Introduction
Hepatocellular carcinoma (HCC) is one of the most
prevalent malignancies worldwide and was the second leading cause
of cancer-associated mortality in 2011 (1). The incidence rate of HCC is the highest
in east and Southeast Asia, as well as in Middle and Western
Africa; however, it is also becoming more prevalent in the United
States and Europe (1). Despite liver
transplantation (LT) remaining one of the most effective radical
treatments, a significant proportion of patients still suffer from
unfavorable outcomes due to a high recurrence rate (~15–20%)
(2,3).
Ultrasonography has been widely used in HCC screening and
monitoring; however, interpretation of test results is subjective
and depends upon the skill and experience of investigators and
therefore this limits its clinical application. Likewise,
radiological examinations including contrast enhanced computed
tomography (CT) or magnetic resonance imaging (MRI) are sometimes
not able to detect small tumors and inconspicuous metastasis
lesions due to low sensitivity, leading to delayed treatment.
α-fetoprotein (AFP) is a widely used serum marker in the clinical
diagnosis and surveillence of HCC. However, it has been previously
reported that pregnancy, benign liver disease and certain
gastrointestinal tumors may contribute to false-positive results
(4). Therefore, the importance of
developing an accurate diagnostic marker or monitoring tool for
patients with HCC should be emphasized.
Circulating tumor cells (CTCs) are cells that have
shed into the vasculature from a primary or metastatic solid tumor
and are carried around the body via blood circulation. Previous
studies have demonstrated that CTCs serve a key function in
metastasis and recurrence in HCC (5,6). Due to
the complete removal of tumor lesion(s) and implantation of
tumor-free grafts through LT, the source of short-term recurrence
and metastasis must be residual extrahepatic CTCs. Therefore,
investigation of CTCs in peripheral blood, also termed liquid
biopsy, is a promising strategy for monitoring patients with HCC
undergoing LT.
CellSearch® system is a traditional
device for CTCs detection (7).
High-level CTCs detected by CellSearch® system have been
implicated in increased recurrence risk in HCC patients after liver
resection (8,9). However, previous evidence demonstrated
that epithelial-mesenchymal transition (EMT) process may contribute
to the low expression of epithelial cell adhesion marker (EpCAM)
and cytokeratins (CKs), and thus reduce the detection sensitivity
(10). The
immunostaining-fluorescence in situ
hybridization® (iFISH®) platform exhibits
good sensitivity in breast (7),
gastric (8), lung (9) and pancreatic cancer (10), and is therefore highly recommended.
However, the size of tumor cells and EpCAM expression varies. iFISH
can eliminate red blood cells (RBCs) and deplete white blood cells
(WBCs) using anti-CD45 antibodies, then in situ phenotypic
and karyotypic identification is performed using centromere probe 8
(CEP8), which effectively improves CTCs detection sensitivity via
subtractive enrichment (11). In the
present study, iFISH® was utilized to separate and
characterize CTCs in patients with HCC undergoing LT. The present
study aims to evaluate the analytical performance of
iFISH® and examine it's clinical value, as well as
compare it to the CellSearch® system. To the best of our
knowledge, this is the first time that this platform has been
applied to patients with HCC undergoing LT.
Materials and methods
Patients and sample collection
Between November 2014 and October 2015, peripheral
blood samples were collected from 30 HCC patients undergoing LT,
and 10 healthy controls in Renji Hospital (Shanghai, China). Blood
was obtained 2 days prior to transplantation for patients with HCC
(baseline), and a median of 90 days (range, 81–97 days) following
LT. At each time point, 7.5 ml peripheral blood was collected from
each subject for iFISH® and Cellsearch®
analyses. Blood samples were processed within 48 h of collection.
Ethical approval for the recruitment of human subjects was obtained
from the Ethics Committee of Shanghai Jiaotong University
Affiliated Renji Hospital (Shanghai, China) and was consistent with
ethical guidelines provided by the Declaration of Helsinki (1975).
Written informed consent was obtained from each patient prior to
collecting blood samples. Clinical information was also collected
including the AFP level, size and number of tumors, Barcelona
Clinic Liver Cancer (BCLC) stage (12), Milan criteria (13), Child-Pugh score and presence of
hepatitis B virus, hepatitis C virus, vascular invasion and portal
vein tumor thrombus. Patients were followed up every 3 months
during the first postoperative year and at least every 3–4 months
thereafter. The mean follow-up was 11.2 months (range, 4–20
months). During the follow-up, 3 patients succumbed to intrahepatic
recurrence and 3 patients succumbed to extrahepatic metastasis. All
patients were monitored prospectively by measuring levels of serum
AFP and performing abdomen ultrasonography monthly in the first 3
months and every 6 months in late stage following LT. Chest x-ray
was also performed every 6 months following LT. For patients with
test results indicating recurrence, CT and/or MRI, and even bone
scanning were used to verify whether intrahepatic recurrence and/or
extrahepatic metastasis had occurred. A diagnosis of recurrence was
based on typical imaging appearances in CT, MRI and/or bone scans,
as well as an elevated AFP level >400 IU/ml.
Subtraction enrichment in
iFISH®
Subtraction enrichment of CTCs was performed
similarly to previous studies (7,10,11). Cytelligen® CTC enrichment
kit (Cytelligen Inc., San Diego, CA, USA) was used to enrich CTCs.
Briefly, a 7.5 ml blood sample was collected into
acid-citrate-dextrose anticoagulant tube, centrifuged at 600 × g
for 5 min at room temperature and the supernatant was discarded.
The sample was mixed with 3 ml Separation Matrix (Cytelligen Inc.,
San Diego, CA, USA), centrifuged at 400 × g for 5 min at room
temperature and the white buffy coat was collected and then
incubated with 150 µl immunomagnetic particles conjugated to
anti-cluster of differentiation (CD)45 antibody (SEH-001R3;
Cytelligen Inc.) for 10 min at room temperature, separated by a
magnetic separator (cat. no. V8151; Promega Corporation, Madison,
WI, USA). The bead-free solution was transferred to a centrifuge
tube and washed twice with CRC washing buffer (SEH-001R1;
Cytelligen Inc.) and, centrifuged at 650 × g for 5 min at room
temperature. The supernatant was removed, then 100 µl Cytelligen
Fixative (SEH-001R4; Cytelligen Inc.) was added at room temperature
for 4 h and the cell suspension was placed into cover glass, 37°C
overnight for drying.
