Introduction
According to the American Cancer Society, breast
cancer (BC) is the most common malignancy in women, accounting for
>246,660 cases and 40,450 fatalities in America alone in 2016
(1). The incidence of BC is rising,
particularly in high-income and developed countries (2). Several subtypes of BC have been
identified, each exhibiting variations in clinical behavior.
Previous research has led to improvements in the early diagnosis
and treatment of BC, including developments in target therapy,
which has prolonged the survival rate of patients (3). However, more effective therapeutic
agents and novel biomarkers are required to improve the prognosis
of patients with BC (4).
Previous studies have demonstrated that expression
of phospholipase A2 (PLA2) is associated with the initiation and
progression of certain types of malignant cancer, including
gastrointestinal, colorectal and prostate carcinomas (5–8). PLA2 is
the name given to enzymes that catalyze the deacylation of
glycerophospholipids at the sn-2 position, producing two lipid
mediators, fatty acid derivatives and lysophospholipids (9,10), which
serve as signaling molecules in various types of cancer. PLA2 has
also been identified as a potential target of cancer therapy. It
has been revealed that mammals possess >30 enzymes with PLA2
activity (9). PLA2 enzymes are
classified into four groups based on differing molecular
mechanisms, including cellular localization, substrate specificity
and calcium dependence. These are: Cytosolic PLA2 (cPLA2),
calcium-independent PLA2 (iPLA2), secreted PLA2 (sPLA2) and
platelet activating factor acetyl hydrolases (11). It has been demonstrated that
sPLA2-IIa, a secretory PLA2 enzyme, is highly expressed in certain
human cells and tissues, and is associated with various diseases,
including cancer (5,12).
Plasma biomarkers can be assessed by low-cost,
non-invasive methods, potentially enabling the early diagnosis of
various diseases (13). The present
study examined the activities of PLA2 and sPLA2 in blood plasma
samples obtained from patients with BC, patients with benign
disease (BD) and healthy participants. The results of a validated,
quantitative and reproducible PLA2 assay (14) utilized in the current study indicated
that the activity of plasma PLA2 and sPLA2 in patients with BC were
significantly higher than that in healthy participants. In
addition, PLA2 and sPLA2 activities were associated with BC tumor
stages. Other potential risk factors for BC development (15–17),
including smoking, alcohol consumption, body-mass index (BMI) and
age, were also assessed in the present study.
Materials and methods
Human sample collection and
processing
Clinical plasma samples were collected from patients
with BC (N=169), patients with BD (N=80) and healthy participants
(N=81) from the First Affiliated Hospital of Xi'an Jiaotong
University (Shaanxi, China) between August 2013 and November 2015.
The mean age was 53.25 years, with a range of 28 to 77 years old.
BC and benign lesions were confirmed using immunohistochemical
staining after ultrasound-guided breast biopsies. Healthy
participants were recruited under the conditions of a free routine
health examination. All participants in this study satisfied the
following inclusion criteria: i) Proven diagnosis of breast cancer
or benign disease by pathology; ii) no adjuvant therapy or surgery
prior to blood sample collection from patients with breast cancer;
iii) female. Exclusion criteria were as follows: i) Previous
history of cancer, infectious disease or other health conditions,
which may have altered the results of the present study; ii)
absence of a complete medical record. Blood samples were collected
in the morning on an empty stomach in the presence of EDTA and
centrifuged at 3,000 × g, at 4°C for 30 min, aliquoted into
siliconized Eppendorf tubes and stored at −80°C. The present study
was reviewed and approved by the ethics committee of The First
Affiliated Hospital of Xi'an University Jiaotong University
(Shaanxi, China) and written informed consent was obtained from all
patients prior to enrollment. All clinical investigations were
conducted according to the principles detailed in the Declaration
of Helsinki (18).
The histopathological staining
procedure of the BC tissues obtained
Tissues were first deparaffinized for 45 sec at 30°C
in 100% xylene then fixed for 45 sec at 30°C in 100% propanol. A
single drop of 5% hematoxylin stain was applied per 50–100
mm2 tissue for 45 sec at 30°C. The slides were then
rinsed vigorously with distilled water at 30°C for 45 sec prior to
2–3 drops per slide of 1% eosin stain for 30 sec at 30°C. The
slides were rinsed again with cold 4°C distilled water for 15 sec,
prior to a final wash for 45 sec in 100% propanol at 30°C and then
45 sec in 100% xylene at 30°C. A Leica DM4 B light microscope (at a
×400 magnification) was used to visualize the staining.
