Introduction
T-cell lymphoma is the one of the most common immune
subtypes of hematopoietic malignancy originating from lymphoid
tissues, accounting for ~15% of all non-Hodgkin's lymphoma in China
between 2000 and 2014 (1). At
present, chemotherapy agents-based strategies remain the first
choice for treatment of T-cell lymphoma, however poor prognosis is
inevitable due to the occurrence of multidrug resistance (MDR)
(2). The typical MDR phenotype is
characterized by the overexpression of the MDR-associated proteins
in the cytomembrane that serve as efflux pumps, to exclude the
intracellular antitumor agents (3,4). As the
prognosis of patients with T-cell lymphoma is greatly impaired by
MDR, novel strategies to alleviate drug resistance and improve
survival rates are urgently required.
Celecoxib is a newly-identified nonsteroidal
anti-inflammatory drug, which is used to treat fever in clinical
practice (4). Previously, celecoxib
was demonstrated to induce apoptosis in multiple malignant tumor
cells, but to have less toxicity and side effects on normal tissue
cells (3). Celecoxib was identified
to enhance the chemosensitivity and reduce the incidence of
acquired MDR in human gastric, colon and breast carcinomas
(4). Considering the function of
celecoxib in reversing MDR in digestive and gynecologic tumors, we
hypothesize that celecoxib may increase the chemosensitivity of
T-cell lymphoma.
To the best of our knowledge, the present study
revealed, for the first time, that celecoxib enhanced the
inhibition effect of conventional chemotherapy drugs on T-cell
lymphoma cell lines and significantly increased the percentages of
apoptotic cells. The half maximal inhibitory concentration
(IC50) of the representative chemotherapy agents
cis-diamminedichloroplatinum (CDDP), epirubicin and vinblastine
(VCR) was significantly decreased in T-cell lymphoma cells treated
with celecoxib compared with those that were not. Additionally, the
expression levels of MDR-associated proteins P-glycoprotein (P-gp),
multidrug resistance-associated protein 1 (MRP1), transcription
factor p65 (p65) and B-cell lymphoma 2 (Bcl-2) were decreased,
whereas the expression level of Bcl-2-associated X protein (Bax)
was increased in celecoxib-treated T-cell lymphoma cell lines
compared with those that were not treated with celecoxib. By
investigating the effect of celecoxib on T-cell lymphoma cells, it
was identified that celecoxib may reduce drug resistance in these
cells by inactivating the nuclear factor (NF)-κB pathway. These
data indicate that the combination of celecoxib and chemotherapy
drugs may be an effective treatment strategy to improve the
curative effect of chemotherapy drugs in T-cell lymphoma.
Materials and methods
Cell lines and regents
The human T-cell lymphoma Jurkat and HuT-78 cell
lines were supplied by the Research Center of the Fourth Hospital
of Hebei Medical University (Shijiazhuang, China) and cultured in
RPMI-1640 complete medium (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) containing 10% fetal calf serum (FCS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified
incubator with 5% CO2. The Annexin V-fluorescein
isothiocyanate (FITC) and propidium iodide (PI) double stain kit
was supplied by BD Pharmingen; BD Biosciences (San Jose, CA, USA).
Rabbit anti-human antibodies against p65 (cat. no., 76311), P-gp
(cat. no., 168337) and MRP1 (cat. no., 84320) were obtained from
Abcam (Cambridge, MA, USA). Rabbit anti-human antibodies against
Bcl-2 (cat. no., 12789-1-AP) and Bax (cat. no., 50599-2-Ig) were
purchased from ProteinTech Group, Inc. (Chicago, IL, USA). Rabbit
anti-human antibodies against β-actin (cat. no., AP0060) were
obtained from Bioworld Technology, Inc. (St. Louis Park, MN, USA).