Immunofluorescent staining of CTCs in
iFISH®
CTCs identification was performed using the
Cytelligen® CTCs identification kit (Cytelligen Inc.)
according to the manufacturers' protocol. Samples were prepared and
fixed as aforementioned. Briefly, the cover glass was removed in
FR2 buffer (FSH-001R2; Cytelligen Inc.) and dehydrated in 100%
ethanol for 2 min in room temperature. CEP 8 SpectrumOrange (Vysis,
Inc.; Abbott Pharmaceutical Co. Ltd., Lake Bluff, IL, USA)
hybridization was performed according to a previously published
protocol (7) Slides were then were
then incubated with antibody preparation solution-1 (FSH-001R6;
Cytelligen Inc.), anti-cytokeratin 18 antibody (HAB-001R1;
Cytelligen Inc.) and anti-CD45 antibody (HAB-001R2; Cytelligen
Inc.). Finally, mounting medium (FSH-001R7; Cytelligen Inc.) with
DAPI was added and observed using a fluorescence microscope
(Magnification, ×40; Nikon Corporation, Tokyo, Japan). CTCs were
defined as cells with features of CK+/CD45-/DAPI+ and hybridization
signal for CEP8 ≥2, CK-/CD45-/DAPI+ and hybridization signal for
CEP8>2. CK-/CD45+/DAPI+ and hybridization signal for CEP8=2 was
defined as a white blood cell (WBC) and CK-/CD45-/DAPI+ and
hybridization signal for CEP8=2 was defined as an indeterminate
cell.
CTCs detected using
CellSearch® system
Samples were detected using CellSearch®
system according to the manufacturer's protocol (Menarini Silicon
Biosystems, Inc., San Diego, CA, USA). Prior to testing, 7.5 ml of
peripheral blood was collected (as aforementioned) into the
CellSave Preservative Tube containing EDTA and pre-prepared
fixative (PBS, 25% proprietary ingredients, 0.1% BSA and 0.1%
sodium azide; included in the CellSearch® CTC Kit;
Veridex LLC, Raritan, NJ, USA) for protecting CTCs morphology.
Subsequently, 6 ml buffer (included in the CellSearch®
CTC kit) was added into the tube and centrifuged at room
temperature with 164 × g for 10 min, and the supernatant was
discarded. The tube was placed in the CellTracks Autoprep System
(Menarini Silicon Biosystems, Inc.). EpCAM-coated magnetic beads
were used to enrich cells which expressed EpCAM on their surface,
and the enriched cells underwent subsequent immunofluorescence
staining.
Immunofluorescence reagent (cat no. 7900001;
Menarini Silicon Biosystems, Inc.) was used according to the
manufacturer's protocol and included anti-CK (CK8, CK18 and CK19;
0.0006%) antibody conjugated to phycoerythrin, anti-CD45 antibody
(0.0012%) conjugated to allophycocyanin and nuclear dye DAPI
(0.005%). The immunostaining of cells was performed at room
temperature for 20 min. Finally, cells were transferred to the
CellTracks Analyzer II (Cat no. 9555; Menarini Silicon Biosystems,
Inc.) for analysis. CTCs were characterized as EpCAM-positive,
CK-positive, DAPI-positive and CD45-negative cells
(EpCAM+/CK+/DAPI+/CD45-).
Validation of iFISH®
platform in cell lines
A cell spiking test was performed to validate the
effectiveness of iFISH® platform in HCC. Huh-7 cells, a
hepatoma cell line, was obtained from the cell bank of Shanghai
Institutes for Biological Sciences, Chinese Academy of Sciences
(Shanghai, China) and cultured in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in
a humidified 5% CO2/95% air atmosphere at 37°C. For the
spiking test, 100 Huh-7 cells were added to each 7.5 ml of
peripheral blood obtained from the 10 healthy volunteers. Then the
aforementioned iFISH® protocol was performed to enrich
and count the CTCs detected. The mean recovery rate was calculated
by the ratio of iFISH-CTCs number to 100 spiked cells number in the
10 samples.
Statistical analysis
All statistical analyses were performed using SPSS
(version 22.0; IBM Corp., Armonk, NY, USA). Data with a normal
distribution are presented as the mean ± the standard deviation,
whereas data without a normal distribution are presented as the
median (range). Difference between groups was analyzed using the
χ2 test, Fisher's exact test or Student's t-test, as
appropriate. If variations within groups were not homogeneous, data
were analyzed using the nonparametric Mann-Whitney U test or
Wilcoxon signed-rank test, as appropriate. The agreement between
CellSearch® and iFISH® was determined by κ
test. Spearman rank correlation analysis was used for nonparametric
correlation analysis. Receiver operating characteristic (ROC)
analysis was used to analyze the sensitivity (SEN) and specificity
of different methods. Positive predictive values and negative
predictive values were also computed. Recurrence-free survival
(RFS) was defined as the time from LT to the date of conformed
recurrence or the final follow-up. Kaplan-Meier survival curves
with log-rank test were used to compare the difference in RFS. The
threshold of AFP level between high-AFP and low-AFP patients was
400 IU/ml (4). Univariate Cox
proportional hazards regression analysis was performed to identify
RFS-associated risk factors. Graphical plots were generated using
GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Technical validation of
iFISH® platform
As iFISH® platform has never been applied
in HCC field, to the best of our knowledge, the analytical
performance of iFISH for CTCs detection was preliminarily examined
in 1 patient and 10 volunteers, as well as cell spiking test. Blood
specimens from 10 healthy volunteers were examined and no
iFISH-CTCs were detected. Furthermore, one patient with HCC was
investigated and exhibited terminal HCC with distant metastases and
a number of 7 iFISH-CTCs and CK-/CD45-/DAPI+/CEP8 >2 was
identified. In the cell spiking test, the mean recovery rate was
81.60±6.04%, suggesting that iFISH platform was practical for
patients with HCC undergoing LT. iFISH-CTCs identified by various
characteristics are presented in Fig.
