Introduction
Being one of the most common malignancies, gastric
cancer (GC) has a high incidence and mortality worldwide (1). The most common histological subtype of
GC is gastric adenocarcinoma (GAC). Despite great advancements in
the diagnosis and therapy of GC in the past decades, the overall
survival (OS) rate of patients with this disease is still poor.
According to a survey, the 5-year survival rate of GC patients is
very poor, with a rate of no more than 35% (2,3). In recent
years, numerous studies on molecular targeted therapy and
associated molecular pathways involved in the gastric
carcinogenesis have shed light on the pathogenesis of GC and
enabled the improvement of GC patient prognosis (4). Consequently, detailed and systematic
analysis of the biomarkers involved in GC is important for the
development of biomarker-driven targeted treatment. Our study has
emerged from this perspective.
Human epidermal growth factor receptor 2
(HER-2/ERBB2/neu), a member of the epidermal growth factor receptor
family of receptor tyrosine kinases, is known as one of the most
important biomarkers in breast cancer (5). For patients undergoing targeted therapy,
HER-2 expression is used as a biomarker for identifying subgroups
that are likely to achieve a survival benefit from trastuzumab
therapy (6). Unfortunately,
trastuzumab resistance is universally observed in both breast
cancer and GC. Moreover, the expression pattern and prognostic
significance of HER-2 in GC remain controversial. The rate of HER-2
overexpression varies across the literature, ranging from 4.4 to
53.4%, with a mean of 17.9% (7).
Consequently, increasing studies have focused on the molecular
mechanisms underlying advanced trastuzumab resistance in GC
patients. Although many studies have reported that activated HER-2
signalling can enhance the capability of cancer cells to migrate
and colonize distant sites by phosphorylating pivotal downstream
small molecules and signalling pathway effectors (8,9), the
definitive relationship between HER-2 and the mTOR signalling
pathway has not yet been reported. Here, for the first time, we
have demonstrated the expression pattern and clinical significance
of the HER-2 signalling pathway downstream small molecule
RAB1A.
The RAB1A protein is a small GTP-binding protein
that is involved in membrane vesicular transport between the Golgi
complex and the endoplasmic reticulum (10). Previous studies have reported that
RAB1A is dysregulated in cancers such as tongue cancer (11) and that it is indicative of
unfavourable survival in patients. In recent years, studies have
demonstrated that RAB1A is a mTORC1 activator in colorectal cancer
(12) and a deregulator of the
phosphoinositide 3-kinase/protein kinase B/mammalian target of
rapamycin complex 1 (PI3K/AKT/mTORC1) pathway in breast cancer
(13). Meanwhile, another study
indicated that RAB1A interacts with other factors to regulate the
initiation of autophagy (14). These
findings indicate that RAB1A plays an important role in tumour
initiation and progression and is involve in the pathogenesis of
cancer. Thus, in the present study, we aimed to examine the
expression pattern of RAB1A and its clinical significance and
association with HER-2 expression in GC.
To date, little is known regarding the relation
between HER-2 and RAB1A expression and its prognostic significance
in human GC. Therefore, the aim of this study was to investigate
HER-2 and RAB1A expression in GC patient samples and to determine
the prognostic significance of and the correlation between the two
biomarkers, which may shed light on the mechanisms associated with
HER-2 resistance during targeted therapy.
Materials and methods
Patients and clinicopathological
information
In this study, we included a total of 280
formalin-fixed paraffin-embedded primary GC specimens and paired
noncancerous tissues from patients diagnosed with primary GAC in
Nanfang Hospital between 2006 and 2009. In addition, we included
120 cases of GAC tissues preserved in −80°C ultra-low temperature
refrigerator in Nanfang Hospital between 2015 and 2016. All
included patients underwent radical operation for GC and had no
history of chemotherapy or radiotherapy before surgery. The cases
were diagnosed by two pathologists at the Department of Pathology,
Nanfang Hospital, Southern Medical University (Guangzhou, China).
Information on clinicopathological variables including age, sex,
Lauren type, lymph node invasion status, tumour size, recurrence,
tumour, node and metastasis (TNM) stage and other factors was
collected by reviewing electronic records at Nanfang Hospital. The
study was approved by the Ethics Committee of Southern Medical
University with informed consent from patients.
Immunohistochemistry (IHC) and
evaluation of immunohistochemical staining
To evaluate HER-2 and RAB1A expression, we obtained
3–4 micrometre-thick serial sections from the archived paraffin
blocks. Immunohistochemical staining for HER-2 was performed using
the HercepTest™ kit (Dako Denmark A/S, Glostrup,
Denmark) according to manufacturer's protocols. For RAB1A
immunohistochemical staining, dewaxing and blocking nonspecific
binding was performed as previously described (15). We used monoclonal antibodies against
RAB1A (1:200 dilution; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) as the primary antibody. Following signal development, the
specimens were washed, dehydrated in alcohol and mounted with
coverslips. RAB1A expression was graded as high expression or low
expression according to the cytoplasmic staining intensity and
average percentage of positively stained area at ×10 magnification.
