally advanced
uterine cervical cancer (FIGO stages IIIA and IIIB). All patients
were <70 years of age and were first treated at Osaka City
University Hospital (Osaka, Japan) between April 1995 and March
2010. Tumor tissue samples were obtained by punch biopsy before
NAC. Patients were classified into two groups based on the response
to NAC: Patients who were successfully treated with NAC, underwent
hysterectomy, and received radiation therapy (NAC + OP+ R group;
n=31), and patients who were unsuccessfully treated with NAC and
received only radiation therapy (NAC + R group; n=25).
Balloon-occluded arterial infusion chemotherapy for NAC is
performed for all patients. We infused Cisplatin (Bristol-Myers
Squibb, Tokyo, Japan) intra-arterially through the catheters over
30 min. Cisplatin was administered three times at doses of 50, 75
or 100 mg/m2, depending on the patient's age and renal
function (26).
Written informed consent was obtained from all
patients prior to tumor biopsy. The study was approved by the
institutional review board of Osaka City University Hospital (IRB
no. 3526).
Immunohistochemical staining
XPA protein expression was examined in
four-micrometre sections from paraffin-embedded tissue samples
using a mouse monoclonal antibody against XPA (cat no. sc-28353;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and a Dako LSAB2
Peroxidase kit (cat no. K0675; Agilent Technologies, Santa Clara,
CA, USA). After routine deparaffnization and rehydration, sections
were immersed in 3% hydrogen peroxide at room temperature for 10
min to block endogenous peroxidase activity. Heat-mediated antigen
retrieval was performed with 10 mM citrate buffer (pH 6.0) by an
autoclave at 110°C for 20 min. After washing with
phosphate-buffered saline (PBS), tumor tissue sections were
incubated with a 1:100 dilution of the anti-XPA antibody overnight
at 4°C. Next, sections were washed in PBS for 15 min and then
incubated for 10 min with biotinylated goat anti-mouse and
anti-rabbit immunoglobulin G secondary antibody (Dako; Agilent
Technologies), followed by an incubation with a
streptavidin-peroxidase complex, and color was developed using
3,3′-diaminobenzidine used as the chromogen. Finally, tissue
sections were counterstained with hematoxylin. A specificity
control was prepared in the same manner except the primary antibody
was omitted.
The expression levels of XPA were quantitatively
analyzed using the weighted score method of Sinicrope et al
(27). The mean percentage of stained
tumor cells was scored on a scale of 0 to 4 as follows: 0, ≤5%; 1,
5–25%; 2, 25–50%; 3, 50–75%; and 4, >75%. Staining intensity was
classified into three categories: 1+, weak; 2+, moderate; and 3+,
intense. The weighted score for each tissue specimen was determined
by multiplying the score of percentage of stained tumor cells by
that of staining intensity.
Cell culture
The human uterine cervical cancer cell line Ca Ski
(cat no. IFO50007; Japanese Collection of Research Biosources Cell
Bank, Osaka, Japan) was cultured in RPMI medium (Gibco; Thermo
Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific) and 1% penicillin and
maintained in a humidified atmosphere with 5% CO2 at
37°C.
RNA interference
Small interfering RNA (siRNA) targeted to XPA
(5′-GUACCGUAAGACUUGUACUtt, 5′-AGUACAAGUCUUACGGUACtt; cat. no.
sc-36853) and a negative control sequence (cat. no. sc-37007) were
obtained from Santa Cruz Biotechnology. Cells were seeded in 6-well
plates overnight and then transfected with siRNAs using
Lipofectamine RNAiMax (Invitrogen, Carlsbad, CA, USA) according to
the manufacturer's instructions. The culture medium was changed 24
h after transfection and cells were used for experiments 24 h after
transfection.
RNA extraction and
reverse-transcription-quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was extracted from the Ca Ski cells using
an RNeasy Mini kit (QIAGEN GmbH, Hilden, Germany) according to the
instruction of the manufacturer. RNA was reverse transcribed using
a High Capacity cDNA Reverse Transcription kit (Applied Biosystems;
Thermo Fisher Scientific). PCR was performed using a TaqMan Gene
Expression Assay (Applied Biosystems; Thermo Fisher Scientific) and
an Applied Biosystems 7500 Fast Real-Time PCR System. XPA mRNA
levels were normalized to glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) mRNA in the same samples. TaqMan probes were Hs00902270_m1
for XPA and Hs99999905_m1 for GAPDH.
Chemosensitivity assay
The sensitivity of Ca Ski cells to cisplatin was
examined using a Cell Counting kit-8 (CCK-8; Dojindo Molecular
Technologies, Kumamoto, Japan). Cells were transfected with
negative control or XPA-specific siRNAs as described above and then
seeded into 96-well tissue culture plates at 2×103
cells/well. After 24 h, the culture medium was removed and vehicle
or cisplatin (0–10 µg/ml) was added for 48 h. Subsequently, 10
µl/well CCK-8 was added and the plates were incubated for 2 h. The
absorbance at 450 nm was then assesed with a microplate reader
(Corona Electric, Ibaraki, Japan). Dose-response curves were
constructed of the percentage viable cells compared with untreated
cells.