1.
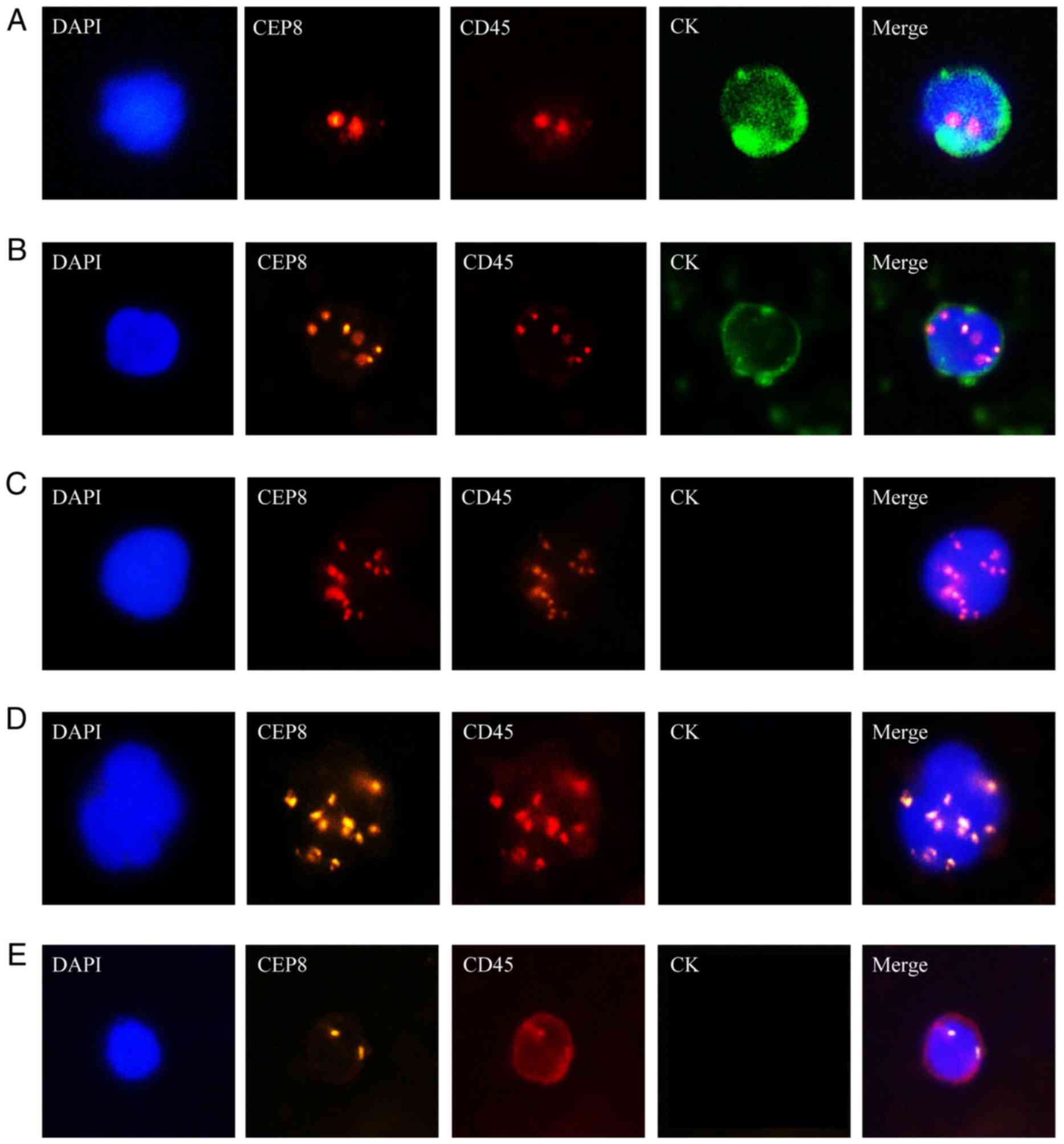 | Figure 1.Identification of CTCs in HCC
patients using iFISH platform. CK: Green, CEP8: Orange, DAPI: Blue,
CD45: Red. (A) CK+/CD45-/DAPI+/CEP8=2; (B)
CK+/CD45-/DAPI+/CEP8>2; (C) CK-/CD45+/DAPI+/CEP8>2. (D) CTC
cluster. (E) CK-/CD45+/DAPI+/CEP8=2, WBC. iFISH,
immunostaining-fluorescence in situ hybridization; CTCs,
circulating tumor cells; HCC, hepatocellular carcinoma; CK,
cytokeratin; CEP8, centromere probe 8; CD, cluster of
differentiation; WBC, white blood cell. |
Detection of CTCs in HCC patients and
healthy volunteers
With the exception of single CTCs detected, CTC
clusters (defined as CTCs aggregated through plakoglobin-dependent
intercellular adhesion) were also detected in certain samples.
These CTC clusters are derived from multicellular groupings of
primary liver tumor cells. A CTC cluster demonstrated 23- to
50-fold increased metastatic potential compared with single CTCs as
indicated in previous studies (14).