The horseradish peroxidase (HRP)-conjugated goat anti-rabbit
secondary antibody (cat no. 29527) was purchased from Rockland
Immunochemicals Inc. (Limerick, PA, USA). TRIzol®
reagent was obtained from Life Technologies (Thermo Fisher
Scientific, Inc.). MTT, Rhodamine-123 and Ficoll separating medium
(density: 1.077 g/ml) were purchased from Sigma-Aldrich; Merck
KGaA. Go Taq® qPCR Master Mix was purchased from Promega
Corporation (Madison, WI, USA). The RevertAid™ First Strand cDNA
Synthesis kit was purchased from MBI Fermentas; Thermo Fisher
Scientific, Inc. CDDP was purchased from Qilu Pharmaceutical Co.,
Ltd. (cat no., EA4A7039A; Jinan, China). Epirubicin was purchased
from Hisun Pharmaceutical Co., Ltd. (cat no., 17035211; Taizhou,
China). VCR was purchased from Shenzhen Main Luck Pharmaceuticals
Inc., (cat. no., 1608V3; Shenzhen, China). Celecoxib was purchased
from Pfizer, Inc. (New York, NY, USA; cat. no., S52338).
MTT assay
The effect of celecoxib on cell viability of Jurkat
and Hut-78 cells was evaluated by the MTT assay. The Jurkat and
Hut-78 cells were plated at a density of 1×105
cells/well in 200 µl RPMI-1640 medium supplemented with 10% FCS and
treated with celecoxib (20, 40 and 80 µmol/l) in 96-well plates
(Gibco; Thermo Fisher Scientific, Inc.). As a control, Jurkat and
Hut-78 cells were not treated with celecoxib. Following incubation
for 24, 48 and 72 h at 37°C in a humidified incubator, 10 µl MTT
solution [5 mg/ml in phosphate-buffered saline (PBS)] was added to
the cells, which were then incubated again for 4 h at 37°C.
Subsequently, 100 µl DMSO was added to dissolve the formazan
crystals. The absorbance at 492 nm was measured using a Titertek
Multiskan™ microplate reader (Flow Laboratories, North Ryde,
Australia) to assess the effect of celecoxib on cell viability.
Lymphocyte cell viability assay
The peripheral blood was extracted from 3 cases of
healthy volunteers without carcinomas, cardiovascular and endocrine
system diseases, who underwent routine physical examination at the
Fourth Hospital of Hebei Medical University (Shijiazhuang, China)
between April 2016 and May 2016. The median age of the volunteers
was 39 years (range, 32–44 years). T lymphocytes was separated from
peripheral blood of healthy volunteers (2 females and 1 male) by
density gradient centrifugation (240 × g for 20 min) at room
temperature, using Ficoll separating medium (density: 1.077 g/ml).
MTT assay was used to determine the cytotoxic effect of celecoxib
on cell viability of T lymphocytes, as aforementioned. As a
control, T lymphocytes were not treated with celecoxib. This
research was approved by the ethic committee of the Fourth Hospital
of Hebei Medical University and written informed consent was
obtained from all participants.
Flow cytometry
The effect of celecoxib on the apoptosis level of
Jurkat and Hut-78 cells was assessed by flow cytometry analysis.
Jurkat and Hut-78 cells (106/ml) treated with celecoxib
at different doses (0, 20, 40 and 80 µmol/l) were incubated with 5
µl Annexin V-FITC and PI double stain at room temperature for 15
min. Following staining, cells were resuspended in ice-cold PBS and
were analyzed using a flow cytometer (FACSCalibur™; BD
Biosciences). Data were analyzed using CellQuest Pro version 5.1
software (BD Biosciences).
Western blotting analysis
Jurkat and Hut-78 cells treated with celecoxib at
different doses (0, 20, 40 and 80 µmol/l) were lysed with 500 µl
lysis buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris-HCl at pH
7.4, 1 mM EDTA, 1 mM EGTA at pH 8.0, 0.2 mM Na3VO4, 0.2 mM
phenylmethyl sulphonyl fluoride and 0.5% NP-40). The protein
concentration in the lysates was determined by BCA assay. The
lysates were then subjected to western blotting analysis to
determine the amount of P-gp, MRP1, Bcl-2, Bax, p65 and β-actin
protein in cells. Briefly, the total proteins (40 µg per well) were
subjected to 10% SDS-PAGE and were electro-transferred onto a
polyvinylidene difluoride membrane. The membranes were blocked in
PBS containing 5% FCS for 2 h at room temperature. Membranes were
incubated with rabbit anti-human primary antibodies at different
dilutions, including monoclonal antibodies to p65 (1:2,000), Bcl-2
(1:1,000), Bax (1:1,000), P-gp (1:1,000), MRP1 (1:1,000) and
β-actin (1:5,000) for 8 h at 4°C. The PVDF membranes were
visualized in the Odyssey infrared imaging system, after incubation
with HRP-conjugated goat anti-rabbit secondary antibodies (1:5,000)
for 2 h at room temperature. The expression levels of these target
proteins were calculated as the ratio of the intensity of the
specified protein to that of β-actin, using Odyssey v3.0 software
(LI-COR Biosciences, Lincoln, NE, USA).