Reagents and inhibitors
The fluorescent substrate of PLA2,
1-O-(6-Dabcyl-aminohexanoyl)-2-O-(12-(5-BODIPY-entanoyl)-aminododecanoyl)-sn-glyceryl
phosphatidylcholine (DBPC) was obtained from Echelon Biosciences,
Inc. (Salt Lake City, UT, USA). The dual inhibitor of cPLA2 and
iPLA2, methyl arachidonyl flourophosphonate (MAFP) was obtained
from Santa Cruz Biotechnology Inc. (Dallas, TX, USA) (19).
Analyses of PLA2 and sPLA2 enzymatic
activity
PLA2 activity was assessed using DBPC; a fluorogenic
phosphatidylcholine substrate (14).
Plasma samples (0.1 ml) were mixed with DBPC (0.2 mg with PBS) to a
final volume of 200 ml and concentration if 0.001 mg/ml). Then put
them in 37°C temperature incubator. The fluorescence of samples was
determined using a Victor3 V plate reader, wavelength 515 nm,
(PerkinElmer Inc., Waltham, MA, USA) at 30 min intervals over 2 h.
Variations in PLA2 activities can be measured by measuring the
change in fluorescence intensity/min/µl of plasma. The following
conditions were selected to distinguish the activity of PLA2 from
the aforementioned subtypes: Normal PLA2 activity was assessed
without exogenous additives and sPLA2 activity was examined in the
presence of 1.2 mM calcium chloride (the natural ionized calcium
concentration in blood) and MAFP (10 µM; a dual inhibitor of cPLA2
and iPLA2).
Freeze-thaw cycling
PLA2 activity stability was analyzed as
aforementioned both prior and subsequent to sample-storage at −80°C
for half a month. This is one Freeze-thaw cycle. The coefficient of
variation (CV)=standard deviation/mean value.
Statistical analysis
Categorical variables were summarized as counts with
percentages and continuous variables were summarized as the mean ±
standard deviation across the healthy control, BD and BC groups.
Pearson's correlation coefficient was used to determine
associations between two variables. A non-parametric test was used
to evaluate the difference between the median values of two groups.
A Kruskal-Wallis test and a post-hoc Nemenyi test were used to
assess the median fluorescence intensity values between the three
groups. One-way analysis of variance with post-hoc
Student-Newman-Keuls test was applied to assess the association
between enzyme activity and univariates, including smoking, alcohol
drinking, BMI and age. Multivariate linear regression analysis was
used to estimate the interaction between dependent variables and
multiple independent variables, including group, smoking,
second-hand smoking (when a patient lives in the household of a
smoker). alcohol consumption, BMI and age. Stepping method criteria
were used to adjust for confounding factors. Receiver operating
characteristic (ROC) curve analysis was performed to test the
diagnostic value of plasma PLA2 activities. All analyses were
performed using SPSS 19.0 (IBM corp., Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient demographic data
All patients with BC were confirmed using
histopathological staining and classified based on
Tumor-Node-Metastasis staging (20).
The mean ages of the healthy controls, patients with BD and
patients with BC were 52.65±8.64, 51.58±7.80 and 54.34±9.78 years,
respectively. The demographic data of age, smoking status, alcohol
consumption and BMI are presented in Table I.
 | Table I.Patient demographic data. |
Table I.
Patient demographic data.
|
| Healthy
controlsa | Patients with
BDb | Patients with
BCc | P-value |
|---|
| Smoking status |
|
|
| 0.523 |
| Current
smoker, n (%) | 8 (9.9) | 6 (7.5) | 20 (11.8) |
|
| Past
smoker, n (%) | 8 (9.9) | 7 (8.8) | 17 (10.1) |
|
| Never
smoked, n (%) | 65 (80.2) | 67 (83.8) | 132 (78.1) |
|
| Alcohol
consumption |
|
|
| 0.339 |
| Does
not consume | 52 (64.2) | 47 (58.8) | 114 (68.3) |
|
|
Consumes | 29 (35.8) | 33 (41.3) | 53 (31.7) |
|
| BMId, kg/m2 | 24.04±3.60 | 24.61±3.58 | 24.98±3.47 | 0.781 |
| Aged, years | 52.65±8.64 | 51.58±7.80 | 54.34±9.78 | 0.065 |
PLA2 and sPLA2 activities are elevated
in patients with BC
The activities of plasma PLA2 (without additives)
and sPLA2 were analyzed using one-way analysis Multivariate linear
regression analysis was used to estimate the interaction between
dependent variables and multiple independent variables. Stepping
method criteria were used to adjust for confounding factors and the
variables of smoking status, alcohol consumption, BMI and age were
ruled out, leaving only the differing group variable. The adjusted
R2 values of PLA2 and sPLA2 were 0.152 and 0.137,
respectively. Multiple comparisons revealed that PLA2 and sPLA2
activity were significantly increased in patients with BC
(P<0.001; Fig. 1).