In the present study, it was estimated there would be 1 CTC cluster
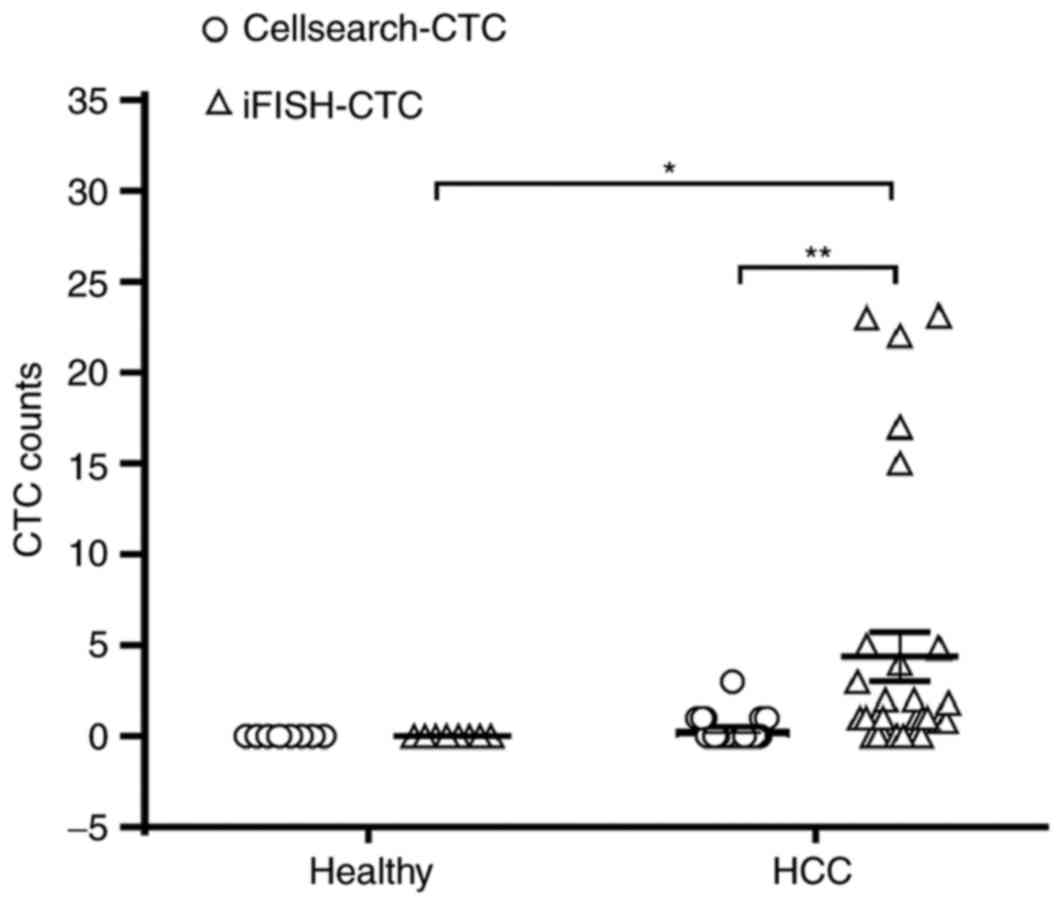
to every 20 CTCs conservatively. As demonstrated in Fig. 2, the number of CTCs detected in
patients with HCC was 0–23/7.5 ml (median, 1/7.5 ml) for
iFISH®, which was significantly increased compared with
that detected by CellSearch® (0–2/7.5 ml, median 0/7.5
ml; P<0.01). CTCs were detected using iFISH® in 21/30
patients (70%), presenting an increased detection rate compared
with that in CellSearch® (8/30 patients, positive rate;
26.67%). The κ agreement coefficient between iFISH® and
CellSearch® was 0.077 (P=0.559), indicating that the
similarity in results between Cellsearch and iFISH was poor. No CTC
was detected in any healthy volunteer for either approach.
To discriminate patents with HCC from healthy
volunteers, ROC curves were applied to determine the detection
efficiency of iFISH and CellSearch®. The SEN of
iFISH® was markedly increased compared with that of
CellSearch® (SEN, 70 vs. 26.67%; P<0.01; Table I).
 | Table I.Comparison of discrimination ability
between iFISH® and CellSearch® systems. |
Table I.
Comparison of discrimination ability
between iFISH® and CellSearch® systems.
| Systems | SEN (%) | SPE (%) | NPV | PPV | 95% CI | P-value |
|---|
|
Cellsearch-CTCs | 26.67 | 1 | 0.312 | 1 | 0.454–0.813 | 0.212 |
| iFISH-CTCs | 70 | 1 | 0.526 | 1 | 0.734–0.966 |
0.001a |
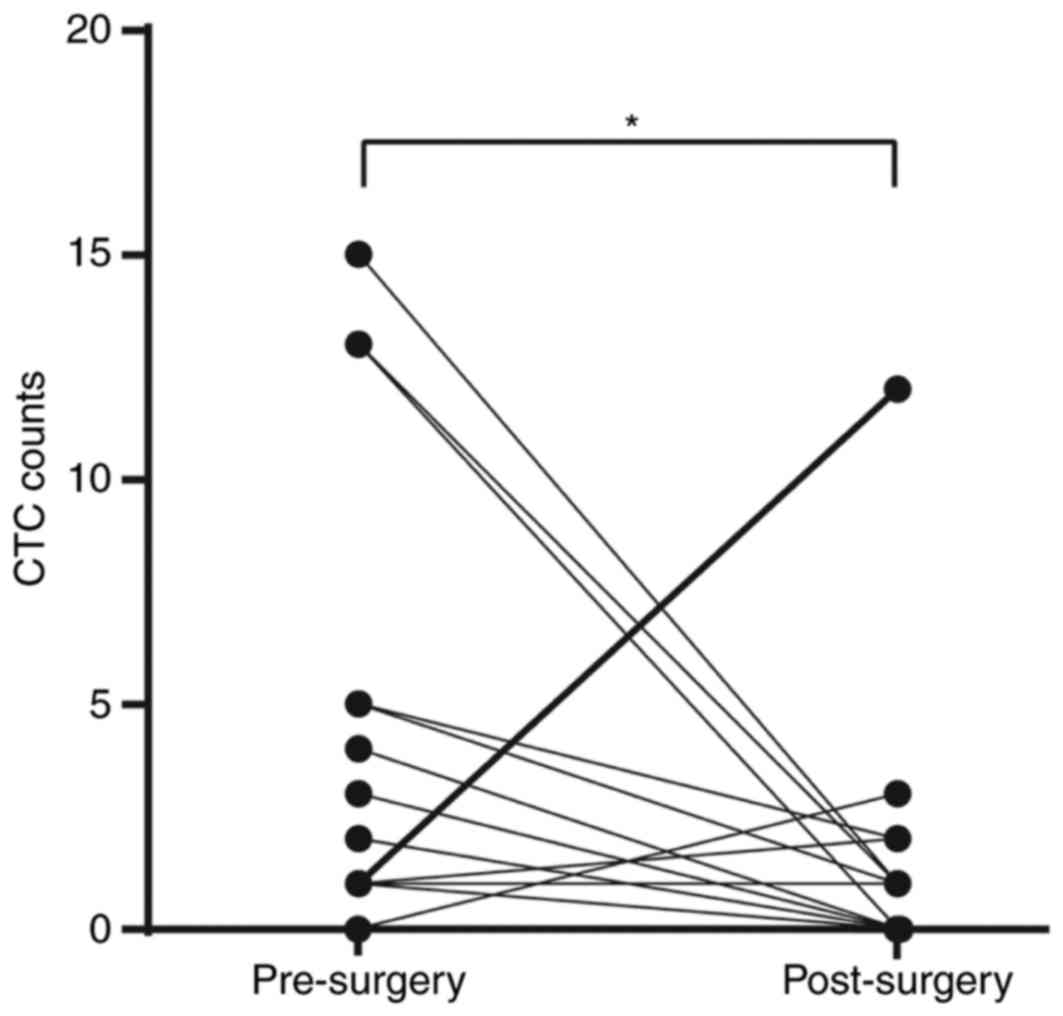
The postoperative levels of CTCs were measured in 23
patients at 3 months following transplantation. Compared with
preoperative results, the number of CTCs detected was decreased in
65.21 and 34.78% (iFISH® and CellSearch®,
respectively) of patients with HCC following LT. However, the
iFISH-CTCs level didn't change in 21.73% of patients and increased
in 13.04%. For Cellsearch-CTCs, the ratio is 56.52 and 8.70%
respectively (Fig. 3). iFISH-CTCs
count was significantly decreased following transplantation
(P<0.05). Notably, the patient whose iFISH-CTCs counts increased
from 2/7.5 ml preoperatively to 12/7.5 ml postoperatively was