RNA preparation and reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
analysis
An RT-qPCR assay was used to evaluated the effect of
celecoxib on expression of P65, Bcl-2, Bax, MDR1 and MRP1 mRNA in
Jurkat and Hut-78 cells. Total RNA was extracted from Jurkat and
Hut-78 cells treated with celecoxib at different doses (0, 20, 40
and 80 µmol/l) using TRIzol reagent and the first strand cDNAs were
generated from 1 µg total RNA using RevertAid™ First Strand cDNA
Synthesis kit and incubation at 42°C for 60 min. The resultant
cDNAs were amplified by RT-qPCR using GoTaq® qPCR
Master. The specific primers sequences (Shanghai Generay Biotech,
Co., Ltd., Shanghai, China) and annealing temperature for the
target genes are summarized in Table
I. The following thermocycling conditions were used for the
PCR: 95°C for 10 min; 40 cycles of 95°C for 15 sec, 60°C for 15 sec
and 60°C for 1 min. The relative level of expression of each target
gene was assessed by the 2−ΔΔCq method (5).
 | Table I.Primer sequences and annealing
temperature for the reverse transcription-quantitative polymerase
chain reaction. |
Table I.
Primer sequences and annealing
temperature for the reverse transcription-quantitative polymerase
chain reaction.
| Gene | Primer sequence | Annealing
temperature, °C |
|---|
| β-actin | F:
5′-GTTGTGATGGGTTCTGA-3′ | 60 |
|
| R:
5′-GAGCAATAGCGTCTGTG-3′ |
|
| MRP1 | F:
5′-CGCTGAGTTCCTGCGTACC-3′ | 60 |
|
| R:
5′-TCTGCGGTGCTGTTGTGG-3′ |
|
| MDR1 | F:
5′-CAGAGGGGATGGTCAGTGTT-3′ | 60 |
|
| R:
5′-CGTGGTGGCAAACAATACAG-3′ |
|
| P65 | F:
5′-GGCGAGAGGAGCACAGATAC-3′ | 60 |
|
| R:
5′-ATCTTGAGCTCGGCAGTGTT-3′ |
|
| Bcl-2 | F:
5′-GTGAACTGGGGGAGGATTGT-3′ | 60 |
|
| R:
5′-GGAGAAATCAAACAGAGGCC-3′ |
|
| Bax | F:
5′-GGCCGGGTTGTCGCCCTTTT-3′ | 60 |
|
| R:
5′-CCGCTCCCGGAGGAAGTCCA-3′ |
|
Rhodamine-123 efflux assay
The rhodamine-123 efflux assay was performed to
evaluate the efflux pump effect of P-gp and MRP1 in the Jurkat and
Hut-78 cells treated with celecoxib at different doses (0, 20, 40
and 80 µmol/l). The celecoxib-treated cells were co-cultured with
Rhodamine-123 (5 mg/ml) for 1 h at room temperature and analyzed
using a FACScan flow cytometer (FACSCalibur™, BD Biosciences) to
analyze the intensity of fluorescence. Data were analyzed by
CellQuest Pro version 5.1 software (BD Biosciences).
Statistical analysis
The data are reported as the mean ± standard
deviation. One-way analysis of variance was performed to determine
the significance between groups. Turkey's method was used for
multiple comparisons. P<0.05 were considered to indicate a
statistically significant difference. Experiments were repeated in
triplicate. All statistical analyses were conducted using the SPSS
software version 13.0 (SPSS, Inc., Chicago, IL, USA).