Reproducibility and stability of the
PLA2 assays
DBPC-based PLA2 assays were utilized. A total of 10
representative plasma samples (randomly selected from each group)
were analyzed 3 times and the coefficient of variation (CV) was
adopted to measure the discrete degree of sample reproducibility.
The average CV of PLA2 and sPLA2 was 1.28 and 2.54%, respectively,
indicating that this method possessed a good repeatability. The
effect of a freeze-thaw cycle on the stability and activity of PLA2
was examined by comparing the results of fresh samples with those
in samples stored at −80°C for half a month. The average CV of PLA2
and sPLA2 was 0.79 and 2.86%, respectively, as previously reported
(19). The PLA2 activity assay
demonstrated good reproducibility and stability, considering the
minimal CV of repeatability and freeze-thaw for the PLA2 and sPLA2
results.
Clinical manifestations and PLA2
activities
PLA2 and sPLA2 activities were significantly
increased in plasma samples obtained from patients with BC compared
with patients with BD and with healthy participants (P<0.05).
This suggests that PLA2 and sPLA2 maybe potential biomarkers for
breast cancer. However, whether they were associated with cancer
advancement remains unclear. To obtain the required statistics, the
activities of PLA2 and sPLA2 in plasma samples from patients with
different tumor stages were compared. The results demonstrated that
natural PLA2 and sPLA2 activities were increased in patients with
late-stage BC (stages III and IV; P<0.001; Fig. 2). This indicated that the enzymatic
activities of PLA2 and sPLA2 induced the progression of BC.
Plasma PLA2 activities possess a good
diagnostic value
To assess the diagnostic capabilities of PLA2 and
sPLA2, an ROC curve analysis was performed using plasma samples
obtained from patients with BC and healthy controls. It was
revealed that PLA2 and sPLA2 exhibited area under the curve values
of 0.783 and 0.748, with sensitivities of 72 and 60.1% and
specificities of 72 and 82%, respectively (Fig. 3). These results indicate that PLA2 and
sPLA2 may possess diagnostic values in discriminating between
patients with BC and healthy individuals.
Impacts of other risk factors on PLA2
activity
Risk factors that may affect the activity of PLA2,