HER-2 immunohistochemical scoring was performed as follows: No
staining or membrane staining in less than 10% of invasive tumour
cells, IHC score, 0; weak membrane staining in 10% or more in
invasive tumour cells, IHC score, 2+; and moderate to strong
complete or basolateral membrane staining in 10% or more of
invasive tumour cells, IHC score, 3+. In this study, an IHC score
of 3+ for HER-2 was considered positive amplification, and IHC
scores of 0 and 1+ for HER-2 were considered negative amplification
according previous literature reports (16). For specimens with an IHC score of 2+,
we performed fluorescence in situ hybridization (FISH) assay
to verify the HER-2 amplification status.
FISH
GC specimens with IHC score of 2+ for HER-2 were
further subjected to FISH assay. FISH was performed using a AmoyDx
HER-2/neu DNA probe kit (ADx-FHE01; Xiamen, China) according to the
manufacturer's protocols. Briefly, neutral formalin fixed and
paraffin-embedded sections (3–4 µm-thick) were placed on adherent
slides and then baked in an oven overnight at 56°C. After
deparaffinization and dehydration at room temperature, the slides
were incubated with a pretreatment solution (with sodium
thiocyanate) and a protease solution for 15 min and subsequently
dehydrated with 70, 85, and 100% alcohol for 5 min each before
being air dried. The probe mixture was denatured at 80°C for 5 min,
added to each slide and sealed under a small glass cover slip
before overnight hybridization at 42°C. Excess probe was washed
away using 0.4X sodium saline citrate (SSC)/0.3% Nonidet (NP40) and
2X SSC buffer. After hybridization, nuclei were counterstained with
4′,6-diamidino-2-phenylindole (DAPI). Slides were analysed for
hybridization signals on a cell-by-cell basis using a multifiltered
fluorescence microscope (Olympus BX51; Olympus Corporation, Tokyo,
Japan) and Imstar FISH assay controller software 2.1 vision
according to standard procedures. The data were expressed as the
ratio of HER-2/neu signal (red) to centromere 17 signal (green),
and the scores were evaluated as follows: An expected ratio of
1–1.8, no gene amplification (negative), a ratio of >2.2 or
HER-2 signal cluster, HER-2/neu gene amplification (positive), and
a ratio of 1.8–2.2, equivocal cases. The presence of polysomy 17
was also recorded in the cells using four spec green signals as
moderate polysomy and >4 spec green signals as high
polysomy.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). To
quantify mRNA expression levels in tissues, the Advantage
RT-for-PCR kit (Clontech Laboratories, Inc., Mountainview, CA, USA)
was used to synthesize cDNA. SYBR-Green PCR master mix (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was
then used for qPCR amplification, followed by detection with an ABI
PRISM 7,900 Sequence Detector and analysis with the ABI SDS 2.3
software (Applied Biosystems; Thermo Fisher Scientific, Inc.).
GAPDH was used as a reference gene. The PCR primer sequences were
as follows: RAB1A forward, 5′-CAGCAGGCCAGGAAAGATT-3′ and reverse,
5′-GGTCAGATCACATTTGTTCCCTA-3′; HER-2 forward,
5′-TGTGACTGCCTGTCCCTACAA-3′ and reverse,
5′-CCAGACCATAGCACACTCGG-3′; and GAPDH forward,
5′-CTCCTCCTGTTCGACAGTCAGC-3′ and reverse,
5′-CCCAATACGACCAAATCCGTT-3′; relative Rab1A and HER-2 expression
levels (defined as fold change) were expressed as 2−ΔCT
(ΔCT = CTRAB1A - CTGAPDH) and
2−ΔCT (ΔCT = CTHER-2 - CTGAPDH)
and then normalized to the relative expression levels detected in
control samples. Each sample was tested in triplicate.
Statistical analysis
Statistical analyses were performed using SPSS
(version 20.0; SPSS, Inc., Chicago, IL, USA) or GraphPad Prism 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). Categorical data were
analysed using Chi-square statistics. Quantitative analysis of the
GAC group and the paired adjacent normal gastric tissue group was
performed using a paired-samples t-test and was presented as the
mean ± standard deviation. The Kaplan-Meier method was used for
survival analysis, and comparisons between different subgroups were
performed using the log-rank test. Multivariate analysis was
carried out by using the Cox proportional hazards model with
adjustment for covariates to identify meaningful prognostic
indicators that are independently associated with survival.
Spearman correlation coefficient analysis was used to detect
associations between variables. All statistics were two-sided, and
P-values <0.05 were considered to be significant.