Statistical analysis
All statistical analyses were conducted with SPSS
software version 21.0 (IBM SPSS, Armonk, NY, USA). Data are
presented as the mean ± standard deviation in the tables and as the
mean ± standard error in the figures. Kaplan-Meier plots and
log-rank analyses were used for prognostic analysis. Weighted
scores were compared using the Mann-Whitney U test. Student's
t-test was used for comparison of significant differences between
group means, and χ2 tests were used for identification
of the association between group categorical variables. P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient characteristics
A total of 56 patients with locally advanced uterine
cervical cancer were divided into two groups based on their
response to therapy: The NAC effective group (NAC + OP + R group;
n=31) and the NAC ineffective group (NAC + R group; n=25). Table I shows the patients'
clinicopathological characteristics. No statistically significant
differences were observed between the two groups.
 | Table I.Characteristics of the patients in the
NAC effective and ineffective groups. |
Table I.
Characteristics of the patients in the
NAC effective and ineffective groups.
| Characteristic | NAC effective | NAC ineffective | P-value |
|---|
| No. of patients | 31 | 25 |
|
| Age (years) |
|
|
0.317a |
| Mean ±
SD | 48.9±13.2 | 52.3±11.5 |
|
|
Range | 24–69 | 36–68 |
|
| FIGO stage |
|
| 0.397b |
|
IIIA | 1 | 0 |
|
|
IIIB | 30 | 25 |
|
| Histology |
|
| 0.433b |
|
SCC | 27 | 21 |
|
| A | 4 | 3 |
|
| AS | 0 | 1 |
|
| Tumor size
(mm) |
|
| 0.956a |
| Mean ±
SD | 41.1±22.7 | 41.4±23.8 |
|
XPA expression in uterine cervical
cancer tissue
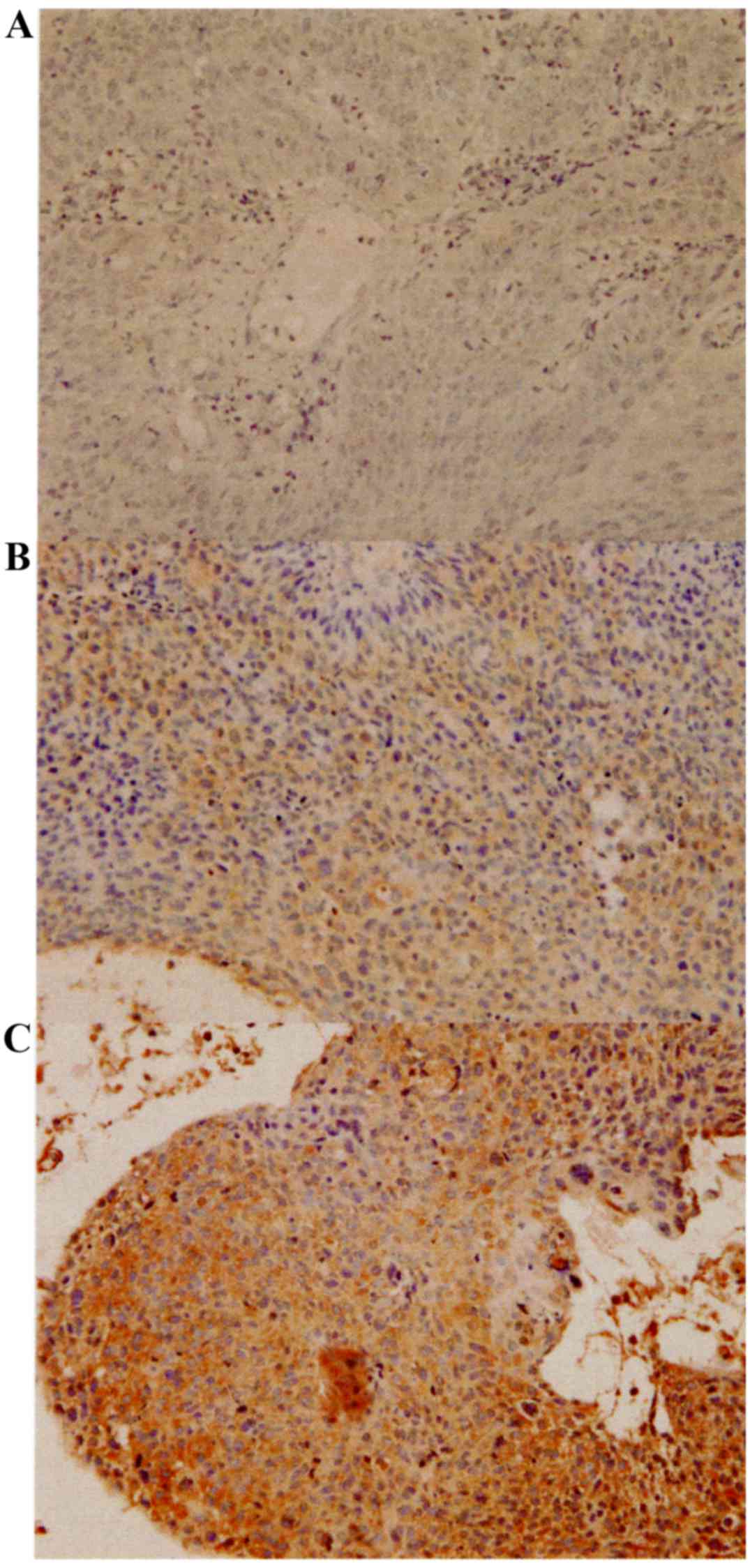
XPA was expressed in both the nuclei and cytoplasm
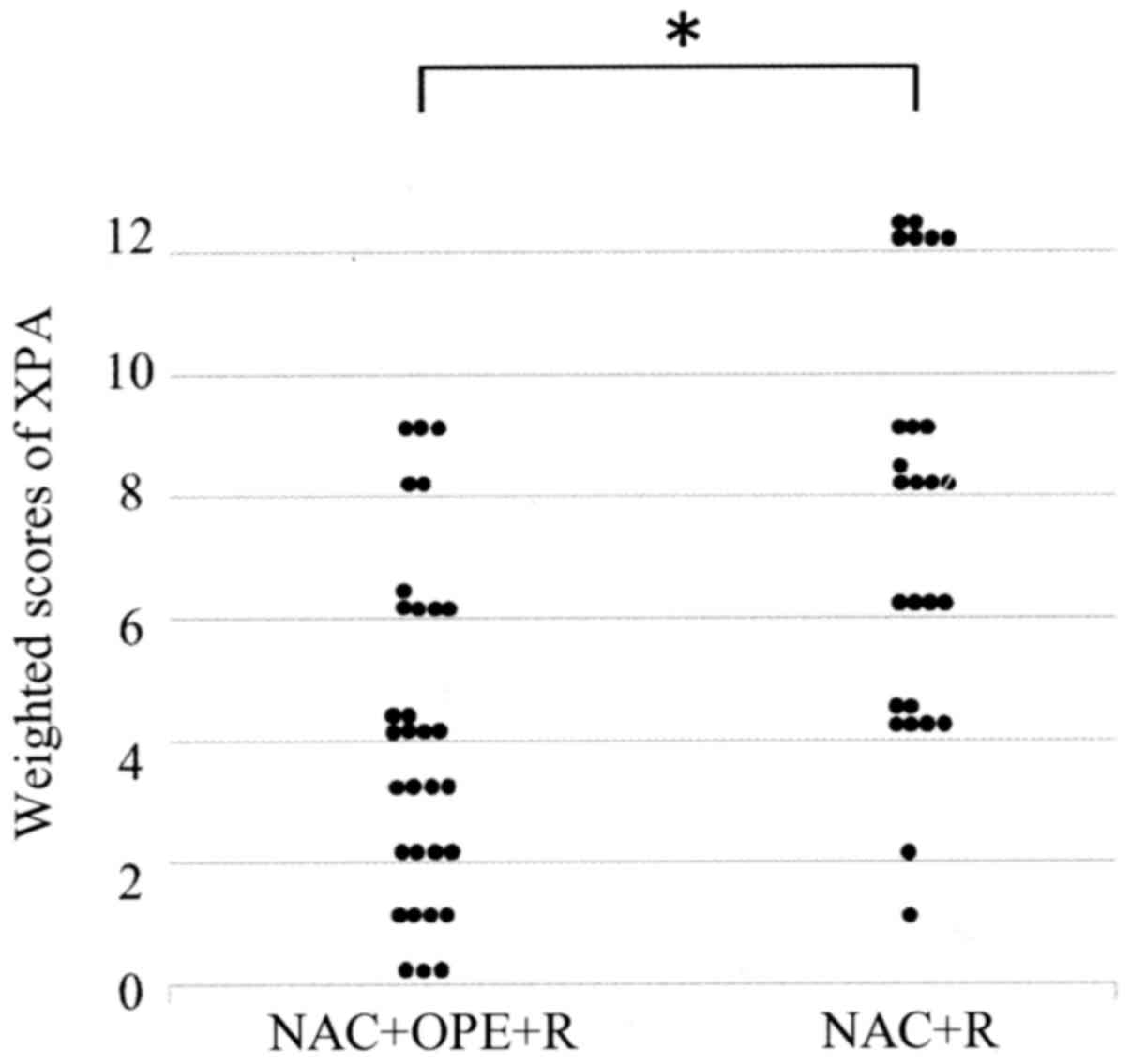
of the tumor cells (Fig. 1). Table II shows the weighted scores for XPA
tissue staining. The mean weighted score of the NAC ineffective
group was significantly higher than that of the NAC effective group
(7.12 and 3.90, respectively; P=0.001) (Fig. 2 and Table
II). Of the 56 patients, 17 had weighted scores of 0–3
(designated low expression) and 39 had weighted scores of 4–12
(high expression). There were no significant differences in patient
characteristics between these two groups (Table III).
 | Table II.Weighted scores for XPA expression in
the NAC effective and ineffective groups. |
Table II.