Results
Celecoxib may inhibit growth and
enhance the sensitivity of T lymphocytes to chemotherapy drugs
Firstly, MTT assays were used to determine the
inhibitory effect of celecoxib on the viability of T-cell lymphoma
cells. As demonstrated in Fig. 1A,
celecoxib significantly suppressed cell viability in Jurkat and
Hut-78 cells in a time- and dose-dependent manner compared with the
control group (P<0.05), indicating that celecoxib may markedly
inhibit the proliferation of T lymphoma cells. Whether celecoxib
had an additive effect on the response to chemotherapy agents in
T-cell lymphoma was additionally explored. As demonstrated in
Fig. 1B, the viability of Jurkat and
Hut-78 cells over 24 h was dose-dependently suppressed by CDDP,
epirubicin and VCR, with significantly increased inhibition
observed in Jurkat and Hut-78 cells treated with chemotherapeutic
drugs combined with celecoxib compared with cells treated with
chemotherapy drugs alone (P<0.05). Additionally, the
IC50 values of chemotherapy agents were significantly
reduced in Jurkat and Hut-78 cells treated with celecoxib in a
dose-dependent manner compared with those that were not treated
with celecoxib (P<0.05) (Fig. 1C).
These results suggested that celecoxib may enhance the sensitivity
of T-cell lymphoma cells to chemotherapy drugs. In contrast, the
viability of normal T cells was not reduced following treatment
with celecoxib at the different doses (P>0.05) (Fig. 1D).
To additionally evaluate the facilitating effect of
celecoxib on the chemosensitivity of T-cell lymphoma, flow
cytometry analysis was performed to determine whether celecoxib
induced apoptosis in Jurkat and Hut-78 cells. As demonstrated in
Fig. 2, the apoptosis rate of Jurkat
and Hut-78 cells treated with celecoxib was significantly increased
compared with the cells in the control group (P<0.01),
indicating that celecoxib-induced proliferation inhibition of
lymphoma cells may be due to a pro-apoptotic effect. Furthermore,
when Jurkat and Hut-78 cells were treated with chemotherapy agents
combined with celecoxib, the percentage of apoptotic cells was also
significantly increased compared with cells treated with
chemotherapy agents alone. These data revealed that celecoxib may
enhance cytotoxicity of chemotherapy drugs by the pro-apoptotic
effect, additionally suggesting that celecoxib is a potential
chemosensitizer for T-cell lymphoma treatment.
Celecoxib inhibits the expression of
the MDR-associated proteins via the NF-κB signaling pathway
To address the underlying mechanism that may be
involved in the celecoxib-mediated pro-apoptotic and
chemosensitizing effect, the changes of MDR-associated proteins
expression in T-cell lymphoma cells was analyzed. As P-gp, encoded
by MDR1, and MRP1 are closely associated with the development of
MDR, while Bcl-2 and Bax are important factors involved in the
induction of apoptosis, their expression was evaluated in Jurkat
and Hut-78 cells treated with celecoxib. The results indicated that
the expression levels of Bcl-2, MDR1 and MRP1 mRNA and proteins
were significantly downregulated, whereas the expression of Bax was
upregulated in tumor cells treated with celecoxib compared with
those that were not treated with celecoxib (P<0.05; Fig. 3A and B).
 | Figure 3.Effect of celecoxib on the expression
of proteins associated with chemotherapy sensitivity in Jurkat and
Hut-78 cells. (A) Reverse transcription quantitative polymerase
chain reaction assays of the expression levels of P65, MDR1, MRP1,
Bcl-2 and Bax mRNAs in Jurkat and Hut-78 cells. (B) Western
blotting assays of the expression levels of P65, P-gp, encoded by
MDR1, MRP1, Bcl-2 and BaX protein expression in Jurkat and Hut-78
cells. β-actin was used as an internal loading control. The
experiments presented are representative of three independent
experiments. *P<0.05 and **P<0.01 vs. the control group. P65,
tumor protein 65; P-gp, P-glycoprotein; MDR1, multidrug resistance
protein 1; MRP1, multidrug resistance-associated protein 1; Bcl-2,
B-cell lymphoma 2, Bax, Bcl-2-associated X protein. |
Furthermore, the effect of celecoxib on the
expression of p65, which is involved in regulating cell apoptosis
and inducing MDR, was assessed. As indicated in Fig. 3A and B, the expression of p65 was
decreased significantly in celecoxib-treated Jurkat and Hut-78
cells. It is well-known that the NF-κB P65 subunit participates in
activating various downstream genes associated with the regulation
of apoptosis and induction of MDR (6–8). Several
studies have revealed that Bcl-2 may suppress the activity of
multiple pro-apoptotic proteins by binding with P65 (3,6).