Results
RAB1A up-regulation and HER-2
amplification were observed in GAC tissues
To evaluate the protein expression of RAB1A and
HER-2 in GAC patients and to analyse the associated
clinicopathological characteristics, we detected the localization
and expression of HER-2 and RAB1A proteins by IHC and evaluated the
relationship of these proteins with clinicopathological features of
patients. In total, 280 GAC patients were enrolled in the present
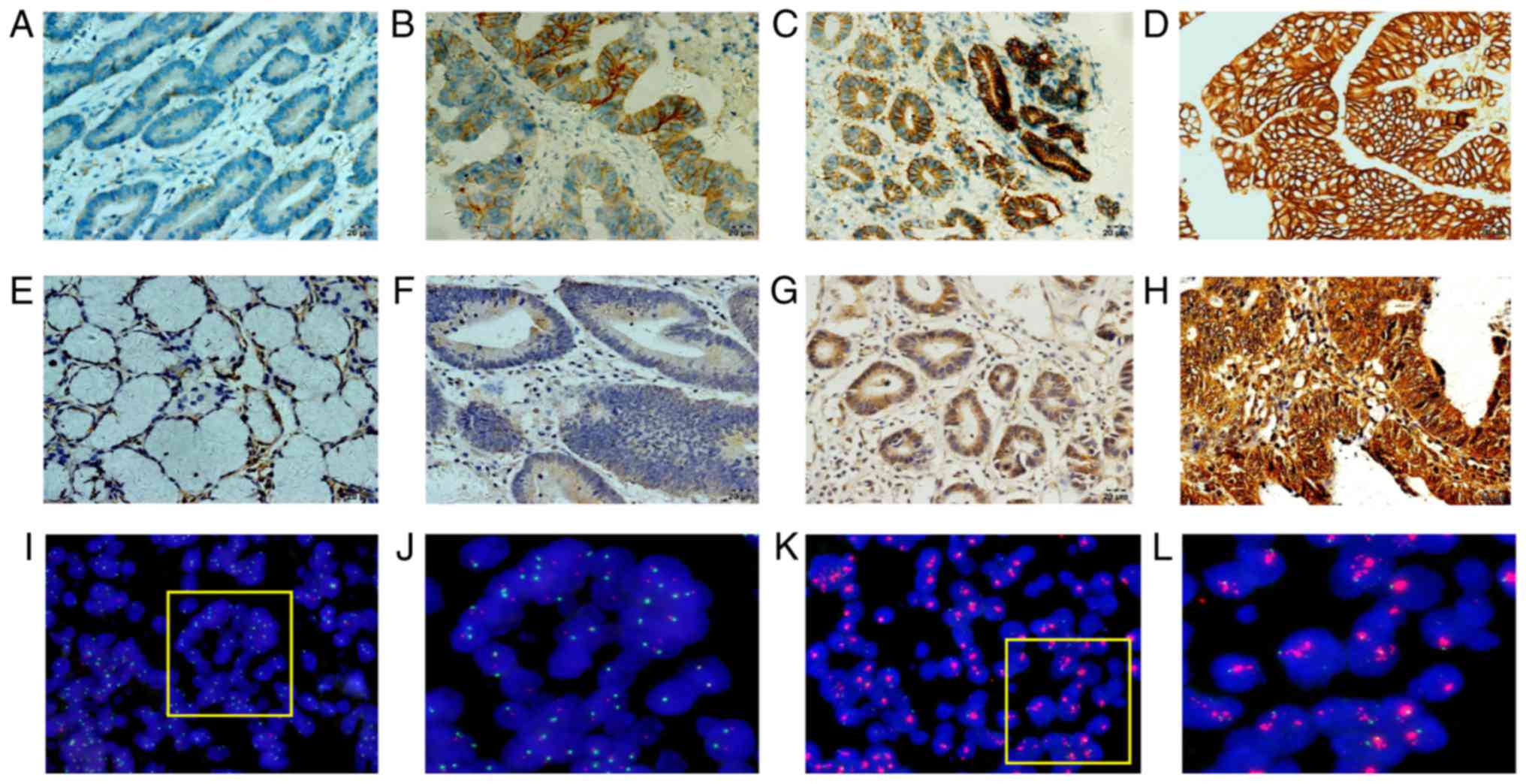
study. As shown in Fig. 1, compared
to that in adjacent noncancerous tissues, RAB1A was overexpressed
in GAC tissues (P<0.001). RAB1A expression was strong in the
cytoplasm of tumour cells in most cases and weak in paired normal
gastric epithelial cells (Fig. 2).
Regarding HER-2 expression, 192 of 280 cases (68.57%) scored 0; 40
cases (14.29%) scored 1+; 19 cases (6.76%) scored 2+; and 29 cases
(10.36%) scored 3+. According to previous studies (16), samples with an IHC score of 0 or 1+
did not have HER-2 amplification, whereas samples with an IHC score
of 3+ did. For samples with an IHC score of 2+, we used FISH assay
to validate the HER-2 amplification status (Fig. 2I-L). Among the 19 tumours with an IHC
score of 2+ for HER-2, only 7 showed amplification by FISH assay
(36.84%). Overall, 36 cases (12.86%) of positive HER-2
amplification was observed. The relationship between RAB1A and
HER-2 expression/amplification level and clinicopathological
parameters is summarized in Table I.