Weighted scores for XPA expression in
the NAC effective and ineffective groups.
|
| No. of
patients |
|---|
|
|
|
|---|
| Weighted score | NAC
effectivea | NAC
ineffectiveb |
|---|
| 0 | 3 | 0 |
| 1 | 4 | 1 |
| 2 | 4 | 1 |
| 3 | 4 | 0 |
| 4 | 6 | 6 |
| 6 | 5 | 4 |
| 8 | 2 | 5 |
| 9 | 3 | 3 |
| 12 | 0 | 5 |
| Total | 31 | 25 |
| Weighted score
(mean) | 3.90 | 7.12 |
 | Table III.Characteristics of patients in the
low and high XPA expression groups. |
Table III.
Characteristics of patients in the
low and high XPA expression groups.
| Characteristic | XPA expression (≤3
score) | XPA expression (≥4
score) | P-value |
|---|
| Number of
patients | 17 | 39 |
|
| Age (years) |
|
| 0.808a |
| Mean ±
SD | 49.8±13.6 | 49.8±13.6 |
|
|
Range | 24–68 | 24–69 |
|
| FIGO stage |
|
| 0.505b |
|
IIIA | 0 | 1 |
|
|
IIIB | 17 | 38 |
|
| Histology |
|
| 0.759b |
|
SCC | 15 | 33 |
|
| A | 2 | 5 |
|
| AS | 0 | 1 |
|
| Tumor size
(mm) |
|
| 0.470a |
| Mean ±
SD | 44.6±18.5 | 39.8±24.8 |
|
NAC effectiveness correlates with XPA
expression
Of the 17 patients with low XPA expression, 15 (88%)
were in the NAC effective group and 2 (12%) were in the NAC
ineffective group. In the high XPA expression group, 16 patients
(41%) were in the NAC effective group and 23 (59%) were in the NAC
ineffective group. Thus, patients in the low XPA expression group
were more sensitive to NAC than those in the high XPA expression
group (P=0.001, Table IV).
 | Table IV.Number of patients with low and high
