Introduction
Hepatocellular carcinoma (HCC) is the most common
form of primary liver cancer and the third and fifth main cause of
cancer-associated mortality in men and women respectively in China,
2015 (1). In previous years, γδ T
cells have been revealed to be feasible candidates for
immunotherapy in the treatment of various types of cancer,
including melanoma, breast cancer and lung cancer. In addition, a
number of studies have demonstrated that γδ T cells may recognize
and lyse numerous types of HCC cell and are involved in the
immunotherapeutic mechanism against HCC (2–7).
Zoledronate may activate and induce the selective amplification of
Vγ9Vδ2 T cells in vitro from peripheral blood mononuclear
cells (PBMCs) taken from patients, making it suitable for clinical
adoptive immunotherapy (8,9). However, the use of this type of cell in
clinical trials has revealed that numerous challenges to be
overcome remain (10).
Human Vγ9Vδ2 T cells comprise 50–95% of peripheral
blood γδ T cells and may be divided into four subsets:
CD45RA+CD27+ naïve (Tnaïve) cells,
CD45RA−CD27+ central memory cells,
CD45RA−CD27− effector memory (TEM)
cells and CD45RA+ CD27− effector memory
(TEMRA) cells (11).
Furthermore, Vγ9Vδ2 T cells may express natural killer receptor
group 2, member D (NKG2D) and recognize major histocompatibility
complex (MHC) class I-related chain A/B and UL16-binding proteins,
which are induced or upregulated on the surface of numerous types
of tumor cell (10). A number of
studies have suggested that γδ T cells may be activated and
regulated by NKG2D (10,12).
Vγ9Vδ2 T cells also exert marked cytotoxic effects
through the perforin/granzyme signaling pathway dependent on
cell-to-cell contact, resulting in the release of interferon
(IFN)-γ and tumor necrosis factor (TNF)-α which enhance antitumor
activity (2–4). A number of studies have demonstrated
that the cytotoxicity of Vγ9Vδ2 T cells primarily depends on the
perforin/granzyme signaling pathway (13,14).
Therefore, the expression levels of perforin and granzyme B, which
are essential in this signaling pathway, may indirectly reflect the
cytotoxicity of Vγ9Vδ2 T cells.
CD4+, CD25+ and
FoxP3+ regulatory T cells (Tregs), which are involved in
the formation of the immunosuppressive network, suppress antitumor
immunity and are the main obstacles faced by cancer immunotherapy.
In vivo and in vitro studies have revealed that Tregs
may suppress the proliferation and function of cytotoxic T cells
(15–17), and impair the function of
HCC-infiltrating γδ T cells (18). Wu
et al (19) demonstrated that
the main innate source of interleukin (IL)-17A was γδ T17 cells and
that these cells may also suppress antitumor immunity in human
colorectal cancer. Furthermore, Ma et al (20) suggested that IL-17A produced by γδ T
cells promoted tumor growth in HCC. However, the effect of in
vitro amplification of circulating γδ T cells in patients with
HCC on the levels of Tregs, γδ T17 cells and IL-17A have yet to be
fully clarified.
On the basis of previous research, the association
between the change in immunosuppressive factors during in
vitro γδ T cell amplification and factors determining the
suitability of patients for immunotherapy remains unclear.
Therefore, the aim of the present study was to characterize the
proportions and functions of circulating γδ T cells, and levels of
immunosuppressive factors in patients with HCC prior to and
following amplification in vitro using zoledronate with
IL-2. In addition, the association between the amplification
ability of γδ T cells and the clinicopathological characteristics
of patients with HCC was investigated.
Materials and methods
Patients and peripheral blood
specimens
Written informed consent was obtained from all
patients prior to the study. Peripheral blood samples (10 ml) from
83 patients with HCC and from 15 healthy donors used as the control
group were collected in the present study. The present study was
approved by the Ethics Committee of Shanxi Medical University
(Taiyuan, China). The inclusion and exclusion criteria of the
patients were as follows: i) patients having a confirmed diagnosis
of HCC according to the National Comprehensive Cancer Network
clinical practice guidelines in Oncology: Hepatobiliary Cancers
(version 2; https://www.nccn.org/professionals/physician_gls/default.aspx);
and ii) patients without other malignancies, autoimmune diseases or
other immune-associated diseases. The clinicopathological
characteristics of the patients are presented in Table I. The clinical stage of the tumors was
confirmed according to the Barcelona-Clinic Liver Cancer system
(21).
 | Table I.Univariate analyses of the quality of
amplification associated with clinicopathological characteristics
and a number of suppressive factors. |
Table I.