diagnosed with extrahepatic metastasis 4 months following surgery.
There was no similar elevation detected in the
Cellsearch®-CTCs count.
Associations between positive rate of
CTCs and clinicopathological characteristics
The associations between positive rates of both
preoperative Cellsearch-CTCs and iFISH-CTCs and clinicopathological
characteristics of HCC patients were examined.
Results identified a significant negative
association between iFISH-CTCs positive rate and high-AFP rate
(Spearmans correlation value=−0.617; P<0.001). However, no
significant associations were observed between CTCs and other
clinicopathological features including AFP level, vascular
invasion, portal vein tumor thrombus, BCLC stage and Milan criteria
(Table II).
 | Table II.Relationship between CTCs positive
rate of iFISH® and Cellsearch® and
clinicopathological features of patients with HCC. |
Table II.
Relationship between CTCs positive
rate of iFISH® and Cellsearch® and
clinicopathological features of patients with HCC.
|
|
| Cellsearch | iFISH |
|---|
|
|
|
|
|
|---|
| Variable | Proportion (%) | Absent | Present | P-value | Absent | Present | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
Male | 27 (90) | 3 | 0 | 0.545 | 1 | 2 | 1 |
|
Female | 3 (10) | 19 | 8 |
| 8 | 19 |
|
| Age, years |
|
|
|
|
|
|
|
|
≤50 | 12 (40) | 5 | 5 | 0.078 | 5 | 5 | 0.115 |
|
>50 | 18 (60) | 17 | 3 |
| 4 | 16 |
|
| Etiology |
|
|
|
|
|
|
|
| HBV (%)
only | 23 (76.67) | 17 | 6 | 0.331 | 7 | 16 | 0.359 |
| HCV (%)
only | 3 (10) | 3 | 0 |
| 0 | 3 |
|
|
Other | 4 (13.33) | 2 | 2 |
| 2 | 2 |
|
| Child-Pugh
score |
|
|
|
|
|
|
|
|
A/B | 19 (63.33) | 13 | 6 | 0.67 | 6 | 13 | 1 |
| C | 11 (36.67) | 9 | 2 |
| 3 | 8 |
|
| AFP, IU/ml |
|
|
|
|
|
|
|
|
≤400 | 20 (66.67) | 13 | 7 | 0.21 | 2 | 18 | 0.002a |
|
>400 | 10 (33.33) | 9 | 1 |
| 7 | 3 |
|
| Tumor size, cm |
|
|
|
|
|
|
|
| ≤5 | 19 (63.33) | 13 | 2 | 0.215 | 4 | 11 | 1 |
|
>5 | 11 (36.67) | 9 | 6 |
| 5 | 10 |
|
| Tumor number |
|
|
|
|
|
|
|
|
Single | 18 (60) | 12 | 6 | 0.419 | 7 | 11 | 0.249 |
|
Multiple | 12 (40) | 10 | 2 |
| 2 | 10 |
|
| Encapsulation |
|
|
|
|