Additionally, previous studies also confirmed that the majority of
the MDR-associated proteins, including P-gp and MRP1, are
positively regulated by the NF-κB pathway, and that blockade of the
NF-κB pathway may suppress their expression (6,7).
Therefore, these results suggested that the celecoxib-mediated
pro-apoptotic and chemosensitizing effect on T lymphocytes are
associated with decreased expression of MDR-associated proteins
Bcl-2, Bax, P-gp and MRP1 via inhibition of the NF-κB pathway.
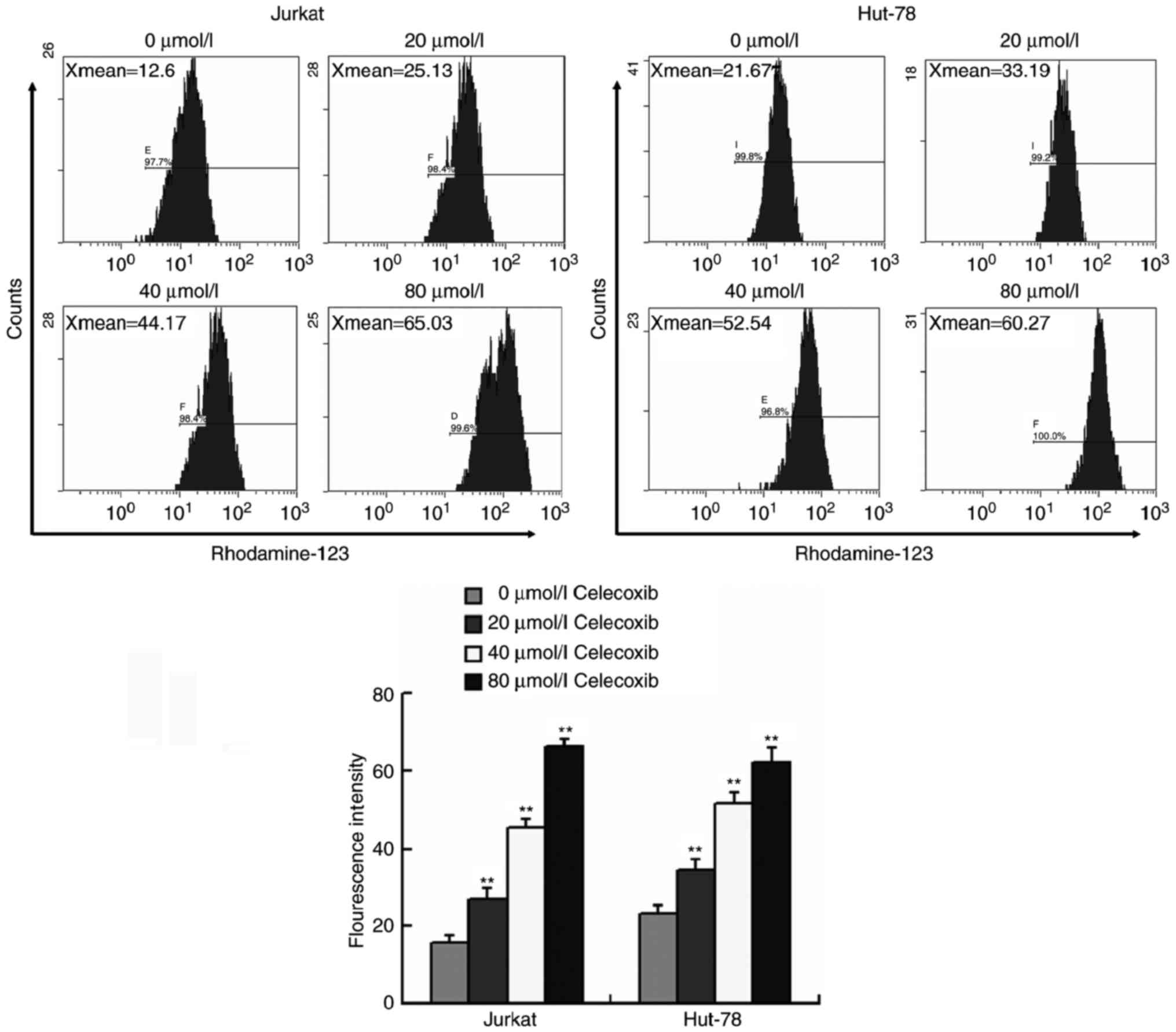
Celecoxib inhibits the efflux of
intracellular Rhodamine-123 in Jurkat and Hut-78 cells
The effect of celecoxib on the exclusion of
intracellular Rhodamine-123 by pump proteins was evaluated by a
Rhodamine-123 efflux assay. The results of flow cytometry analysis
indicated that the intracellular concentration of Rhodamine-123 was
significantly increased in Jurkat and Hut-78 cells treated with
celecoxib compared with those that were not treated with celecoxib
(P<0.01, Fig. 4), indicating that
celecoxib may impair the efflux effect of the P-gp and MRP1 in
Jurkat and Hut-78 cells, inhibiting the exclusion and promoting the
intracellular accumulation of chemotherapy agents.
Discussion
At present, T-cell lymphoma has one of the highest
mortality rates of all non-Hodgkin lymphoma; the average mortality
rate of the patients with T-cell lymphoma remains >70% (1). Whilst the traditional chemotherapy
strategies have markedly improved in the previous 3 decades, MDR
remains a great challenge in lymphoma treatment. It was suggested
that the application of anticancer agents may induce the
overexpression of various MDR-associated proteins and blockage of
apoptosis in tumor cells (7).
However, the exact mechanism that induces MDR has not been