including smoking, alcohol consumption, age and BMI, were analyzed
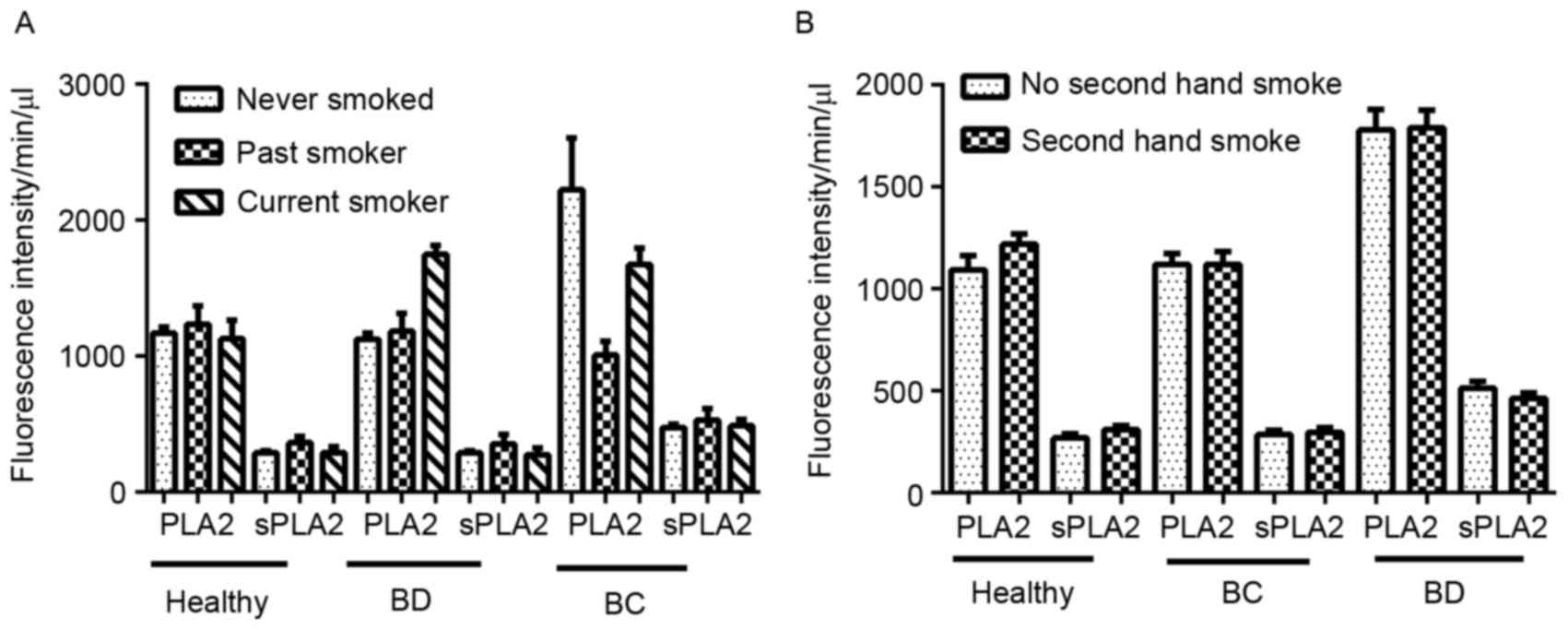
in the present study. A total of 330 patients were assessed for
smoking history: no smoking history, 80.2% (healthy controls),
83.8% (BD), 78.1% (BC); current smokers, 9.9% (healthy controls),
7.5% (BD), 11.8% (BC); previous smokers 9.9% (healthy controls),
8.8% (BD), 11.8% (BC). There was no difference among the 3 groups
regarding smoking status and PLA2/sPLA2 (P>0.05; Fig. 4A and B).
Alcohol consumption was determined for a total of
328 patients. The percentage of patients that consumed alcohol in
healthy control patients, patients with BD and patients with were
35.8, 41.3 and 31.7%, respectively. There was no difference of
PLA2/sPLA2 activity among the 3 groups comparing alcohol
consumption with no alcohol consumption: healthy group, P=0.412
(PLA2), P=0.375 (sPLA2); BD group, P=0.805 (PLA2), P=0.259 (sPLA2);
BC group, P=0.168 (PLA2), P=0.291 (sPLA2) (Fig. 5).
The body mass indexes (BMIs) of 324 patients were
also determined, revealing an even distribution of BMI values, and
no statistically significant difference of PLA2 and sPLA2
activities comparing patients of a BMI ≤25 with BMI >25: healthy
group, P=0.348 (PLA2), P=0.085 (sPLA2); BD group, P=0.572 (PLA2),
P=0.865 (sPLA2); BC group, P=0.414 (PLA2), P=0.710 (sPLA2)
(Fig. 6).
Age is an established risk factor for BC as
menopause may affect its development. To remove this confounding
factor, patients in BC, BD and healthy controls were then divided
into two subgroups: Those aged <50 and those aged ≥50 years.
PLA2 and sPLA2 remained unchanged in two age subgroups among BC, BD
and healthy controls (Fig. 7).
Discussion
The present study determined that the activities of
PLA2 and sPLA2 in plasma may serve as novel biomarkers for patients
with BC. One drew similar conclusions regarding colorectal cancer,
lung cancer, bladder cancer and pancreas cancer (19). The present study assessed PLA2
obtained without additives, so that the activity of PLA2 was
considered ‘natural’. Optimal conditions, i.e. in the presence of
1.2 mM calcium chloride (the natural ionized calcium concentration
in blood) and 10 mM MAFP were also selected for the assessment of
sPLA2 activity.
