Introduction
Epithelial ovarian cancer (EOC) is associated with
the second highest incidence and highest mortality rate among
gynecological malignancies worldwide. At present, the survival rate
remains poor for patients with advanced EOC (1,2). There
were ~21,880 new cases of EOC diagnosed and 13,850 mortalities in
the United States in 2010 (3). The
poor prognosis of EOC is primarily due to its advanced stage at the
time of diagnosis (4). EOC mortality
occurs predominantly due to metastasis; without effective screening
tests or the appearance early symptoms, the majority of patients
with EOC are diagnosed with metastatic disease (2). Approximately 75% of patients are
diagnosed with advanced (stage III/IV) ovarian carcinoma, which is
characterized by peritoneal or distant metastases, respectively,
and for which the 5-year survival rate is 15–20%; the rate for
patients diagnosed during the early stage (stage I/II) is 80–90%
(5). The majority of cases may be
curable if the disease is diagnosed at the early stage. However,
the molecular mechanism of EOC aggressiveness has yet to be fully
characterized. At present, there are no reliable and accurate
markers that predict aggressive phenotypes. Therefore, establishing
novel approaches to increase the sensitivity and decrease
resistanceto EOC therapy remains critical.
Forkhead box (FOX) A1 representsa potential
candidate gene for therapeutic targeting in human EOC; FOXA1 is a
transcription factorthat is expressed widely and functions in the
development of numerous types of human tissue (6,7). FOXA1,
also known as hepatocyte nuclear factor 3α, belongs to a
superfamily of winged helix transcription factors and has been
identified as a hepatocyte-enriched transcription factor required
for the expression of transthyretin and α-1-antitrypsin (8–10). FOXA1
has attracted attentionas it interacts with cis-regulatory regions
in heterochromatin to enhance the interaction of ERa with chromatin
(11). FOXA1 also serves important
functions during multiple phases of mammalian life, including in
the regulation of the development of the endodermal layer
andorganogenesis, as well as in metabolism and homeostasis in the
adult (12–14). FOXA1 is an endodermal ‘pioneer
transcription factor’, which bindsto the promoters and enhancersto
enable chromatin access to other tissue-specific transcription
factors (15,16). FOXA1 is highly expressed in lung
tissue (6). Previous studies have
revealed that FOXA1 expression is increased in liver, colon,
thyroid and esophageal cancer (17–19). FOXA1
is also essential for the estrogen signaling pathway in breast
cancer cells, which is associated with a favorable prognosis
(20). It has been suggested that
FOXA1 influences androgen receptor (AR) binding to chromatinin
androgen-dependent and androgen-independent prostate cancer
(21,22). However, no studies have reported on
the function of FOXA1 in epithelial ovarian tumors to the best of
our knowledge.
To the best of our knowledge, there have also been
no studies published on the association between FOXA1 expression
and clinical features to determine its clinicopathological
significance in human EOC. Therefore, the present study assessed
whether the expression of FOXA1 may serve as a novel biomarker for
the prognosis of patients with OC and whether it may be suitable
for development as a target for therapy. FOXA1 gene expression in
EOC samples was compared with benign ovarian tumor surface
epithelia and normal ovarian tissues. The association between FOXA1
expression and clinicopathological data in a group of patients with
EOC was also analyzed. Finally, the prognostic potential of FOXA1
protein expression in EOC was evaluated.
Materials and methods
Pathological samples
Formalin-fixed, paraffin-embedded ovarian tissues
were harvested from 110 cases of primary epithelial ovarian
carcinoma and 24 benign ovarian tumor surface epithelium samples.
The patients with ovarian tumors underwent surgery from January
2003 to December 2007 at the Affiliated Hospital of Nantong
University (Nantong, China). The 10 healthy individual ovary
samples were also harvested for comparison. The clinical stage of
all the patients with EOC was determined using the FIGO staging
system (23); 64 cases were low stage
tumors (I–II) and 46 were high stage tumors (III–IV). Neither
preoperative radiation nor chemotherapy had been received by the
patients with EOC. Clinical information on each patient, including
age, histological type, grade based on the World Health
Organization (WHO) criteria (24),
FIGO stage and tumor size, was collected from medical records,
including from a 5-year follow-up. The mean age of the patients was
54.5 years (range, 29–78 years). The protocol of the present study
was approved by the Institutional Review Board at the Affiliated
Hospital of Nantong University. Informed written consent was
obtained for each patient.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
EOC fresh-frozen at −80°C and paired normal ovarian
epithelium samples (n=16 of each) were obtained from January 2011
to August 2012 at the Affiliated Hospital of Nantong University.