clarified at present. Strategies of avoiding MDR and improving
sensitivity to chemotherapy remain the most critical problems in
treatment of T-cell lymphoma.
Whilst celecoxib has been identified as an effective
anti-tumor agent, characterized by inhibiting proliferation in a
variety of tumor cells (8), little is
known about its effect on chemosensitivity in T-cell lymphoma.
Previous studies have revealed that celecoxib may prevent
chemotherapy drug resistance in digestive and gynecologic
carcinomas (9–12), but whether it serves a similar role in
T-cell lymphoma has not been determined. The present study
demonstrated that celecoxib sensitized Jurkat and Hut-78 cells to
various chemotherapy drugs by triggering apoptosis and inhibiting
the expression of MDR-associated proteins.
Firstly, the inhibitory effect of celecoxib on
T-cell lymphoma cells was investigated using an MTT assay. The
results demonstrated that celecoxib significantly suppressed the
viability of Jurkat and Hut-78 cells and facilitated the
sensitivity of cells to conventional chemotherapy drugs, including
CDDP, epirubicin and VCR. The IC50 value of the
chemotherapy drugs was significantly decreased in celecoxib-treated
cells compared with those that were not treated with celecoxib.
Additionally, the results of the flow cytometry analysis
demonstrated that celecoxib may induce apoptosis and enhanced
cytotoxicity of the chemotherapy drugs in Jurkat and Hut-78 cells.
These data demonstrated that the chemotherapy agents combined with
celecoxib improved the efficacy of chemotherapy, suggesting that
celecoxib may be a potential chemosensitizer in the clinical
treatment of T-cell lymphoma. The present study may provide novel
insight into the therapeutic effects of celecoxib, and assist in
overcoming chemotherapy resistance in T-cell lymphoma.