The present study assessed the confounding factors
that are associated with PLA2 activity. In the present study, it
was determined that patient age, BMI, smoking status or alcohol
consumption did not differ significantly between the three groups
of patients assessed. In addition, Buhmeida et al (21) demonstrated that there were no
significant associations between PLA2 expression and age, sex,
depth of invasion or lymph node status. Furthermore, Yamashita
et al (22) did not identify
an association between steroid hormone receptor status and the
concentration of PLA2 in BC tissues. These results were also
consistent with those produced by Mannello et al (12). However, induced abortion, oral
contraceptive use, family history of BC, delayed child birth and
reduced duration of breast feeding have been identified as risk
factors based on previous studies. Oral contraceptive use may
induce the proliferation of breast cells, therefore increasing the
risk of breast development (23). It
has also been determined that a window of susceptibility to breast
cancer exists, in the time period between puberty onset and first
full-term pregnancy (15). The
process of pregnancy transforms pubescent breast tissue into fully
mature tissue that contains type-4 lobules (24). The conclusion was that these factors
are strongly associated with an increased risk of BC development
(25). Hence, the combination of
estrogen receptor status and plasma PLA2 activity may serve as a
biomarker to predict the survival of patients with BC.
A lack of screening for BC may be the reason for
increased rates of mortality among patients with BC (26). A previous study identified that there
has been a large increase in metastatic breast malignancy (to the
bone, brain and lungs) in young women at the time of diagnosis
(15). Therefore, a more effective
and convenient screening method is required to reduce mortality and
prolong overall survival. Although image-based detection methods,
including infrared ray scanning, B ultrasound and mammography are
effective for the early detection of BC, it has been determined
that ~40% of BC cases remain undiagnosed (27). In addition, certain modern imaging
techniques, including breast molybdenum target mammography, produce
false positive results, are expensive, inconvenient and require
painful patient examinations (13,28). Early
detection of BC has contributed to a 3% annual decline in patient
mortality rate (12). The development
of non-invasive screening techniques for BC is required. Molecular
diagnostic approaches are often non-invasive and may serve to
improve the specificity and sensitivity of BC screening (15). It has been demonstrated that
serological biomarkers are effective in the early diagnosis of
various types of cancer, and are also cost-effective and convenient
(19,29). Furthermore, serological biomarkers may
functionally detect cancers at early stages and elucidate the
molecular mechanisms that underlie the development of cancer,
potentially leading to the establishment of novel therapeutic
strategies (13,29).
The present study determined that measuring PLA2
activity using a fluorescent plate reader is convenient and is able
to be developed into an automated test. Furthermore, only a small
quantity of plasma (1–10 ml) is required to perform the test, the
results of which are obtained in 1–2 h. Additional biomarkers or
more specific modalities, including imaging techniques, may then be
implemented for further examinations. An additional advantage to
the PLA2 activity test is its reproducibility, stability and
automated mechanism. A previous study established that blood
biomarkers are sensitive to the handling, processing and storage of
patient samples (13). For example,
samples are particularly sensitive to freeze-thaw cycles. This
explains why various sensitive and specific biomarkers discovered
using proteomics are not utilized in clinical settings. The present
study compared the CV of PLA2 and sPLA2 following experimental
repeats and the induction of freeze-thaw cycling. The results
demonstrated that PLA2 activities are highly consistent and
independent of certain confounding factors, including freeze-thaw
cycling, making them more likely to be effective biomarkers.
The activity of PLA2 may be restricted in patients
with benign inflammatory diseases as these enzymes may be elevated
owing to their involvement in inflammation. Inflammation is a
response to internal and external environmental stimuli that
functions to eliminate foreign agents and restore the physiology of
normal tissue. However, when inflammation becomes chronic, it can
result in the development of cancer (30,31).
Inflammation following viral infection can drive the progression of
cancer (32). Epidemiological data
has indicated that >25% of various types of cancer are
associated with chronic infection and inflammation (29). It has been demonstrated that the
administration of non-steroidal anti-inflammatory drugs is
associated with a reduction in the risk of developing various types
of cancer, including BC (33). A
previous study identified that sPLA2 regulates the activation of
Ras and extracellular signal-regulated kinase and induces the
phosphorylation of the epidermal growth factor receptor via a
protein kinase C-dependent pathway, which may lead to a poor
patient prognosis in an inflammatory microenvironment (34).