The mean age of the patients was 34.5 years (range, 23–64 years).
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was used to extract total RNA from the frozen
samples. Total RNA was then reverse transcribed using a RevertAid™
First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol at 42°C
for 30 min. For RT-qPCR, the analysis of mRNA levels was performed
using SYBR-Green Reagents (Toyobo Life Science, Osaka, Japan) using
an iQ5 Multicolor Real-time PCR Detection System, and all mRNA
levels were normalized to GAPDH (n=3). The FOXA1 primer sequences
were as follows: Forward, 5′-GTTGAAGACTCCAGCCTCCTC-3′ and reverse,
5′-CTGCCCAGAACATCATCCCT-3′. GAPDH primers sequences were as
follows: Forward, 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse,
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ (Invitrogen; Thermo Fisher
Scientific, Inc.). GAPDH mRNA levels were used as an internal
control following melt curve analysis (25). Amplification conditions were as
follows: Taq activation at 94°C for 2 min; 35 cycles of 94°C for 20
sec, 58°C for 20 sec andelongation at 72°C for 30 sec.
Immunohistochemical analysis
The 4 µm sections were deparaffinized using a graded
ethanol series (Xylene 3 times for 3 min, then 100, 95 and 70%, 3
times for 2 min each), and 0.3% hydrogen peroxide was used to block
endogenous peroxidase activity at room temperature for 15 min. For
antigen retrieval, the sections were placed in 10 mM citrate buffer
(pH 6.0) and heated to 121°C in an autoclave for 20 min. Goat serum
(10%; Sangon Biotech Co., Ltd., Shanghai, China) was used to block
non-specific reactions for 1 h at room temperature. The sections
were then rinsed in phosphate-buffered saline (pH 7.2) and
incubated with anti-human FOXA1 antibody (dilution, 1:200; cat. no.
ab23738; Abcam, Cambridge, MA, USA) overnight at 4°C. Then, the
sections were incubated with secondary antibody (dilution 1:1,000;
cat. no. ab205718; Abcam, Cambridge, MA, USA) at room temperature
for 30 min. Sections incubated without antibody were used as the
negative control. All slides were processed using the peroxidase
anti-peroxidase method (Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA). The sections were counterstained with hematoxylin,
dehydrated, and coverslips were added subsequent to rinsing with
water. The stained sections were observed under a phase contrast
microscope (magnification, ×400). All immunostained sections were
assessed blindly. High-power fields (n=5) for each specimen were
randomly selected to assess FOXA1 expression, and nuclear staining
was observed under high-power magnification. To determine the mean
percentage of immunostained cells, >500 cells were counted. The
analysis was repeated twice in half of the samples to decrease the
likelihood of technical errors; the results of these repeats were
similar. If the nuclei were stained >5% and the plasma was not,
FOXA1 protein expression was considered positive. Staining of
<5% of the cells was judged as negative, 5–30% as weak (low
expression), 31–70% as moderate and >70% as strong (high
expression).
Statistical analysis
SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA)
was used to perform statistical analysis. Mean ± standard deviation
was used to represent the results. To compare clinicopathological
data, the Fisher's exact test, the χ2 test, and
two-sample t-tests were used. Overall survival time was defined as
the interval between primary surgery and patient mortality or the
final follow-up. Survival curves were plotted using the
Kaplan-Meier method. The association between clinical
characteristics and survival time in patients with EOC was assessed
using the log rank test. P<0.05 was considered to indicate a
statistically significant difference.
Results
FOXA1 mRNA expression differs between
EOC and normal ovarian epithelium tissues
In the present study, total RNA was extracted from
paired, frozen EOC and normal ovarian epithelium tissues, and
subjected to RT-qPCR to measure FOXA1 mRNA expression, normalized
to GAPDH mRNA level. FOXA1 expression was increased in EOC
specimens compared with the corresponding normal tissue (Fig. 1A and B). The mean relative expression
of FOXA1 mRNA in EOC and the corresponding benign ovarian tumor
surface epithelium tissues was 1.389±0.1225 and 1.029±0.0626,
respectively, indicating a significant difference (P=0.014;
Fig. 1C).