High RAB1A expression was significantly associated with tumour size
(P<0.001), lymph node invasion (P<0.001), recurrence
(P<0.001) and TNM stage (P<0.001) and World Health
Organisation (WHO) Classification (P=0.040). However, no
correlation was observed with age, sex, WHO Classification, Lauren
type or Helicobacter pylori infection status. HER-2 positive
amplification was closely associated with Lauren type (P<0.001),
tumour size (P<0.001) and lymph node invasion. However, no
difference was observed regarding the amplification status
stratified by age, sex, H. pylori infection status, WHO
Classification, recurrence or TNM stage.
 | Table I.Correlation between
clinicopathological parameters and RAB1A expression levels/human
epidermal growth factor receptor 2 amplification in 280 patients
with gastric cancer. |
Table I.
Correlation between
clinicopathological parameters and RAB1A expression levels/human
epidermal growth factor receptor 2 amplification in 280 patients
with gastric cancer.
|
|
| HER-2
amplification | RAB1A expression |
|---|
|
|
|
|
|
|---|
| Clinicopathologic
characteristics | n | Positive | Negative | P-value | Positive | Negative | P-value |
|---|
| Age (years) |
|
|
| 0.580 |
|
| 0.157 |
| ≥60 | 191 | 26 (72.2) | 165 (67.6) |
| 124 (65.3) | 67 (74.4) |
|
|
<60 | 89 | 10 (27.8) | 79 (32.4) |
| 66 (34.7) | 23 (25.6) |
|
| Sex |
|
|
| 0.290 |
|
| 0.329 |
|
Female | 108 | 11 (30.6) | 97 (39.8) |
| 77 (40.5) | 31 (34.4) |
|
| Male | 172 | 25 (69.4) | 147 (60.2) |
| 113 (59.5) | 59 (65.6) |
|
| Lauren |
|
|
|
<0.001a |
|
| 0.157 |
|
Intestinal | 90 | 27 (75.0) | 63 (25.8) |
| 60 (31.6) | 30 (33.3) |
|
|
Diffuse | 114 | 6 (16.7) | 108 (44.3) |
| 72 (37.9) | 42 (46.7) |
|
|
Mixed | 76 | 3 (8.3) | 73 (29.9) |
| 58 (30.5) | 18 (20.0) |
|
| Tumor size
(cm) |
|
|
|
<0.001a |
|
|
<0.001a |
| ≥5 | 93 | 25 (69.4) | 68 (27.9) |
| 75 (39.5) | 18 (20.0) |
|
|
<5 | 187 | 11 (30.6) | 176 (72.1) |
| 115 (60.5) | 72 (80.0) |
|
| Lymph node
invasion |
|
|
|
<0.001a |
|
|
<0.001a |
|
Positive | 81 | 19 (52.8) | 62 (25.4) |
| 69 (36.3) | 12 (13.3) |
|
|
Negative | 199 | 17 (47.2) | 182 (74.6) |
| 121 (63.7) | 78 (86.7) |
|
| TNM stage |
|
|
| 0.066 |
|
|
<0.001a |
|
I–II | 171 | 27 (75.0) | 144 (59.0) |
| 137 (72.1) | 34 (37.8) |
|
|
III–IV | 109 | 9 (25.0) | 100 (41.0) |
| 53 (27.9) | 56 (62.2) |
|
| WHO
Classification |
|
|
| 0.265 |
|
| 0.040a |
|
High | 39 | 8 (22.2) | 31 (12.7) |
| 24 (12.6) | 15 (16.7) |
|
|
Medium | 152 | 19 (52.8) | 133 (54.5) |
| 113 (59.5) | 39 (43.3) |
|
|
Low | 89 | 9 (25.0) | 80 (32.8) |
| 53 (27.9) | 36 (40.0) |
|
| Recurrence |
|
|
| <0.216 |
|
|
<0.001a |
|
Yes | 70 | 12 (33.3) | 58 (23.8) |
| 60 (31.6) | 10 (11.1) |
|
| No | 210 | 24 (66.7) | 186 (76.2) |
| 130 (68.4) | 80 (88.9) |
|
| Helicobacter
pylori infection |
|
|
| 0.742 |
|
| 0.556 |
|
Yes | 231 | 29 (80.6) | 202 (82.8) |
| 155 (81.6) | 76 (84.4) |
|
| No | 49 | 7 (19.4) | 42 (17.2) |
| 35 (18.4) | 14 (15.6) |
|
Association of RAB1A but not HER-2
with adverse prognosis in GAC patients
To evaluate the clinical significance of RAB1A and
HER-2 overexpression/amplification, we performed survival analyses
in these 280 patients. The median level of RAB1A expression was
used as a cutoff to divide the 280 patients into two groups.
Patients who expressed RAB1A at levels higher than the cutoff value
were assigned to the high-expression group, while patients with
expression lower than the cutoff value were assigned to the
low-expression group. Kaplan-Meier analysis demonstrated that
patients with high expression of RAB1A had worse OS than did
patients with low expression of RAB1A (P<0.001). However, the
Kaplan-Meier curves did not reveal any difference in survival
between patients with or without HER-2. Meanwhile, we analysed the
co-expression of HER-2 and RAB1A; Kaplan-Meier analysis
demonstrated that GAC patients co-expressing both HER-2 and RAB1A
had a significantly poorer OS than did GAC patients expressing
either HER-2 or RAB1A (P=0.001) (Fig.