XPA expression in the NAC effective and NAC ineffective groups. |
Table IV.
Number of patients with low and high
XPA expression in the NAC effective and NAC ineffective groups.
|
| Number of patients
(%) |
|---|
|
|
|
|---|
| XPA expression | NAC + OP + R
n=31 | NAC + R n=25 | P-value |
|---|
| Low expression (≤3
score) | 15 (88%) | 2 (12%) | 0.001a |
| High expression (≥4
score) | 16 (41%) | 23 (59%) |
|
Survival
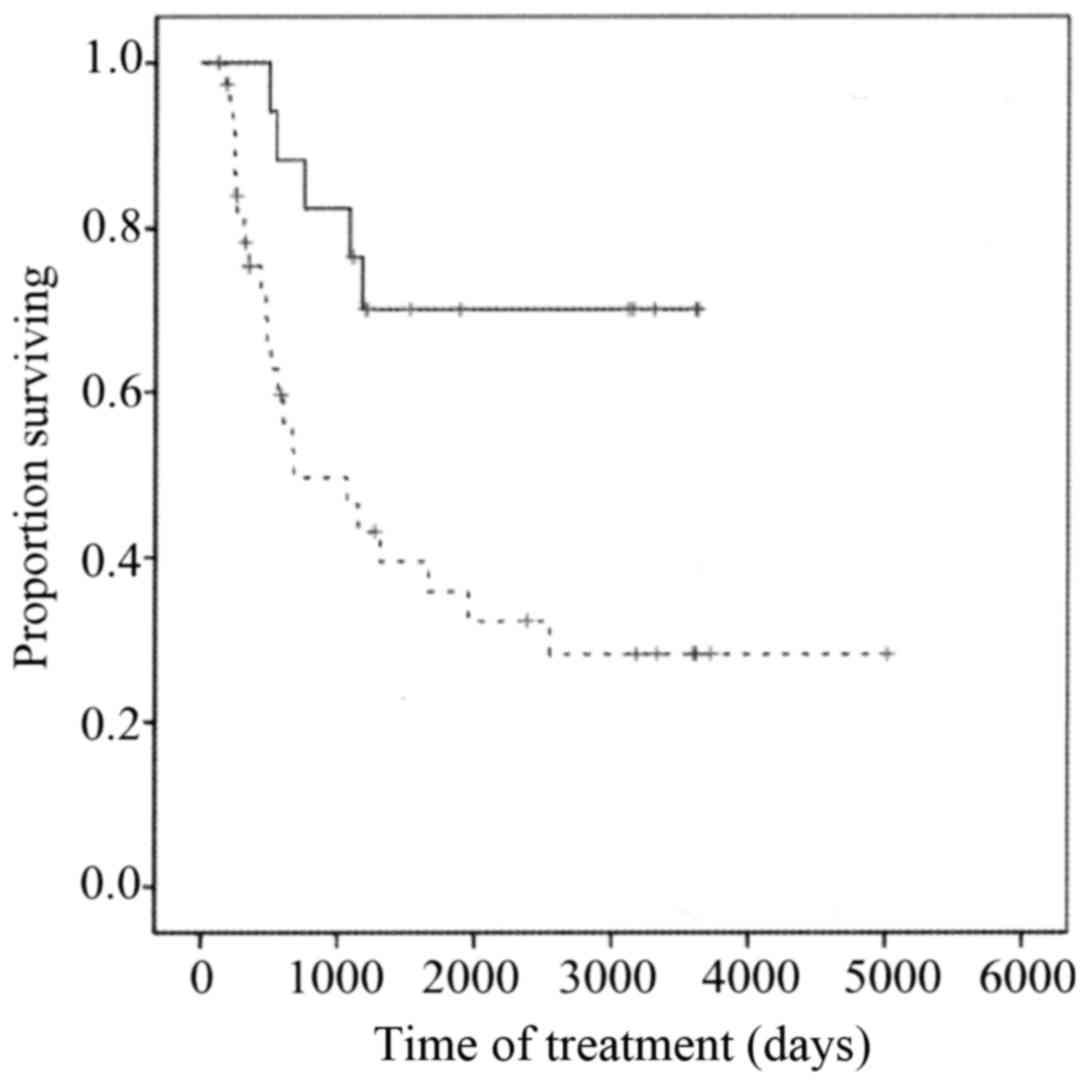
Overall survival was significantly longer for the
NAC effective group than for the NAC ineffective group (P<0.001)
(Fig. 3) and was significantly longer
for the low XPA expression group than for the high XPA expression
group (P=0.01) (Fig. 4).
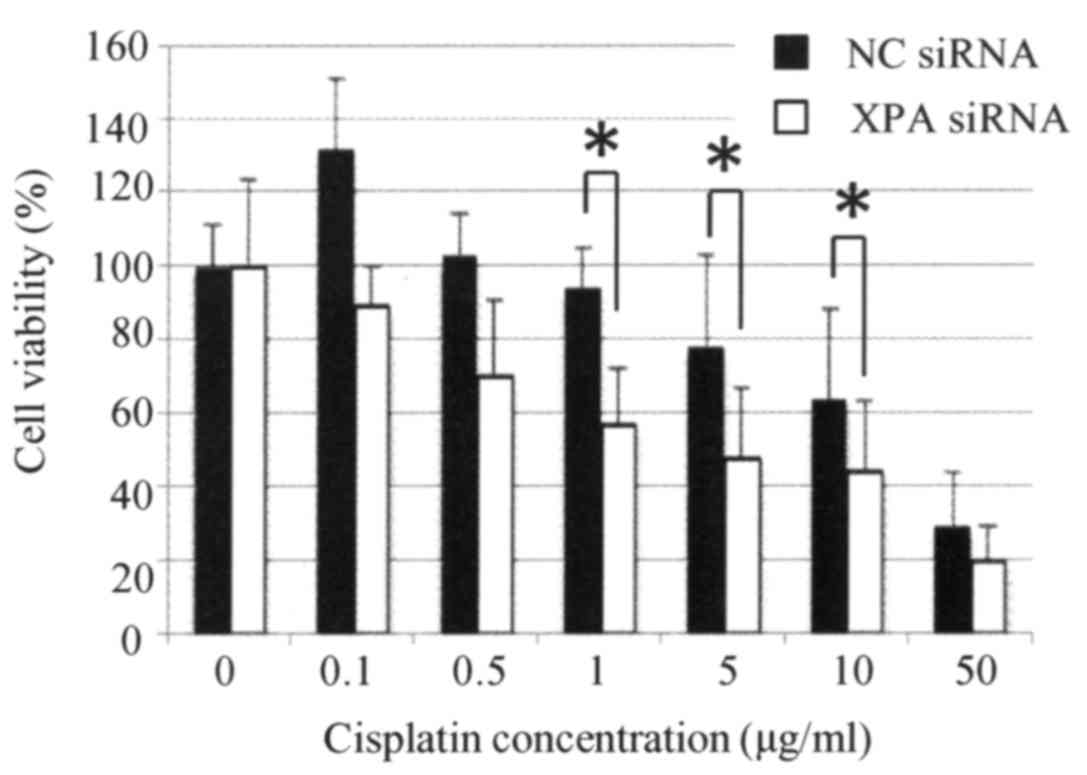
Knockdown of XPA enhances the
sensitivity of a uterine cervical cancer cell line to cisplatin
treatment
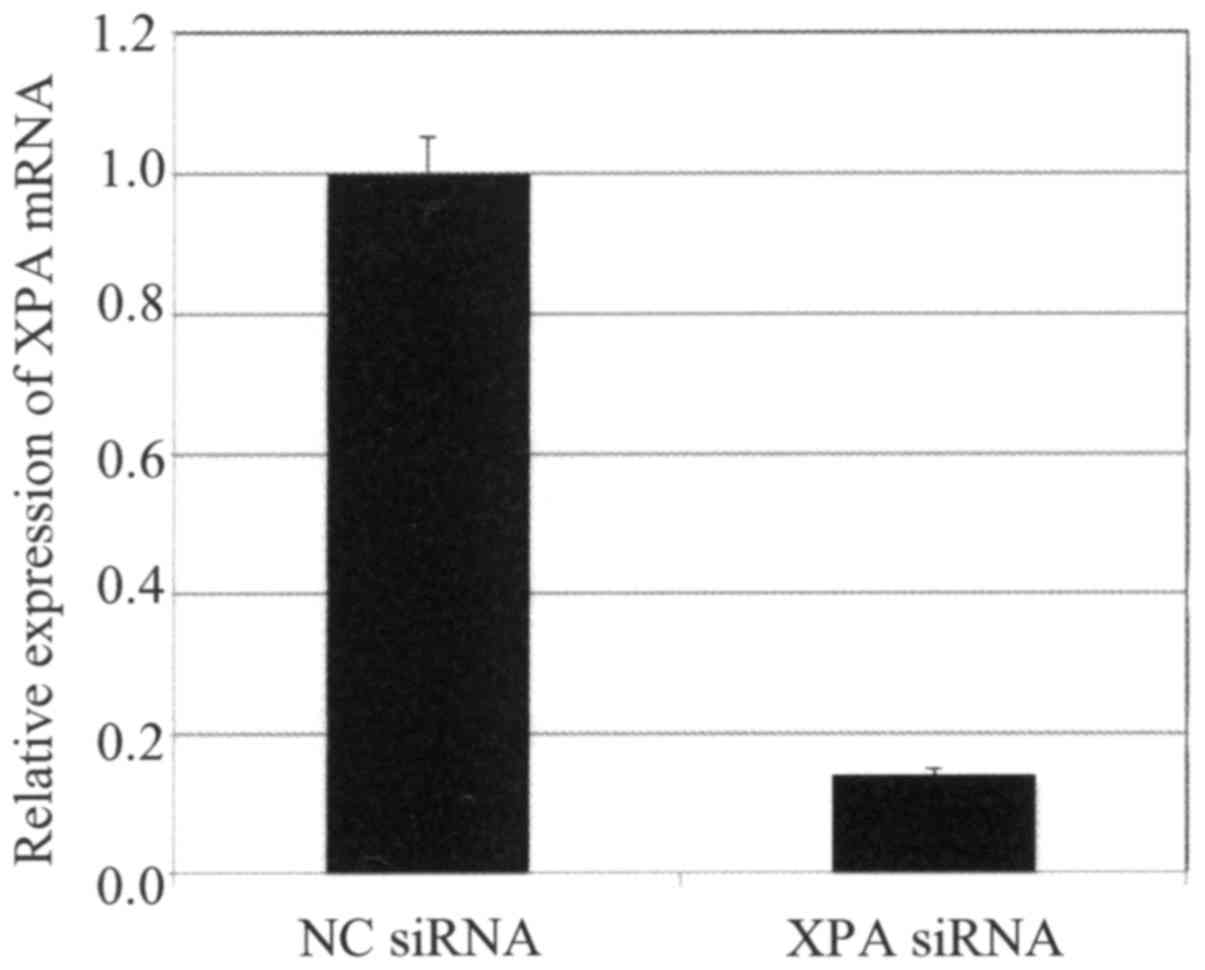
qRT-PCR analysis of Ca Ski cells confirmed that XPA
expression was effectively suppressed by transfection with a
specific targeting siRNA but not by a non-targeting control siRNA
(Fig. 5). Cells transfected with
XPA-specific siRNA were significantly more sensitive to cisplatin
than were the control cells (P<0.05) (Fig. 6).
Discussion
CCRT is considered the standard treatment for
patients with locally advanced uterine cervical cancer. Effective
NAC can reduce the tumor size, allowing the patient to undergo