Expression of FOXA1 protein in
epithelial ovarian tumor and non-cancerous tissues
To confirm the increased expression of FOXA1 in EOC
tissues compared with non-cancerous tissues, immunohistochemical
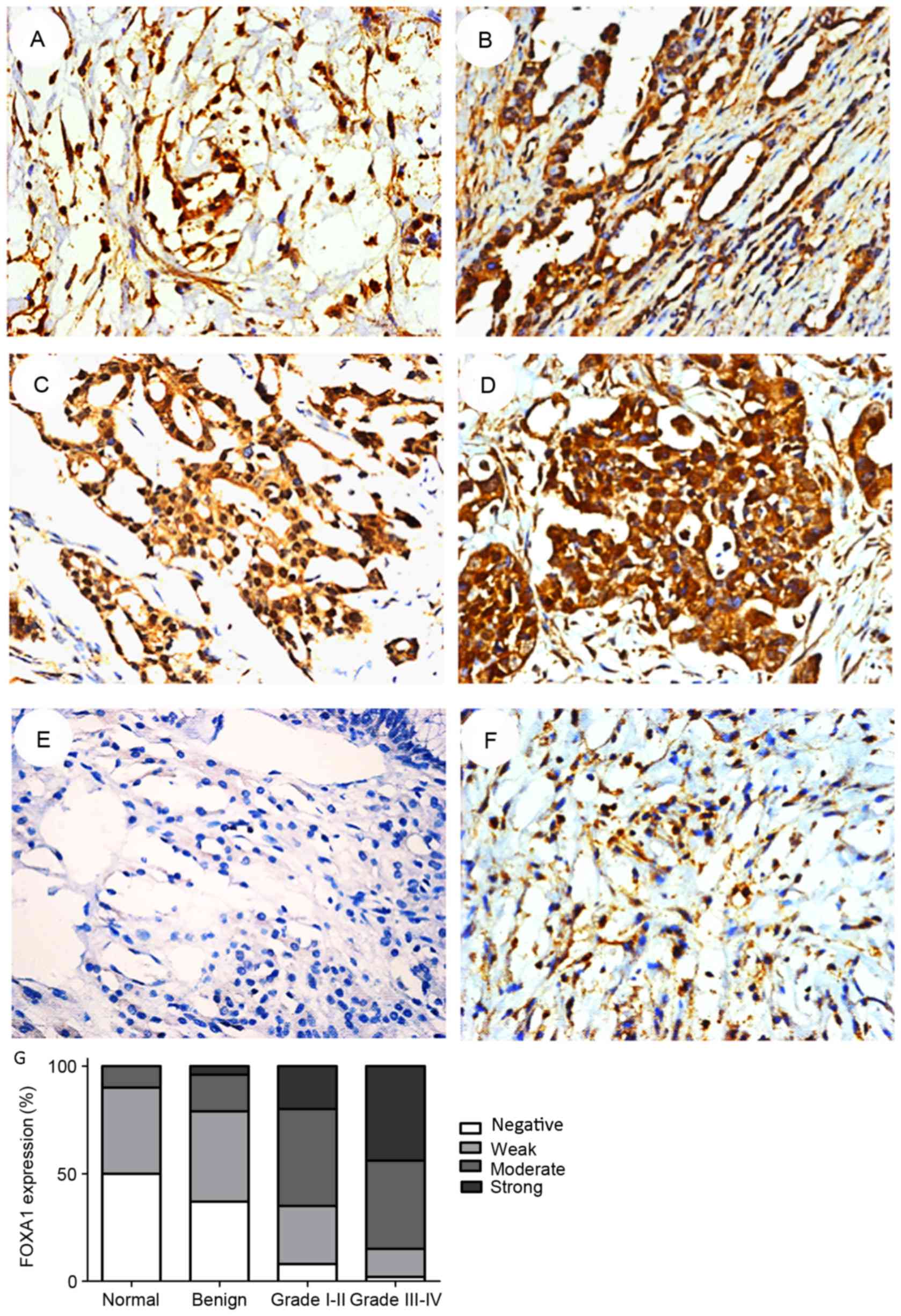
staining was performed. FOXA1 expression was primarily observed in
the nuclei and not inthe cytoplasm (Fig.