In conclusion, the results of the present study
demonstrated that elevated PLA2 and sPLA2 activities were detected
in patients with BC, particularly at late disease stages. Following
the analysis of confounding factors, it was determined that disease
type was as an independent factor, exhibiting no association with
age, smoking, alcohol or BMI. The results indicated that plasma
PLA2 activity might be a potential prognostic biomarker for
patients with BC. Elevated PLA2 and sPLA2 activities may serve a
vital role in breast carcinogenesis and could represent a novel
therapeutic target for patients with BC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81502295) and
Clinical Innovation Funds of The First Affiliated Hospital of XJTU
(grant no. 14YB11).
Glossary
Abbreviations
Abbreviations:
|
BC
|
breast cancer
|
|
BD
|
benign disease
|
|
PLA2
|
phospholipase A2
|
|
sPLA2-IIa
|
group IIa secretory phospholipase
A2
|
|
AA
|
arachidonic acid
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hassan LM, Mahmoud N, Miller AB, Iraj H,
Mohsen M, Majid J, Reza SM and Mojgan M: Evaluation of effect of
self-examination and physical examination on breast cancer. Breast.
24:487–490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Curigliano G, Spitaleri G, Dettori M,
Locatelli M, Scarano E and Goldhirsch A: Vaccine immunotherapy in
breast cancer treatment: Promising, but still early. Expert Rev
Anticancer Ther. 7:1225–1241. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li SY, Li R, Chen YL, Xiong LK, Wang HL,
Rong L and Luo RC: Aberrant PTPRO methylation in tumor tissues as a
potential biomarker that predicts clinical outcomes in breast
cancer patients. BMC Genet. 15:672014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Huang CJ, Yu GZ, Wang JJ, Wang R,
Li YM and Wu Q: Expression of group IIA phospholipase A2 is an
independent predictor of favorable outcome for patients with
gastric cancer. Hum Pathol. 44:2020–2027. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li S, Zhao X, Wu Z, Li Y, Zhu L, Cui B,
Dong X, Tian S, Hu F and Zhao Y: Polymorphisms in arachidonic acid
metabolism-related genes and the risk and prognosis of colorectal
cancer. Fam Cancer. 12:755–765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mirtti T, Laine VJ, Hiekkanen H, Hurme S,
Rowe O, Nevalainen TJ, Kallajoki M and Alanen K: Group IIA
phospholipase A as a prognostic marker in prostate cancer:
Relevance to clinicopathological variables and disease-specific
mortality. APMIS. 117:151–161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Menschikowski M, Hagelgans A, Nacke B,
Jandeck C, Mareninova OA, Asatryan L and Siegert G: Epigenetic
control of group V phospholipase A2 expression in human malignant
cells. Tumour Biol. 37:8097–8105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quach ND, Arnold RD and Cummings BS:
Secretory phospholipase A2 enzymes as pharmacological targets for
treatment of disease. Biochem Pharmacol. 90:338–348. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin Y, Yang F and Du L: Nanoassemblies
containing a fluorouracil/zidovudine glyceryl prodrug with
phospholipase A2-triggered drug release for cancer treatment.
Colloids Surf B Biointerfaces. 112:421–428. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu C, Sun Z, Li C, Guo R, Li L, Jin L,
Wan R and Li S: Urocortin affects migration of hepatic cancer cell
lines via differential regulation of cPLA2 and iPLA2. Cell Signal.
26:1125–1134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mannello F, Qin W, Zhu W, Fabbri L, Tonti
GA and Sauter ER: Nipple aspirate fluids from women with breast
cancer contain increased levels of group IIa secretory
phospholipase A2. Breast Cancer Res Treat. 111:209–218. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kondo T: Inconvenient truth: Cancer
biomarker development by using proteomics. Biochim Biophys Acta.
1844:861–865. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai Q, Zhao Z, Antalis C, Yan L, Del
Priore G, Hamed AH, Stehman FB, Schilder JM and Xu Y: Elevated and
secreted phospholipase A2 activities as new potential
therapeutic targets in human epithelial ovarian cancer. FASEB J.
26:3306–3320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schneider AP II, Zainer CM, Kubat CK,
Mullen NK and Windisch AK: The breast cancer epidemic: 10 facts.
Linacre Q. 81:244–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lanfranchi AE and Fagan P: Breast cancer
and induced abortion: A comprehensive review of breast development
and pathophysiology, the epidemiologic literature, and proposal for
creation of databanks to elucidate all breast cancer risk factors.