3). To evaluate whether RAB1A expression with or without HER-2
amplification is an independent prognostic factor for GAC, we
performed univariate and multivariate analyses. Univariate analysis
demonstrated that Lauren type (P<0.001), lymph node invasion
(P<0.001), recurrence (P<0.001), RAB1A overexpression
(P=0.002), WHO Classification (P=0.001), HER-2 amplification
(P<0.001) and co-expression of both RAB1A and HER-2 (P<0.001)
were significantly associated with OS of GC patients (Table II). However, multivariate analysis
using the Cox proportional hazards model for all variables that
were significant in univariate analyses showed that only the
co-expression of RAB1A and HER-2 was an independent prognostic
factor for GAC patients (Table
II).
 | Table II.Univariate and multivariate analyses
of various potential prognostic factors in 280 patients with
gastric cancer. |
Table II.
Univariate and multivariate analyses
of various potential prognostic factors in 280 patients with
gastric cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | 1.152
(0.732–1.811) | 0.541 | – | – |
| Sex | 1.096
(0.730–1.645) | 0.659 | – | – |
| Tumor size | 1.214
(0.800–1.843) | 0.361 | – | – |
| TNM stage | 0.991
(0.663–1.482) | 0.966 | – | – |
| Helicobacter
pylori infection | 1.162
(0.681–1.983) | 0.582 | – | – |
| Lauren type | 0.505
(0.383–0.667) |
<0.001a | 0.679
(0.445–1.036) | 0.072 |
| Lymph node
invasion | 3.184
(2.141–4.736) |
<0.001a | 0.743
(0.422–1.307) | 0.302 |
| RAB1A
overexpression | 1.938
(1.277–2.943) | 0.002a | 0.932
(0.619–1.442) | 0.669 |
| Recurrence | 2.567
(1.715–3.841) |
<0.001a | 0.787
(0.425–1.454) | 0.444 |
| HER-2
amplification | 9.241
(5.973–14.296) |
<0.001a | 1.291
(1.081–2.365) | 0.253 |
| WHO
classification | 1.687
(1.261–2.258) | 0.001a | 1.400
(0.813–2.413) | 0.225 |
| RAB1A/HER-2
co-expression | 9.241
(5.973–14.296) |
<0.001a | 6.662
(3.448–12.871) |
<0.001a |
Positive correlation of RAB1A
expression with HER-2 amplification in GAC patients
To confirm whether there was correlation between
HER-2 amplification and RAB1A expression in GAC tissues, we
analysed the IHC data for RAB1A and HER-2 combined with HER-2 FISH
results (Fig. 2). As described in
Table III, 47.84% (111/232) of
patients with an IHC score of 0 or 1+ had weak staining for RAB1A,
and 75.00% (27/36) of patients with an IHC score of 3+ for HER-2 or
HER-2 amplification had strong staining for RAB1A. Thus, HER-2
amplification was found to be significantly positively associated
with RAB1A expression in GAC tissues (P=0.036). Furthermore, we
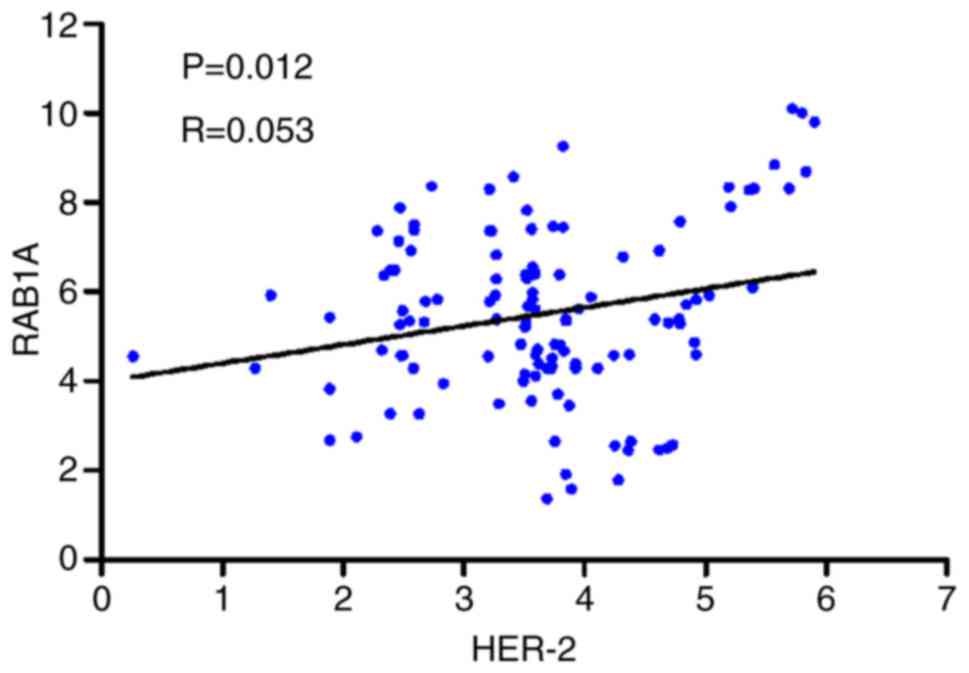
compared RAB1A and HER-2 mRNA expression in 120 GAC tissues by
RT-PCR. The correlation coefficient was 0.053 (P=0.012), and the
scatter diagram is shown in Fig. 4.