|
|
|
|
Complete | 11 (36.67) | 15 | 5 | 1 | 8 | 12 | 0.204 |
|
None | 19 (63.33) | 7 | 3 |
| 1 | 9 |
|
| Vascular
invasion |
|
|
|
|
|
|
|
| No | 23 (76.67) | 17 | 6 | 1 | 6 | 17 | 0.64 |
|
Yes | 7 (23.33) | 5 | 2 |
| 3 | 4 |
|
| Portal vein tumor
thrombus |
|
|
|
|
|
|
|
| No | 24 (80) | 18 | 6 | 0.645 | 7 | 17 | 1 |
|
Yes | 6 (20) | 4 | 2 |
| 2 | 4 |
|
| BCLC stage |
|
|
|
|
|
|
|
|
0+A | 24 (80) | 13 | 4 | 0.698 | 5 | 12 | 1 |
|
B+C | 6 (20) | 9 | 4 |
| 4 | 9 |
|
| Milan criteria |
|
|
|
|
|
|
|
|
Within | 10 (33.33) | 15 | 5 | 1 | 6 | 14 | 1 |
|
Beyond | 20 (66.67) | 7 | 3 |
| 3 | 7 |
|
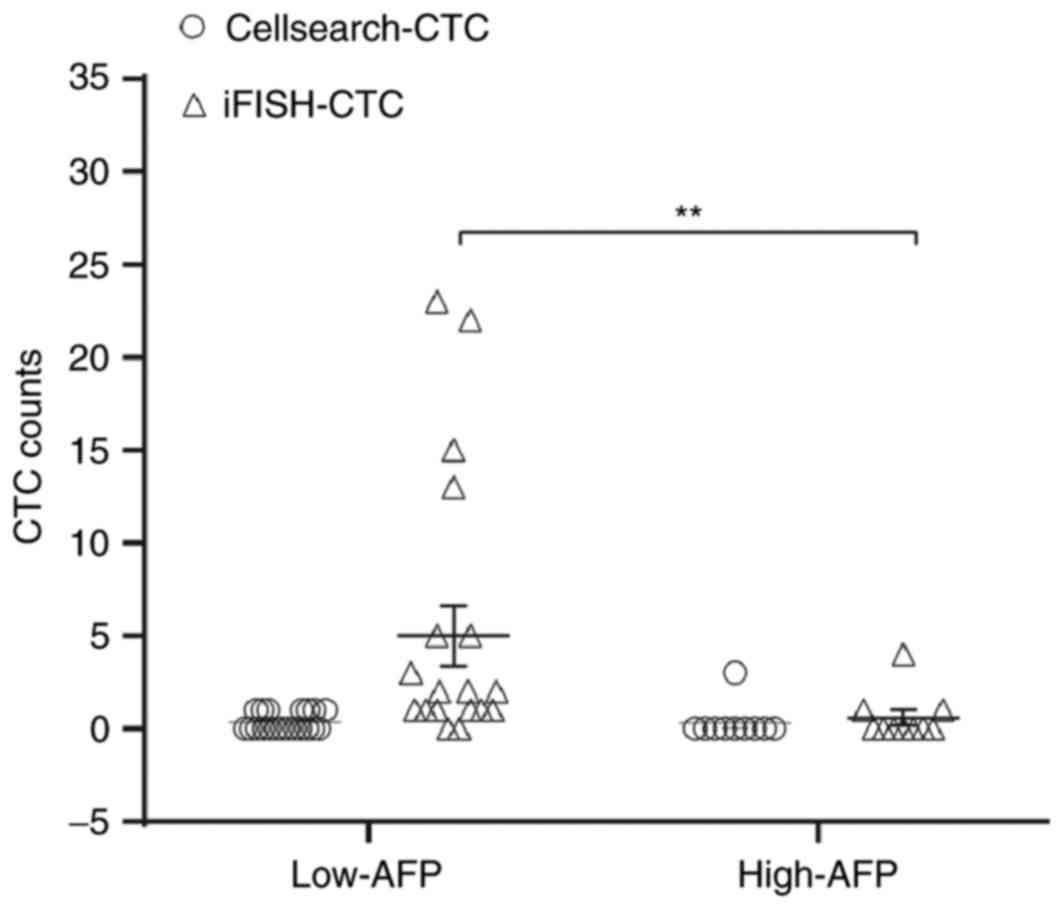
Notably, results demonstrated that the median
preoperative iFISH-CTCs number in low-AFP and high-AFP patients was
1.5/7.5 ml (range, 0–23) and 0/7.5 ml (range, 0–4), respectively,
which suggested that the low-AFP subgroup had a significantly
increased iFISH-CTCs level compared with the high-AFP group
(P<0.01, Fig. 4). Noticeably,
positive iFISH-CTCs were detected in 90% of patients with low-AFP
levels. Spearman correlation analysis demonstrated that AFP levels
were negatively associated with iFISH-CTCs levels (R=−0.385;
P<0.05).
Prognostic value of CTCs in HCC
patients
At a medium follow-up of 15 months (range 4–20), 11
patients with HCC (36.67%) were diagnosed with intrahepatic
recurrence (n=7) or extrahepatic metastasis using CT or MRI
scanning. Furthermore, metastatic lesions were located in numerous
locations including the bone (n=1), lung (n=1), abdominal cavity
(n=1) and the adrenal gland (n=1), which were detected by CT, MRI
or bone scanning. Univariate Cox proportional hazards regression
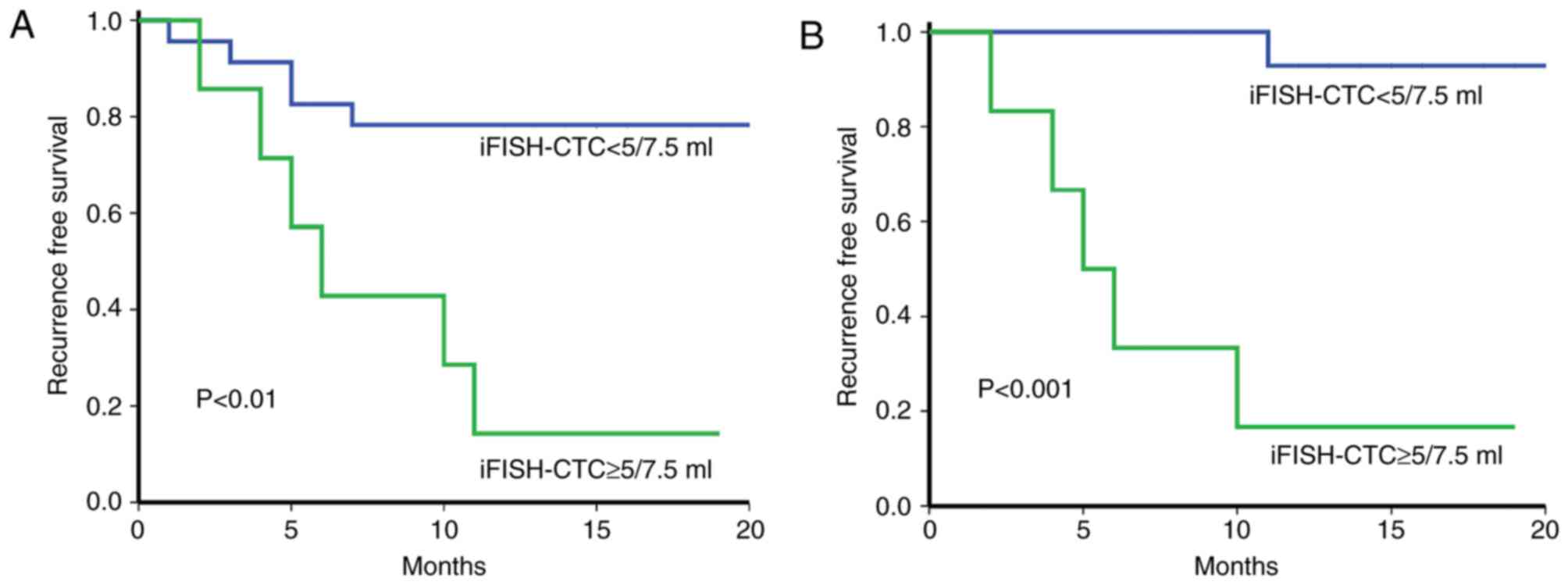
analysis identified that preoperative iFISH-CTCs (≥5/7.5 ml) and
vascular invasion were prognostic factors for RFS (Table III). The median RFS in patients with
iFISH-CTCs <5/7.5 ml was significantly increased compared with
CTC ≥5/7.5 ml (15 vs. 5.5 months). Kaplan-Meier analysis
demonstrated that preoperative iFISH-CTCs ≥5/7.5 ml was associated
with poor RFS (hazard ratio=5.142, P<0.01; Table III, Fig.