Issues Law Med. 29:3–133. 2014.PubMed/NCBI
|
|
17
|
Linos E, Spanos D, Rosner BA, Linos K,
Hesketh T, Qu JD, Gao YT, Zheng W and Colditz GA: Effects of
reproductive and demographic changes on breast cancer incidence in
China: A modeling analysis. J Natl Cancer Inst. 100:1352–1360.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
World Medical Association, . World medical
association declaration of helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai H, Chiorean EG, Chiorean MV, Rex DK,
Robb BW, Hahn NM, Liu Z, Loehrer PJ, Harrison ML and Xu Y: Elevated
phospholipase A2 activities in plasma samples from multiple
cancers. PLoS One. 8:e570812013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sobin LH and Wittekind C: TNM
classification of malignant tumors. International Union Against
Cancer (UICC) (5th). 54–58. 1997.
|
|
21
|
Buhmeida A, Bendardaf R, Hilska M, Laine
J, Collan Y, Laato M, Syrjänen K and Pyrhönen S: PLA2 (group IIA
phospholipase A2) as a prognostic determinant in stage II
colorectal carcinoma. Ann Oncol. 20:1230–1235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamashita S, Yamashita J, Sakamoto K,
Inada K, Nakashima Y, Murata K, Saishoji T, Nomura K and Ogawa M:
Increased expression of membrane-associated phospholipase A2 shows
malignant potential of human breast cancer cells. Cancer.
71:3058–3064. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lovett JL, Chima MA, Wexler JK, Arslanian
KJ, Friedman AB, Yousif CB and Strassmann BI: Oral contraceptives
cause evolutionarily novel increases in hormone exposure: A risk
factor for breast cancer. Evol Med Public Health. 2017:97–108.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Russo J, Moral R, Balogh GA, Mailo D and
Russo IH: The protective role of pregnancy in breast cancer. Breast
Cancer Res. 7:131–142. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kamińska M, Ciszewski T, Łopacka-Szatan K,
Miotła P and Starosławska E: Breast cancer risk factors. Prz
Menopauzalny. 14:196–202. 2015.PubMed/NCBI
|
|
26
|
Webb ML, Cady B, Michaelson JS, Bush DM,
Calvillo KZ, Kopans DB and Smith BL: A failure analysis of invasive
breast cancer: Most deaths from disease occur in women not
regularly screened. Cancer. 120:2839–2846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Howell A: The emerging breast cancer
epidemic: Early diagnosis and treatment. Breast Cancer Res. 12
Suppl 4:S102010. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Killelea BK, Long JB, Chagpar AB, Ma X,
Wang R, Ross JS and Gross CP: Evolution of breast cancer screening
in the Medicare population: Clinical and economic implications. J
Natl Cancer Inst. 106:pii: dju1592014. View Article : Google Scholar
|
|
29
|
Indovina P, Marcelli E, Pentimalli F,
Tanganelli P, Tarro G and Giordano A: Mass spectrometry-based
proteomics: The road to lung cancer biomarker discovery. Mass
Spectrom Rev. 32:129–142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vendramini-Costa DB and Carvalho JE:
Molecular link mechanisms between inflammation and cancer. Curr
Pharm Des. 18:3831–3852. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gurel B, Lucia MS, Thompson IM Jr, Goodman
PJ, Tangen CM, Kristal AR, Parnes HL, Hoque A, Lippman SM,
Sutcliffe S, et al: Chronic inflammation in benign prostate tissue
is associated with high-grade prostate cancer in the placebo arm of
the prostate cancer prevention trial. Cancer Epidemiol Biomarkers
Prev. 23:847–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deivendran S, Marzook KH and Radhakrishna
Pillai M: The role of inflammation in cervical cancer. Adv Exp Med
Biol. 816:377–399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Q, Tan Q, Zheng Y, Chen K, Qian C, Li
N, Wang Q and Cao X: Blockade of Fas signaling in breast cancer
cells suppresses tumor growth and metastasis via disruption of Fas
signaling-initiated cancer-related inflammation. J Biol Chem.
289:11522–11535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hernández M, Martín R, García-Cubillas MD,
Maeso-Hernández P and Nieto ML: Secreted PLA2 induces proliferation
in astrocytoma through the EGF receptor: Another
inflammation-cancer link. Neuro Oncol. 12:1014–1023. 2010.
View Article : Google Scholar : PubMed/NCBI
|