To additionally explore the pro-apoptotic and
chemosensitizing effect of celecoxib on T-cell lymphoma cells, the
role of celecoxib in regulating the expression of MDR-associated
proteins was investigated. It has been demonstrated previously that
the Bcl2 family members protect against the activation of multiple
signaling pathways leading to apoptosis of tumor cells, whereas the
activation of Bax family members will induce cell apoptosis,
indicating that Bcl2/Bax axis played a crucial part in regulating
the common apoptosis pathway (8–10). In the
present study, the celecoxib-treated lymphoma cells exhibited
significantly decreased expression of Bcl-2 and increased
expression of Bax compared with those that were not treated with
celecoxib, suggesting that effect on the Bcl-2/BaX protein ratio
may be an important contributing factor to celecoxib-induced
apoptosis. Additionally, it has been identified that the abnormal
expression of P-gp and MRP1 on the surface of the cell membrane may
impair tumor chemosensitivity and lead to MDR by removing
chemotherapy drugs from tumor cells (13–16).
Overexpression of P-gp and MRP1 in tumor tissues has been
associated with poor response to clinical chemotherapy in T-cell
lymphoma (15,17). The results of the present study also
demonstrated that the expression of P-gp and MRP1 was significantly
downregulated in Jurkat and Hut-78 cells treated with celecoxib
compared with those that were not treated with celecoxib,
indicating that celecoxib may inhibit the efflux of intracellular
anticancer agents. In addition, the intracellular concentration of
Rhodamine-123 in Jurkat and Hut-78 cells treated with celecoxib was
significantly increased, indicating that celecoxib weakened the
efflux function of P-gp and MRP1.
To elucidate the signaling pathway affected by
celecoxib, the effect of celecoxib on the activity of the NF-κB
pathway was evaluated. P65, the primary functioning subunit of
activated NF-κB, is an important indicator for predicting MDR in
tumors, as it promotes cell viability, causes apoptosis resistance
and enhances expression of various MDR-associated proteins
including P-gp and MRP1 (7).
Furthermore, previous studies have also confirmed that the
expression of Bcl-2 may be directly upregulated by activation of
the NF-κB signaling pathway, thereby inhibiting Bax expression and
leading to an anti-apoptotic effect in tumor cells (8–11). In the
present study, the results suggested that the expression of P65,
Bcl2, P-gp and MRP1 was significantly decreased, while the
expression of Bax was increased in celecoxib-treated Jurkat and
Hut-78 cells t compared with those that were not treated with
celecoxib. Therefore, P65 may be a key factor affected by celecoxib
in regulating the expression of the apoptosis- and MDR-associated
proteins.
To conclude, the present study identified that
celecoxib may induce apoptosis and improve the sensitivity of
T-cell lymphoma cells to chemotherapy by decreasing the expression
of P-gp, MRP1 and Bcl-2/Bax ratio via the NF-κB pathway, and by
increasing the intracellular accumulation of anticancer drugs.
Celecoxib may therefore be a potential candidate for improving the
curative effect of chemotherapy drugs in T-cell lymphomas.
Acknowledgements
The present study was partially supported by the
Natural Science Foundation of China (grant no., 81372200), the
Natural Science Foundation of China of Hebei Province (grant no.,
H2015206376) and the Health and Family Planning Commission of Hebei
Province (grant nos., 20150305 and 20160171).
Glossary
Abbreviations
Abbreviations:
|
CDDP
|
cis-diamminedichloroplatinum
|
|
VCR
|
vinblastine
|
|
IC50
|
half-maximal inhibitory
concentration
|
|
MDR
|
multidrug resistance
|
References
|
1
|
Wang C, McKeithan TW, Gong Q, Zhang W,
Bouska A, Rosenwald A, Gascoyne RD, Wu X, Wang J, Muhammad Z, et
al: IDH2R172 mutations define a unique subgroup of patients with
angioimmunoblastic T-cell lymphoma. Blood. 126:1741–1752. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fang P, Zhang X, Gao Y, Ding CR, Cui F and
Jiao SC: Reversal effect of melanoma differentiation associated
gene-7/interleukin-24 on multidrug resistance in human
hepatocellular carcinoma cells. Anat Rec. 295:1639–1646. 2012.