2). As revealed by immunohistochemical analysis, FOXA1
expression was upregulated in EOC. Only 10.0% of the normal ovarian
tissues demonstrated moderate or strong positive expression of
FOXA1: ~20.8 and 73.6% of the benign ovarian tumor surface
epithelium tissues and EOC specimens, respectively, revealed
moderate or strong positive expression of FOXA1 (Table I). Furthermore, the percentage of
FOXA1 positive cells increased with an increasing WHO grade in EOC
tissue: 84.8% of high-grade (III–IV) and 65.6% of low-grade (I–II)
OC tissues exhibited moderate or strong expression of FOXA1
(Table I). Therefore, the total
expression of FOXA1 in EOC tissues was significantly increased
compared with non-cancerous tissues (P<0.001), and increased
with increasing tumor grade (P=0.024) (Table II). The results indicated that the
mean value of FOXA1 immunoreactivity in benign ovarian tumor
surface epithelium tissues was not significantly differentcompared
with normal ovarian tissues (P=0.450).
 | Table I.Expression of FOXA1 in normal ovarian
and ovarian cancer samples of different grades. |
Table I.
Expression of FOXA1 in normal ovarian
and ovarian cancer samples of different grades.
|
|
| FOXA1 expression |
|---|
|
|
|
|
|---|
| Group | Cases, n | Negative | Weak | Moderate | Strong |
|---|
| Normal | 10 | 5 | 4 | 1 | 0 |
| Benign | 24 | 9 | 10 | 4 | 1 |
| Grade I/II | 64 | 5 | 17 | 29 | 13 |
| Grade III/IV | 46 | 1 | 6 | 19 | 20 |
 | Table II.FOXA1 expression and
clinicopathological parameters in 110 ovarian cancer specimens,
including a comparison to normal and benign specimens. |
Table II.
FOXA1 expression and
clinicopathological parameters in 110 ovarian cancer specimens,
including a comparison to normal and benign specimens.
|
|
| FOXA1 expression,
n |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Cases, n | Low | High | P-value |
|---|
| Age, years |
|
|
| 0.424 |
| ≤55 | 54 | 21 | 33 |
|
|
>55 | 56 | 26 | 30 |
|
| Status |
|
|
| <0.001 |
|
Normal | 10 | 9 | 1 |
|
|
Benign | 24 | 19 | 5 |
|
|
Invasive | 110 | 29 | 81 |
|
| Clinical stage |
|
|
| 0.024 |
| I–II | 64 | 22 | 42 |
|
|
III–IV | 46 | 7 | 39 |
|
| Histological
subtype |
|
|
| 0.768 |
| Serous
papillary adenocarcinoma | 80 | 36 | 44 |
|
|
Endometrioid
adenocarcinoma | 12 | 3 | 9 |
|
| Clear
cell carcinoma | 9 | 4 | 5 |
|
|
Mucinous carcinoma | 5 | 2 | 3 |
|
|
Transitional cell
carcinoma | 4 | 2 | 2 |
|
|
Differentiation |
|
|
| 0.003 |
|
Good | 48 | 30 | 18 |
|
|
Moderate | 26 | 12 | 14 |
|
|
Poor | 36 | 9 | 27 |
|
| Tumor diameter,
cm |
|
|
| 0.179 |
| ≤5 | 49 | 28 | 21 |
|
|
>5 | 61 | 27 | 34 |
|
| Tumor location |
|
|
| 0.061 |
|
Unilateral | 59 | 36 | 23 |
|
|
Bilateral | 51 | 22 | 29 |
|
FOXA1 expression in EOC is associated
with a reduced survival time
To determine the association between FOXA1
expression and clinicopathological characteristics in EOC, the
clinical data of 110 patients with EOC were analyzed (Table II). Increased expression of FOXA1 in
EOC was significantly associated with the tumor WHO grade (P=0.024)
and differentiation status (P=0.003). There was no significant
association between FOXA1 expression and age, histological subtype,
tumor diameter or location. Furthermore, increased FOXA1 expression
in non-cancerous epithelial ovarian tissues was not significantly
associated with the clinical characteristics of EOC (data not
shown). However, the overall survival time of the patients in the
FOXA1 low expression and high expression groups differed
significantly (P=0.013) (Fig. 3). The
patients with low expression of FOXA1 exhibited increased overall
survival time compared with those with high expression of FOXA1,
indicating that FOXA1 expression may exhibit prognostic value for
patients with EOC.