The results of statistical analysis indicated a significant
correlation between RAB1A and HER-2 in GAC.
 | Table III.Correlation between RAB1A
overexpression and HER-2 amplification. |
Table III.
Correlation between RAB1A
overexpression and HER-2 amplification.
|
| HER-2 |
|
|---|
|
|
|
|
|---|
|
| FISH (N) IHC
0+/1+ | FISH (N) IHC
2+ | FISH (A) IHC
3+ | P-value |
|---|
| RAB1A |
|
|
|
|
| Low
expression (n=125) | 111 | 5 | 9 | 0.036a |
| High
expression (n=155) | 121 | 7 | 27 |
|
| Total |
|
|
|
|
| 280 | 232 | 12 | 36 |
|
Discussion
GC is an important health problem worldwide, and
despite the development of various diagnostic and treatment
strategies, the morbidity associated with this disease remains
high. Traditional methods of diagnostic and treatment strategies
are limited in GCs. In recent years, molecular classification based
on the HER-2 status and targeted therapies have been introduced for
GC because of potential therapeutic implications. However, given
that the rate of clustering of positive cells in tissue biopsies is
low or at least 10% of positive neoplastic cells in surgical
resection specimens are needed for these type of therapies
(17), immunohistochemical scoring
for HER-2 may not have adequate sensitivity. Thus, further
classifications based on molecular alterations are urgently
required. Based on this perspective, we conducted this study to
evaluate the expression pattern of and correlation between RAB1A
and HER-2 and their clinical significance in GAC patients. Previous
studies have demonstrated that the HER-2 protein/gene is
overexpressed/amplified in various human cancers, including ovarian
cancer (18), prostate cancer
(19), GC (20), lung cancer (21) and bladder cancer (22) and that HER-2 is an important biomarker
for therapeutic assessment for the use of trastuzumab treatment in
breast cancer and GC patients. In our study, 12.86% of the patients
had HER-2 amplification, but we could not identify any association
between HER-2 amplification and adverse prognosis in GAC patients.
The reason that HER-2 is not associated with survival might be that
the small sample size in our research. According to the
literatures, HER-2 overexpression are associated with poor
prognosis in GC patients in large-scale population study (23,24). HER-2
amplification was closely associated with Lauren type, tumour size,
lymph node invasion and the co-expression of RAB1A and HER-2
indicated worse survival than did the expression of RAB1A and HER-2
alone. Moreover, the co-expression of RAB1A and HER-2 was
identified as a prognostic factor in GAC patients. Our results were
partially consistent with those of other studies involving GC
patients. Variations in the rate of HER-2-positivity and other
related factors are reflective of heterogeneous testing modalities
and other variables. However, because GC phenotyping is
heterogenous and because histopathologic and molecular
characteristics in patients can lead to inconsistencies in
therapeutic effects, our findings would aid in the identification
of molecular signatures for HER-2-associated GAC.
Previous reports have demonstrated that RAB1A
regulates the sorting of early endocytic vesicles from the
endoplasmic reticulum to the Golgi complex (25) and have implicated RAB1A in Parkinson's
disease (26). Additionally, RAB1A
has been shown to combine with some small molecules or complexes to
regulate cellular ageing and autophagy (27,28). In
recent years, evidence on the role of RAB1A in tumours has begun to
emerge. RAB1A overexpression has been reported in human tongue
cancer (11) and lung cancer
(29). In our previous study, RAB1A
was identified as an oncogene in colorectal and liver cancer. The
overexpression of RAB1A was significantly associated with poor
prognosis in colorectal and liver cancer patients. Moreover, RAB1A
gene amplification was shown to promote oncogenic transformation
and malignant growth by hyperactivating mTORC1 signalling. In the
present study, we detected the expression status and analysed the
clinical significance of RAB1A and HER-2. RAB1A was found to be
overexpressed in GAC tissues, and the high expression of RAB1A was
associated with lymph node invasion, TNM stage, tumour size,
recurrence and WHO Classification consistent with the role of RAB1A
in inducing migration, invasion and metastasis in cancer cells as
shown in our earlier study. These results further confirmed the
significance of RAB1A in cancer pathogenesis and provided new
perspectives for molecular therapy for GC.