hysterectomy and potentially improving their prognosis (7). However, the prognosis can worsen if NAC
is unsuccessful because the change in treatment plan from surgery
to radiotherapy can delay implementation of the core treatment
(8–10). Therefore, it is important to identify
biomarkers that can predict the efficacy of NAC in patients with
locally advanced uterine cervical cancer.
The antitumor mechanism of platinum-containing drugs
such as cisplatin results from covalent binding to DNA and
formation of platinum-DNA adducts, which interfere with DNA
replication and ultimately induce apoptosis (28). Although platinum-based chemotherapy
often has good initial efficacy, cancer cells may acquire
resistance to this therapy. Some of the potential mechanisms of
resistance are reduced intracellular accumulation of cisplatin
(29–31), inactivation of apoptotic pathways
(32), increased DNA damage repair
capacity (18,33), increased detoxification of cisplatin
(34), and other epigenetic changes
occurring at the molecular and cellular levels (35,36). Among
these, NER, which mediates DNA damage repair, is believed to be one
the most crucial determinants (18).
XPA is an indispensable factor for NER (17,18).
Several reports have shown that XPA mediates the initial
recognition and verification of DNA lesions, stabilizes repair
intermediates, and is involved in the induction of other NER
factors (19–24). Therefore, it is likely that
upregulation of XPA expression would increase platinum resistance.