Discussion
OC is the second most common type of gynecological
cancer worldwide, and a major cause of cancer-associated mortality
in women (2). Unlike other
reproductive malignancies, including prostate cancer and breast
carcinoma, EOC lacks an established biomarker for screening.
Identifying biomarkers for EOC may reveal novel therapeutic targets
and provide a potential screening test. The present study assessed
the expression of FOXA1 in EOC to determine its diagnostic or
prognostic value.
The FOX proteins are a large family that is divided
into 17 subclasses (A-Q) according to the amino acid sequence of
their conserved Forkhead domains (26). They are associated with embryonic
development, cell cycle regulation, cellular proliferation,
transformation, immune regulation, differentiation, longevity and
multiple other biological processes; mutation and expression
abnormalities to proteins of this family may be associated with
developmental abnormalities, metabolic diseases and tumor
occurrence (27,28). FOXA1, a member of the FOXA subclass of
FOX transcription factors, mediates the nuclear steroid receptor
signaling pathwayby regulating androgen receptor and estrogen
receptor activity (26). FOXA1 serves
a major function in modulating nuclear steroid receptor activity in
breast and prostate cancer, and it has been suggested that FOXA1
may be associated with pro-tumorigenic phenotypes (29). FOXA1 is necessary for the estrogen
signaling pathway to function in breast cancer cells, and its
expression has been associated with improved prognosis in patients
with breast cancer (19,20). It has also been suggested that FOXA1
influences AR binding to chromatin in androgen-dependent and
androgen-independent prostate cancer (21,22). As
EOC is also hormone-dependent, this prompted the assessment of
FOXA1 expression in EOC.
The present study demonstrated that the expression
of FOXA1 mRNA and protein in EOC tissues was significantly
increased compared with that in non-malignant tissues. The results
of immunohistochemical analysis were consistent with those of
RT-qPCR analysis, which suggested that the expression of FOXA1 may
serve an important function in EOC tumorigenesis. The present study
also revealed that the alteration to FOXA1 expression was
associated with the WHO grade of EOC, clinicopathological
characteristics and patient survival time. The increased expression
of FOXA1 was associated with an increased WHO grade, poor
differentiation and reduced overall survival time irrespective of
age, histological type, tumor size or location. The results of the
present study suggest that FOXA1 expressionmaypredict survival
timein patients with OC. FOXA1may represent a novel prognostic
factor and potential therapeutic target for patients with OC as an
important transcription factor in EOCand other types of
malignancy.
In conclusion, the present study revealed that FOXA1
was overexpressed in EOC tissues compared with normal tissue. The
level of FOXA1 expression was associated with the tumor grade,
differentiation status and prognosis. The results of the present
study indicated that FOXA1 may serve an important function in EOC
and may be a potential therapeutic target and prognostic marker.
However, the function of FOXA1 and its role in tumorigenesis in EOC
have yet to be fully characterized, and require additional
study.
References
|
1
|
Auersperg N, Wong AS, Choi KC, Kang SK and
Leung PC: Ovarian surface epithelium: Biology, endocrinology, and
pathology. Endocr Rev. 22:255–288. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ozols RF: Treatment goals in OC. Int J
Gynecol Cancer. 15 Suppl 1:S3–S11. 2005. View Article : Google Scholar
|
|
5
|
Schink JC: Current initial therapy of
stage III and IV OC: challenges for managed care. Semin Oncol. 26(1
Suppl 1): S2–S7. 1999.
|
|
6
|
Lin L, Miller CT, Contreras JI, Prescott
MS, Dagenais SL, Wu R, Yee J, Orringer MB, Misek DE, Hanash SM, et
al: The hepatocyte nuclear factor 3 alpha gene, HNF3alpha (FOXA1),
on chromosome band 14q13 is amplified and overexpressed in
esophageal and lung adenocarcinomas. Cancer Res. 62:5273–5279.
2002.PubMed/NCBI
|
|
7
|
Wolf I, Bose S, Williamson EA, Miller CW,
Karlan BY and Koeffler HP: FOXA1: Growth inhibitor and a favorable
prognostic factor in human breast cancer. Int J Cancer.
120:1013–1022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lantz KA and Kaestner KH: Winged-helix
transcription factors and pancreatic development. Clin Sci (Lond).