The PI3K/Akt/mTOR pathway is one of the main
downstream signalling pathways of HER-2. When HER-2 is activated by
various factors, it mediates signal transduction through
heterodimerization and autophosphorylation of its tyrosine kinases,
leading to subsequent activation of downstream pathways, including
the PI3K/Akt/mTOR and Ras/Raf/mitogen-activated protein kinase
(MAPK) pathways (30). Previous
studies in breast cancer have demonstrated that the PI3K/AKT/mTORC1
pathway is activated after adjuvant endocrine therapy (31). These findings prompted us to
investigate whether RAB1A is involved in cancer pathogenesis
because RAB1A is a direct activator of the mTORC1 pathway and
because mTORC1 is a downstream signalling molecule of HER-2. Based
on this hypothesis, we analysed RAB1A protein expression and HER-2
amplification in 280 GAC specimens and observed a significant
positive correlation between these two factors. Additionally, we
compared the mRNA expression of RAB1A and HER-2 in 120 GAC
specimens and observed a significant positive correlation between
the two biomarkers. Therefore, RAB1A is likely involved in
downstream signalling pathways of HER-2 in GAC. Moreover, we
confirmed that both HER-2 and RAB1A were overexpressed in GAC
patients. Furthermore, the co-expression of HER-2 and RAB1A were
found to predict adverse prognosis and could thus be used as an
independent prognostic indicator. Moreover, both RAB1A and HER-2
were associated with lymph node invasion and recurrence. In future
studies, we intend to detect the expression pattern of mTORC1 and
its pivotal downstream molecules, such as 4EBP1 and p70S6K. Also,
we intend to verify prognostic value of HER-2 and it's clinical
significance in large-scale of GAC patients. Further studies
regarding HER-2 and its associated molecular pathways may reveal
potential targets for therapeutic intervention, especially for
HER-2-targeted therapy-resistant cancers.
In conclusion, for the first time, we assessed the
expression levels of RAB1A in GAC tissues and showed that RAB1A
expression was significantly higher in GAC tissues than in normal
tissues. Overexpression of RAB1A was closely associated with the
degree of tumour invasion and metastasis as well as poor prognosis.
Meanwhile, the pattern of HER-2 expression observed in our study
was similar to that in other studies, and HER-2
overexpression/amplification was not correlated with poor prognosis
in GAC patients. Furthermore, the expression of RAB1A was
positively correlated with HER-2 amplification, and the
co-expression of RAB1A and HER-2 indicated poor OS, suggesting that
the two factors likely play a synergistic role in tumour
progression, metastasis and angiogenesis. Our results indicate that
multiple signalling pathways might determine the prognosis of
cancer patients, and these pathways may be potential targets for
combined therapeutic intervention, especially in HER-2-targeted
therapy-resistant GC patients.
Glossary
Abbreviations
Abbreviations:
|
HER-2
|
human epidermal growth factor receptor
2
|
|
GC
|
gastric cancer
|
|
IHC
|
immunohistochemistry
|
|
FISH
|
fluorescence in situ
hybridization
|
|
OS
|
overall survival
|
References
|
1
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Dicker D, Pain A, Hamavid H,
Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et
al: The global burden of cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chon SH, Berlth F, Plum PS, Herbold T,
Alakus H, Kleinert R, Moenig SP, Bruns CJ, Hoelscher AH and Meyer
HJ: Gastric cancer treatment in the world: Germany. Transl
Gastroenterol Hepatol. 2:532017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song Z, Wu Y, Yang J, Yang D and Fang X:
Progress in the treatment of advanced gastric cancer. Tumour Biol.
39:10104283177146262017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jou E and Rajdev L: Current and emerging
therapies in unresectable and recurrent gastric cancer. World J
Gastroenterol. 22:4812–4823. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tong ZJ, Shi NY, Zhang ZJ, Yuan XD and
Hong XM: Expression and prognostic value of HER-2/neu in primary
breast cancer with sentinel lymph node metastasis. Biosci Rep.
37(pii): BSR201701212017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gajria D and Chandarlapaty S:
HER2-amplified breast cancer: Mechanisms of trastuzumab resistance
and novel targeted therapies. Expert Rev Anticancer Ther.
11:263–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abrahao-Machado LF and Scapulatempo-Neto
C: HER2 testing in gastric cancer: An update. World J
Gastroenterol. 22:4619–4625. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hashimoto K, Tsuda H, Koizumi F, Shimizu
C, Yonemori K, Ando M, Kodaira M, Yunokawa M, Fujiwara Y and Tamura
K: Activated PI3K/AKT and MAPK pathways are potential good
prognostic markers in node-positive, triple-negative breast cancer.
Ann Oncol. 25:1973–1979. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shan X, Wen W, Zhu D, Yan T, Cheng W,
Huang Z, Zhang L, Zhang H, Wang T, Zhu W, et al: miR 1296-5p
inhibits the migration and invasion of gastric cancer cells by
repressing ERBB2 expression. PLoS One. 12:e01702982017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mukhopadhyay A, Nieves E, Che FY, Wang J,
Jin L, Murray JW, Gordon K, Angeletti RH and Wolkoff AW: Proteomic
analysis of endocytic vesicles: Rab1a regulates motility of early
endocytic vesicles. J Cell Sci. 124:765–775. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimada K, Uzawa K, Kato M, Endo Y, Shiiba
M, Bukawa H, Yokoe H, Seki N and Tanzawa H: Aberrant expression of
RAB1A in human tongue cancer. Br J Cancer. 92:1915–1921. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomas JD, Zhang YJ, Wei YH, Cho JH,
Morris LE, Wang HY and Zheng XF: Rab1A is an mTORC1 activator and a
colorectal oncogene. Cancer Cell. 26:754–769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Davis NM, Sokolosky M, Stadelman K, Abrams
SL, Libra M, Candido S, Nicoletti F, Polesel J, Maestro R, D'Assoro
A, et al: Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in
breast cancer: Possibilities for therapeutic intervention.