Indeed, XPA expression has been reported to correlate with the
resistance of nasopharyngeal carcinoma and lung cancer cell lines
to platinum-based therapy (17,18,25).
The present study reveals a significant relationship
between XPA expression and the effectiveness of NAC in patients
with locally advanced uterine cervical cancer. Patients with low
XPA expression tended to be more sensitive to NAC and underwent
surgery after NAC. This is consistent with the longer overall
survival times of the low XPA expression group and NAC effective
group compared with the high XPA expression group and NAC
ineffective group, respectively. We also found that downregulation
of XPA expression increased the cisplatin sensitivity of cultured
uterine cervical cancer cells, suggesting that XPA is a
cisplatin-resistance factor. This is the first report of a
correlation between XPA expression and NAC efficacy for locally
advanced uterine cervical cancer. However, this study included only
56 patients. One of the limitations of this study was the small
number of patients. We need further investigations with a larger
number of cases to know the critical fact.
In summary, XPA expression may be a predictive
marker of the effectiveness of NAC for patients with locally
advanced uterine cervical cancer. This finding could help to
improve the prognosis of these patients.
Acknowledgements
The authors would like to thank Dr Anne M. O'Rourke,
for editing a draft of this manuscript.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Japan Society of Gynecologic Oncology, .
Formulation Committee of the Treatment Guidelines for Cervical
Cancer, 2011. Kanehara & Co.; Tokyo: 2011, (In Japanese).
|
|
4
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in Oncology. Cervical Cancer
Version II. 2013.
|
|
5
|
Morris M, Eifel PJ, Lu J, Grigsby PW,
Levenback C, Stevens RE, Rotman M, Gershenson DM and Mutch DG:
Pelvic radiation with concurrent chemotherapy compared with pelvic
and para-aortic radiation for high-risk cervical cancer. N Engl J
Med. 340:1137–1143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eifel PJ, Winter K, Morris M, Levenback C,
Grigsby PW, Cooper J, Rotman M, Gershenson D and Mutch DG: Pelvic
irradiation with concurrent chemotherapy versus pelvic and
para-aortic irradiation for high-risk cervical cancer: An update of
radiation therapy oncology group trial (RTOG) 90–01. J Clin Oncol.
22:872–880. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishiko O, Sumi T, Yasui T, Matsumoto Y,
Kawamura N, Ogita S, Kamino T, Nakamura K and Yamada R:
Balloon-occluded arterial infusion chemotherapy, simple total
hysterectomy and radiotherapy as a useful combination-therapy for
advanced cancer of the uterine cervix. Oncol Rep. 7:141–144.
2000.PubMed/NCBI
|
|
8
|
Souhami L, Gil RA, Allan SE, Canary PC,
Araújo CM, Pinto LH and Silveira TR: A randomized trial of
chemotherapy followed by pelvic radiation therapy in stage IIIB
carcinoma of the cervix. J Clin Oncol. 9:970–977. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tattersall MH, Lorvidhaya V, Vootiprux V,
Cheirsilpa A, Wong F, Azhar T, Lee HP, Kang SB, Manalo A, Yen MS,
et al: Randomized trial of epirubicin and cisplatin chemotherapy
followed by pelvic radiation in locally advanced cervical cancer.
Cervical cancer study group of the asian oceanian clinical oncology
association. J Clin Oncol. 13:444–451. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishiko O, Sumi T, Yasui T, Matsumoto Y,
Ogita S, Kaminou T, Nakamura K and Yamada R: Tumor marker and MR
imaging criteria for evaluating the efficacy of cyclic
balloon-occluded arterial infusion for advanced cancer of the
uterine cervix. Oncol Rep. 7:827–830. 2000.PubMed/NCBI
|
|
11
|
Ishiko O, Sumi T, Yoshida H, Ogita S and
Yamada R: Expression of apoptosis regulatory proteins in advanced
cancer of the uterine cervix after cyclic balloon-occluded arterial
infusion chemotherapy. Int J Oncol. 18:1151–1155. 2001.PubMed/NCBI
|
|
12
|
Okamoto E, Sumi T, Misugi F, Nobeyama H,
Hattori K, Yoshida H, Matsumoto Y, Yasui T, Honda K and Ishiko O:
Expression of apoptosis-related proteins in advanced uterine
cervical cancer after balloon-occluded arterial infusion
chemotherapy as an indicator of the efficiency of this therapy. Int
J Mol Med. 15:41–47. 2005.PubMed/NCBI
|
|
13
|
Nobeyama H, Sumi T, Misugi F, Okamoto E,
Hattori K, Matsumoto Y, Yasui T, Honda K, Iwai K and Ishiko O:
Association of HPV infection with prognosis after neoadjuvant
chemotherapy in advanced uterine cervical cancer. Int J Mol Med.