108:195–204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Costa RH, Grayson DR and Darnell JE Jr:
Multiple hepatocyte-enriched nuclear factors function in the
regulation of transthyretin and alpha 1-antitrypsin genes. Mol Cell
Biol. 9:1415–1425. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lai E, Prezioso VR, Smith E, Litvin O,
Costa RH and Darnell JE Jr: HNF-3A, a hepatocyte-enriched
transcription factor of novel structure is regulated
transcriptionally. Genes Dev. 4:1427–1436. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carroll JS and Brown M: Estrogen receptor
target gene: An evolving concept. Mol Endocrinol. 20:1707–1714.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Friedman JR and Kaestner KH: The Foxa
family of transcription factors in development and metabolism. Cell
Mol Life Sci. 63:2317–2328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee CS, Friedman JR, Fulmer JT and
Kaestner KH: The initiation of liver development is dependent on
Foxa transcription factors. Nature. 435:944–947. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Besnard V, Wert SE, Hull WM and Whitsett
JA: Immunohistochemical localization of Foxa1 and Foxa2 in mouse
embryos and adult tissues. Gene Expr Patterns. 5:193–208. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cirillo LA, Lin FR, Cuesta I, Friedman D,
Jarnik M and Zaret KS: Opening of compacted chromatin by early
developmental transcription factors HNF3 (FoxA) and GATA-4. Mol
Cell. 9:279–289. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gualdi R, Bossard P, Zheng M, Hamada Y,
Coleman JR and Zaret KS: Hepatic specification of the gut endoderm
in vitro: Cell signaling and transcriptional control. Genes Dev.
10:1670–1682. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakshatri H and Badve S: FOXA1 as a
therapeutic target for breast cancer. Expert Opin Ther Targets.
11:507–514. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nucera C, Eeckhoute J, Finn S, Carroll JS,
Ligon AH, Priolo C, Fadda G, Toner M, Sheils O, Attard M, et al:
FOXA1 is a potential oncogene in anaplastic thyroid carcinoma. Clin
Cancer Res. 15:3680–3689. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Badve S, Turbin D, Thorat MA, Morimiya A,
Nielsen TO, Perou CM, Dunn S, Huntsman DG and Nakshatri H: FOXA1
expression in breast cancer-correlation with luminal subtype A and
survival. Clin Cancer Res. 13:4415–4421. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thorat MA, Marchio C, Morimiya A, Savage
K, Nakshatri H, Reis-Filho JS and Badve S: Forkhead box A1
expression in breast cancer is associated with luminal subtype and
good prognosis. J Clin Pathol. 61:327–332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lupien M, Eeckhoute J, Meyer CA, Wang Q,
Zhang Y, Li W, Carroll JS, Liu XS and Brown M: FoxA1 translates
epigenetic signatures into enhancer-driven lineage-specific
transcription. Cell. 132:958–970. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J,
Chen Z, Beroukhim R, Wang H, Lupien M, et al: Androgen receptor
regulates a distinct transcription program in androgen-independent
prostate cancer. Cell. 138:245–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeppernick F and Meinhold-Heerlein I: The
new FIGO staging system for ovarian, fallopian tube and primary
peritoneal cancer. Arch Gynecol Obstet. 290:839–842. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hauptmann S, du Bois A, Meinhold-Herlein
I, Pfisterer J and Avril S: Histological grading of epithelial
ovarian cancer. Review and recommendation. Pathologe. 35:497–503.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vinayagamoorthy T, Maryanski D,
Vinayagamoorthy D, Hay KS, Yo J, Carter M and Wiegel J: Improved
internal control for molecular diagnosis assays. MethodsX.
2:159–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kaestner KH, Knochel W and Martinez DE:
Unified nomenclature for the winged helix/forkhead transcription
factors. Genes Dev. 14:142–146. 2000.PubMed/NCBI
|
|
27
|
Lehmann OJ, Sowden JC, Carlsson P, Jordan
T and Bhattacharya SS: Fox's in development and disease. Trend
Genet. 19:339–344. 2003. View Article : Google Scholar
|
|
28
|
Tan Y, Raychaudhuri P and Costa RH: Chk2
mediates stabilization of the FoxM1 transcription factor to
stimulate expression of DNA repair genes. Mol Cell Biol.
27:1007–1016. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Robinson JL and Carroll JS: FoxA1 is a key
mediator of hormonal response in breast and prostate cancer. Front
Endocrinol (Lausanne). 3:682012.PubMed/NCBI
|