View Article : Google Scholar
|
|
3
|
Futagami S, Suzuki K, Hiratsuka T, Shindo
T, Hamamoto T, Ueki N, Kusunoki M, Miyake K, Gudis K, Tsukui T and
Sakamoto C: Chemopreventive effect of celecoxib in gastric cancer.
Inflammopharmacology. 15:1–4. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu HB, Shen FM and Lv QZ: Celecoxib
enhanced the cytotoxic effect of cisplatin in chemo-resistant
gastric cancer xenograft mouse models through a
cyclooxygenase-2-dependent manner. Eur J Pharmacol. 776:1–8. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan ZX, Zheng Z, Xue W, Zhao MZ, Fei XC,
Wu LL, Huang LM, Leboeuf C, Janin A, Wang L, et al: MicroRNA181a is
overexpressed in T-cell leukemia/lymphoma and related to
chemoresistance. Biomed Res Int. 2015:1972412015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuo MT, Liu Z, Wei Y, Lin-Lee YC, Tatebe
S, Mills GB and Unate H: Induction of human MDR1 gene expression by
2-acetylaminofluorene is mediated by effectors of the
phosphoinositide 3-kinase pathway that activate NF-kappaB
signaling. Oncogene. 21:1945–1954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Kang F, Li J, Zhang J and Shan B:
Overexpression of p65 attenuates celecoxib-induced cell death in
MDA-MB-231 human breast cancer cell line. Cancer Cell Int.
13:142013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang L, Liu LH, Shan BE, Zhang C, Sang MX
and Li J: Celecoxib promotes apoptosis of breast cancer cell line
MDA-MB-231 through down-regulation of the NF-kappaB pathway. Ai
Zheng. 28:569–574. 2009.(In Chinese). PubMed/NCBI
|
|
10
|
Arunasree KM, Roy KR, Anilkumar K, Aparna
A, Reddy GV and Reddanna P: Imatinib-resistant K562 cells are more
sensitive to celecoxib, a selective COX-2 inhibitor: Role of COX-2
and MDR-1. Leuk Res. 32:855–864. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Z, Duan ZJ, Chang JY, Zhang ZF, Chu R,
Li YL, Dai KH, Mo GQ and Chang QY: Sinomenine sensitizes
multidrug-resistant colon cancer cells (Caco-2) to doxorubicin by
downregulation of MDR-1 expression. PLoS One. 9:e985602014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan M and Nuriding H: Reversal effect of
vitamin D on different multidrug-resistanT cells. Genet Mol Res.
13:6239–6247. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rauf A, Uddin G, Raza M, Ahmad B, Jehan N,
Siddiqui BS, Molnar J, Csonka A and Szabo D: Reversal of multidrug
resistance in mouse lymphoma cells by extracts and flavonoids from
pistacia integerrima. Asian Pac J Cancer Prev. 17:51–55. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu D, Shi HC, Wang ZX, Gu XW and Zeng YJ:
Multidrug resistance-associated biomarkers PGP, GST-pi, Topo-II and
LRP as prognostic factors in primary ovarian carcinoma. Br J Biomed
Sci. 68:69–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu WY, Huang YY, Liu XG, He JY, Chen DD,
Zeng F, Zhou JH and Zhang YK: Prognostic evaluation of CapG,
gelsolin, P-gp, GSTP1, and Topo-II proteins in non-small cell lung
cancer. Anat Rec. 295:208–214. 2012. View
Article : Google Scholar
|
|
16
|
Sakamoto A, Matsumaru T, Yamamura N,
Uchida Y, Tachikawa M, Ohtsuki S and Terasaki T: Quantitative
expression of human drug transporter proteins in lung tissues:
Analysis of regional, gender, and interindividual differences by
liquid chromatography-tandem mass spectrometry. J Pharm Sci.
102:3395–3406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshimori M, Takada H, Imadome K, Kurata
M, Yamamoto K, Koyama T, Shimizu N, Fujiwara S, Miura O and Arai A:
P-glycoprotein is expressed and causes resistance to chemotherapy
in EBV-positive T-cell lymphoproliferative diseases. Cancer Med.
4:1494–1504. 2015. View
Article : Google Scholar : PubMed/NCBI
|