14:101–105. 2004.PubMed/NCBI
|
|
14
|
Benedetti Panici P, Bellati F, Manci N,
Pernice M, Plotti F, Di Donato V, Calcagno M, Zullo MA, Muzii L and
Angioli R: Neoadjuvant chemotherapy followed by radical surgery in
patients affected by FIGO stage IVA cervical cancer. Ann Surg
Oncol. 14:2643–2648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Köberle B, Grimaldi KA, Sunters A, Hartley
JA, Kelland LR and Masters JR: DNA repair capacity and cisplatin
sensitivity of human testis tumour cells. Int J Cancer. 70:551–555.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Riedl T, Hanaoka F and Egly JM: The
comings and goings of nucleotide excision repair factors on damaged
DNA. EMBO J. 22:5293–5303. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martin LP, Hamilton TC and Schilder RJ:
Platinum resistance: The role of DNA repair pathways. Clin Cancer
Res. 14:1291–1295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Z, Roginskaya M, Colis LC, Basu AK,
Shell SM, Liu Y, Musich PR, Harris CM, Harris TM and Zou Y:
Specific and efficient binding of xeroderma pigmentosum
complementation group A to double-strand/single-strand DNA
junctions with 3′- and/or 5′-ssDNA branches. Biochemistry.
45:15921–15930. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mer G, Bochkarev A, Gupta R, Bochkareva E,
Frappier L, Ingles CJ, Edwards AM and Chazin WJ: Structural basis
for the recognition of DNA repair proteins UNG2, XPA and RAD52 by
replication factor RPA. Cell. 103:449–456. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Batty DP and Wood RD: Damage recognition
in nucleotide excision repair of DNA. Gene. 241:193–204. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Liu Y, Yang Z, Utzat C, Wang G,
Basu AK and Zou Y: Cooperative interaction of human XPA stabilizes
and enhances specific binding of XPA to DNA damage. Biochemistry.
44:7361–7368. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guzder SN, Sommers CH, Prakash L and
Prakash S: Complex formation with damage recognition protein Rad14
is essential for Saccharomyces cerevisiae Rad1-Rad10 nuclease to
perform its function in nucleotide excision repair in vivo. Mol
Cell Biol. 26:1135–1141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shell SM and Zou Y: Other proteins
interacting with XP proteins. Adv Exp Med Biol. 637:103–112. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu X, Hu J, Han HY, Hua YJ, Zhou L, Shuai
WD, Du WY, Kuang CM, Chen S, Huang W and Liu RY: High expression of
XPA confers poor prognosis for nasopharyngeal carcinoma patients
treated with platinum-based chemoradiotherapy. Oncotarget.
6:28478–28490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsuji K, Yamada R, Kawabata M, Mitsuzane
K, Sato M, Iwahashi M, Kitayama S and Nakano R: Effect of balloon
occluded arterial infusion of anticancer drugs on the prognosis of
cervical cancer treated with radiation therapy. Int J Radiat Oncol
Biol Phys. 32:1337–1345. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sinicrope FA, Ruan SB, Cleary KR, Stephens
LC, Lee JJ and Levin B: Bcl-2 and p53 oncoprotein expression during
colorectal tumorigenesis. Cancer Res. 55:237–241. 1995.PubMed/NCBI
|
|
28
|
Wang D and Lippard SJ: Cellular processing
of platinum anticancer drugs. Nat Rev Drug Discov. 4:307–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ishida S, Lee J, Thiele DJ and Herskowitz
I: Uptake of the anticancer drug cisplatin mediated by the copper
transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci USA.
99:pp. 14298–14302. 2002; View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morimoto A, Serada S, Enomoto T, Kim A,
Matsuzaki S, Takahashi T, Ueda Y, Yoshino K, Fujita M, Fujimoto M,
et al: Annexin A4 induces platinum resistance in a chloride-and
calcium-dependent manner. Oncotarget. 5:7776–7787. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Q, Shi S, He W, Padilla MT, Zhang L,
Wang X, Zhang B and Lin Y: Retaining MKP1 expression and
attenuating JNK-mediated apoptosis by RIP1 for cisplatin resistance
through miR-940 inhibition. Oncotarget. 5:1304–1314. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu RY, Dong Z, Liu J, Yin JY, Zhou L, Wu
X, Yang Y, Mo W, Huang W, Khoo SK, et al: Role of eIF3a in
regulating cisplatin sensitivity and in translational control of
nucleotide excision repair of nasopharyngeal carcinoma. Oncogene.
30:4814–4823. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mistry P, Kelland LR, Abel G, Sidhar S and
Harrap KR: The relationships between glutathione,
glutathione-S-transferase and cytotoxicity of platinum drugs and
melphalan in eight human ovarian carcinoma cell lines. Br J Cancer.
64:215–220. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen DW, Pouliot LM, Hall MD and Gottesman
MM: Cisplatin resistance: A cellular self-defense mechanism
resulting from multiple epigenetic and genetic changes. Pharmacol
Rev. 64:706–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu RY, Dong Z, Liu J, Zhou L, Huang W,
Khoo SK, Zhang Z, Petillo D, Teh BT, Qian CN and Zhang JT:
Overexpression of asparagine synthetase and matrix
metalloproteinase 19 confers cisplatin sensitivity in
nasopharyngeal carcinoma cells. Mol Cancer Ther. 12:2157–2166.
2013. View Article : Google Scholar : PubMed/NCBI
|