5A). For patients with low-AFP (AFP<400 IU/ml) levels, the
prognostic value was increased (hazard ratio=26.4, P<0.001;
Fig. 5B) comparing with high-AFP
patients.
 | Table III.Univariate analysis of factors
predictive for RFS. Preoperative iFISH-CTCs ≥5/7.5 ml and vascular
invasion were prognostic factors for RFS. |
Table III.
Univariate analysis of factors
predictive for RFS. Preoperative iFISH-CTCs ≥5/7.5 ml and vascular
invasion were prognostic factors for RFS.
| Factors | Hazard ratio | 95% CI | P-value |
|---|
| Univariate
analysis |
|
|
|
| AFP, IU/ml (≤400
vs. >400) | 2.072 | 0.629–6.824 | 0.231 |
| Cellsearch-CTC
(negative vs. positive) | 0.536 | 0.115–2.486 | 0.425 |
| iFISH-CTC (<5
vs. >5) | 5.142 | 1.528–17.305 | 0.008b |
| Milan criteria
(within vs. beyond) | 6.11 | 0.78–47.877 | 0.085 |
| Portal vein tumor
thrombus (negative vs. positive) | 2.982 | 0.857–10.373 | 0.086 |
| Tumor number
(single vs. multiple) | 2.109 | 0.559–7.959 | 0.271 |
| Vascular invasion
(negative vs. positive) | 4.32 | 1.290–14.467 | 0.018a |
Discussion
In the present study, the analytical performance and
clinical value of iFISH® in patients with HCC undergoing
LT was evaluated. The detected iFISH-CTCs count was markedly
increased compared with that of Cellsearch-CTCs, and the positive
rate of iFISH-CTCs was significantly increased compared with
Cellsearch-CTCs. Furthermore, the positive rate of Cellsearch-CTCs
in the present study was significantly decreased compared with
previous studies investigating patients with HCC who underwent
hepatectomy (15–17). One possible reason for this is that
the tumor lesion was drawn out thoroughly during LT; however,
following hepatectomy, lesions may remain in residual liver lobes.
Thus, for patients with HCC undergoing LT, the increased detection
sensitivity may facilitate in the clinical application of the
iFISH® platform.
The ability of iFISH® system to
discriminate between HCC patients and healthy volunteers may
provide a promising method for diagnosing and/or monitoring HCC.
Currently, AFP remains the mainstream serum screening biomarker for
HCC. However, ~30–40% of patients with HCC are AFP-negative
(18,19), and it has been previously demonstrated
that pregnancy, benign liver disease and certain gastrointestinal
tumors may contribute to false-positive result (4,20).
Therefore, guidelines in the USA and Europe have recommended
ultrasonography alone without AFP examination as the routine
surveillance method for HCC (21,22).
Notably, in the present study results demonstrated that there was
an increased negative association between iFISH-CTCs positive rate
and high-AFP rate. This association was evident as one or more
iFISH-CTCs were detected in 90% of low-AFP patients. Thus,
iFISH-CTCs levels may provide an effective supplement for the AFP
approach, especially for those patients with low-AFP levels.
The present study also examined the dynamic change
of CTCs during the perioperative period. Surgical manipulation
including squeezing or traction on the tumor has been proven to
facilitate tumor cell dissemination into circulation (23). To the best of our knowledge, this is
the first time that a significant decrease of iFISH-CTCs load was
observed following LT, which may be caused by the radical removal
of the primary tumor. However, future studies should be implemented
to investigate the value of monitoring CTCs changes along with
postoperative treatment. According to the present study, the
regular surveillance of iFISH-CTCs following LT counts may be
effective for early-detection of HCC recurrence, and subsequently
inform personalized antitumor therapy.
The prognostic value of iFISH® platform
was also investigated. Results of the present study demonstrated
that iFISH®-CTCs (≥5/7.5 ml) was a prognostic factor for
patients with HCC undergoing LT. Despite this,
Cellsearch®-CTCs have been previously reported as a
strong predictor for HCC recurrence following curative resection
(15–17); however, the prognostic value of
Cellsearch®-CTCs was not favorable in the present study,
which may be due to the low detection rate. Additionally, it was
also identified that in addition to vascular invasion, portal vein
tumor thrombus, BCLC stage and Milan criteria, iFISH-CTCs is an
independent prognostic predictor for patients with HCC undergoing
LT. Notably, Milan criteria has been previously reported as a
well-known predictor for HCC recurrence following transplantation
(13). However, in the present study,
results demonstrated that only 50% (10/20) of patients exceeding
Milan criteria suffered relapse. Considering the lack of serum
biomarker in Milan Criteria, iFISH-CTCs count may be a promising
option.