Oncotarget. 5:4603–4650. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Webster CP, Smith EF, Bauer CS, Moller A,
Hautbergue GM, Ferraiuolo L, Myszczynska MA, Higginbottom A, Walsh
MJ, Whitworth AJ, et al: The C9orf72 protein interacts with Rab1a
and the ULK1 complex to regulate initiation of autophagy. EMBO J.
35:1656–1676. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu BH, Li XX, Yang Y, Zhang MY, Rao HL,
Wang HY and Zheng XF: Aberrant amino acid signaling promotes growth
and metastasis of hepatocellular carcinomas through Rab1A dependent
activation of mTORC1 by Rab1A. Oncotarget. 6:20813–20828.
2015.PubMed/NCBI
|
|
16
|
Hofmann M, Stoss O, Shi D, Büttner R, van
de Vijver M, Kim W, Ochiai A, Rüschoff J and Henkel T: Assessment
of a HER2 scoring system for gastric cancer: Results from a
validation study. Histopathology. 52:797–805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ouyang W, Xu L, Huang Z, Guo J, Cai J, Gao
X and Wang Z: Role of HER family members in predicting prognoses in
epithelial ovarian cancer: A meta-analysis. Tumori. 101:595–602.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murray NP, Reyes E, Fuentealba C, Jacob O
and Orellana N: Possible role of HER-2 in the progression of
prostate cancer from primary tumor to androgen independence. Asian
Pac J Cancer Prev. 16:6615–6619. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lordick F, Al-Batran SE, Dietel M, Gaiser
T, Hofheinz RD, Kirchner T, Kreipe HH, Lorenzen S, Möhler M, Quaas
A, et al: HER2 testing in gastric cancer: Results of a German
expert meeting. J Cancer Res Clin Oncol. 143:835–841. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim EK, Kim KA, Lee CY and Shim HS: The
frequency and clinical impact of HER2 alterations in lung
adenocarcinoma. PLoS One. 12:e01712802017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cormio L, Sanguedolce F, Cormio A,
Massenio P, Pedicillo MC, Cagiano S, Calò G, Pagliarulo V, Carrieri
G and Bufo P: Human epidermal growth factor receptor 2 expression
is more important than Bacillus Calmette Guerin treatment in
predicting the outcome of T1G3 bladder cancer. Oncotarget.
8:25433–25441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kurokawa Y, Matsuura N, Kimura Y, Adachi
S, Fujita J, Imamura H, Kobayashi K, Yokoyama Y, Shaker MN,
Takiguchi S, et al: Multicenter large-scale study of prognostic
impact of HER2 expression in patients with resectable gastric
cancer. Gastric Cancer. 18:691–697. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lei YY, Huang JY, Zhao QR, Jiang N, Xu HM,
Wang ZN, Li HQ, Zhang SB and Sun Z: The clinicopathological
parameters and prognostic significance of HER2 expression in
gastric cancer patients: A meta-analysis of literature. World J
Surg Oncol. 15:682017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hutagalung AH and Novick PJ: Role of Rab
GTPases in membrane traffic and cell physiology. Physiol Rev.
91:119–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Winslow AR, Chen CW, Corrochano S,
Acevedo-Arozena A, Gordon DE, Peden AA, Lichtenberg M, Menzies FM,
Ravikumar B, Imarisio S, et al: α-synuclein impairs macroautophagy:
Implications for Parkinson's disease. J Cell Biol. 190:1023–1037.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu X, Fu B, Chen D, Hong Q, Cui J, Li J,
Bai X and Chen X: miR-184 and miR-150 promote renal glomerular
mesangial cell aging by targeting Rab1a and Rab31. Exp Cell Res.
336:192–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ramírez-Peinado S, Ignashkova TI, van Raam
BJ, Baumann J, Sennott EL, Gendarme M, Lindemann RK, Starnbach MN
and Reiling JH: TRAPPC13 modulates autophagy and the response to
Golgi stress. J Cell Sci. 130:2251–2265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Liu F, Qin X, Huang T, Huang B,
Zhang Y and Jiang B: Expression of Rab1A is upregulated in human
lung cancer and associated with tumor size and T stage. Aging
(Albany NY). 8:2790–2798. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wilks ST: Potential of overcoming
resistance to HER2-targeted therapies through the PI3K/Akt/mTOR
pathway. Breast. 24:548–555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Beelen K, Hoefnagel LD, Opdam M, Wesseling
J, Sanders J, Vincent AD, van Diest PJ and Linn SC: PI3K/AKT/mTOR
pathway activation in primary and corresponding metastatic breast
tumors after adjuvant endocrine therapy. Int J Cancer.
135:1257–1263. 2014. View Article : Google Scholar : PubMed/NCBI
|