To conclude, iFISH® platform presents an
increased analytical sensitivity compared with the
Cellsearch® platform and has the potential to be a
dynamic monitoring tool for CTCs, whose level in peripheral
circulation may be a prognostic marker for patients with HCC
undergoing LT.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AFP
|
α-fetoprotein
|
|
CTC
|
circulating tumor cells
|
|
Cellsearch-CTCs
|
CTCs counted by
CellSearch®
|
|
CEP8
|
Chromosome 8
|
|
CKs
|
cytokeratins
|
|
CT
|
contrast enhanced computed
tomography
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
EMT
|
epithelial-mesenchymal transition
|
|
EpCAM
|
epithelial cell adhesion marker
|
|
HCC
|
hepatocellular carcinoma
|
|
iFISH
|
immunostaining-fluorescence in
situ hybridization
|
|
iFISH-CTCs
|
CTCs counted by iFISH
|
|
LT
|
liver transplantation
|
|
MRI
|
magnetic resonance imaging
|
|
RBCs
|
red blood cells
|
|
RFS
|
recurrence-free survival
|
|
ROC
|
receiver operating characteristic
|
|
SEN
|
sensitivity
|
|
WBCs
|
white blood cells
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Trojan J, Zangos S and Schnitzbauer AA:
Diagnostics and treatment of hepatocellular carcinoma in 2016:
Standards and developments. Visc Med. 32:116–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Welker MW, Bechstein WO, Zeuzem S and
Trojan J: Recurrent hepatocellular carcinoma after liver
transplantation-an emerging clinical challenge. Transpl Int.
26:109–118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rich N and Singal AG: Hepatocellular
carcinoma tumour markers: Current role and expectations. Best Pract
Res Clin Gastroenterol. 28:843–853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gorges TM, Tinhofer I, Drosch M, Röse L,
Zollner TM, Krahn T and von Ahsen O: Circulating tumour cells
escape from EpCAM-based detection due to epithelial-to-mesenchymal
transition. BMC Cancer. 12:1782012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schulze K, Gasch C, Staufer K, Nashan B,
Lohse AW, Pantel K, Riethdorf S and Wege H: Presence of
EpCAM-positive circulating tumor cells as biomarker for systemic
disease strongly correlates to survival in patients with
hepatocellular carcinoma. Int J Cancer. 133:2165–2171. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sheng Y, Wang T, Li H, Zhang Z, Chen J, He
C, Li Y, Lv Y, Zhang J, Xu C, et al: Comparison of analytic
performances of Cellsearch and iFISH approach in detecting
circulating tumor cells. Oncotarget. 8:8801–8806. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang J, Wang DD, Yang M, Chen D, Pang L,
Guo S, Cai J, Wery JP, Li L, Li HQ and Lin PP: Comprehensive
characterization of chemotherapeutic efficacy on metastases in the
established gastric neuroendocrine cancer patient derived xenograft
model. Oncotarget. 6:15639–15651. 2015.PubMed/NCBI
|
|
9
|
Ma C, Lv Y, Jiang R, Li J, Wang B and Sun
L: Novel method for the detection and quantification of malignant
cells in the CSF of patients with leptomeningeal metastasis of lung
cancer. Oncol Lett. 11:619–623. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao Y, Zhu Y, Zhang Z, Zhang C, Huang X
and Yuan Z: Clinical significance of pancreatic circulating tumor
cells using combined negative enrichment and
immunostaining-fluorescence in situ hybridization. J Exp Clin
Cancer Res. 35:662016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin PP: Integrated EpCAM-independent
subtraction enrichment and iFISH strategies to detect and classify
disseminated and circulating tumors cells. Clin Transl Med.
4:382015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Llovet JM, Bru C and Bruix J: Prognosis of
hepatocellular carcinoma: The BCLC staging classification. Semin
Liver Dis. 19:329–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mazzaferro V, Regalia E, Doci R, Andreola
S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A and
Gennari L: Liver transplantation for the treatment of small
hepatocellular carcinomas in patients with cirrhosis. N Engl J Med.
334:693–699. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aceto N, Bardia A, Miyamoto DT, Donaldson
MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, et al:
Circulating tumor cell clusters are oligoclonal precursors of
breast cancer metastasis. Cell. 158:1110–1122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Felden J, Schulze K, Krech T, Ewald F,
Nashan B, Pantel K, Lohse AW, Riethdorf S and Wege H: Circulating
tumor cells as liquid biomarker for high HCC recurrence risk after
curative liver resection. Oncotarget. 8:89978–89987.
2017.PubMed/NCBI
|
|
16
|
Guo W, Yang XR, Sun YF, Shen MN, Ma XL, Wu
J, Zhang CY, Zhou Y, Xu Y, Hu B, et al: Clinical significance of
EpCAM mRNA-positive circulating tumor cells in hepatocellular
carcinoma by an optimized negative enrichment and qRT-PCR-based
platform. Clin Cancer Res. 20:4794–4805. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu
SJ, Shi RY, Hu B, Zhou J and Fan J: Circulating stem cell-like
epithelial cell adhesion molecule-positive tumor cells indicate
poor prognosis of hepatocellular carcinoma after curative
resection. Hepatology. 57:1458–1468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Trevisani F, D'intino PE, Morselli-Labate
AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, De Notariis S,
Roda E and Bernardi M: Serum alpha-fetoprotein for diagnosis of
hepatocellular carcinoma in patients with chronic liver disease:
Influence of HBsAg and anti-HCV status. J Hepatol. 34:570–575.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Debruyne EN and Delanghe JR: Diagnosing
and monitoring hepatocellular carcinoma with alpha-fetoprotein: New
aspects and applications. Clin Chim Acta. 395:19–26. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abdul-Hamid S, Fox R and Martin I:
Maternal serum screening for trisomy 21 in women with a false
positive result in last pregnancy. J Obstet Gynaecol. 24:374–376.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
European Association for the Study of The
Liver, ; European Organisation For Research and Treatment of
Cancer, . EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Shi ZL, Yang X and Yin ZF:
Targeting of circulating hepatocellular carcinoma cells to prevent
postoperative recurrence and metastasis. World J Gastroenterol.
20:142–147. 2014. View Article : Google Scholar : PubMed/NCBI
|