Univariate analyses of the quality of
amplification associated with clinicopathological characteristics
and a number of suppressive factors.
|
| High | Low |
|
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristic | n (%) | n (%) | χ2 | P-value |
|---|
| Sex |
|
| 0.232 | 0.656 |
|
Male | 15 (44.4) | 28 (53.8) |
|
|
|
Female | 16 (51.6) | 24 (46.2) |
|
|
| BCLC stage |
|
| 22.270 | <0.001 |
| A | 24 (77.4) | 13 (25.0) |
|
|
| B | 5 (16.1) | 19 (36.5) |
|
|
| C | 2 (6.2) | 20 (38.5) |
|
|
| Tumor size, cm |
|
| 7.574 | 0.007 |
|
>5 | 10 (32.3) | 33 (63.5) |
|
|
| ≤5 | 21 (67.7) | 19 (36.5) |
|
|
| Tumor number |
|
| 4.310 | 0.044 |
| 1 | 21 (67.7) | 23 (44.2) |
|
|
| ≥2 | 10 (32.3) | 29 (55.8) |
|
|
| DOD, months |
|
| 16.929 | <0.001 |
|
≥20 | 24 (77.4) | 16 (30.8) |
|
|
|
<20 | 7 (22.6) | 36 (69.2) |
|
|
| TBIL, µmol/l |
|
| 0.361 | 0.646 |
|
≥17.1 | 17 (54.8) | 32 (61.5) |
|
|
|
<17.1 | 14 (45.2) | 20 (38.5) |
|
|
| AFP, ng/ml |
|
| 19.136 | <0.001 |
|
≤20 | 23 (74.2) | 13 (25.0) |
|
|
|
>20 | 8 (25.8) | 39 (75.0) |
|
|
| Albumin, g/l |
|
| 3.832 | 0.041 |
|
≥55 | 20 (64.5) | 22 (42.3) |
|
|
|
<55 | 11 (35.5) | 30 (57.7) |
|
|
| Ascites |
|
| 0.066 | 0.824 |
|
Yes | 17 (54.8) | 27 (51.9) |
|
|
| No | 14 (45.2) | 25 (48.1) |
|
|
| TACE |
|
| 1.745 | 0.263 |
|
Yes | 4 (12.9) | 13 (25.0) |
|
|
| No | 27 (87.1) | 39 (75.0) |
|
|
| ALT, U/l |
|
| 0.148 | 0.819 |
|
≥40 | 15 (48.4) | 22 (42.3) |
|
|
|
<40 | 16 (51.6) | 30 (57.7) |
|
|
| AST, U/l |
|
| 0.086 | 0.819 |
|
≥40 | 12 (38.7) | 21 (40.4) |
|
|
|
<40 | 19 (61.3) | 31 (59.6) |
|
|
| PT, sec |
|
| 0.001 | 0.998 |
|
≥14 | 15 (48.4) | 24 (46.2) |
|
|
|
<14 | 16 (51.6) | 28 (53.8) |
|
|
| Tregs, % |
|
| 17.566 | <0.001 |
|
<0.91±0.54 | 23 (74.2) | 14 (26.9) |
|
|
|
≥0.91±0.54 | 8 (25.8) | 38 (73.1) |
|
|
| γδ T17 cells,
% |
|
| 7.961 | 0.006 |
|
<0.68±0.17 | 20 (64.5) | 17 (32.7) |
|
|
|
≥0.68±0.17 | 11 (35.5) | 35 (67.3) |
|
|
| Age, years |
|
| 0.021 | 0.989 |
|
<40 | 1 (3.2) | 2 (3.8) |
|
|
|
40–55 | 12 (38.7) | 20 (38.5) |
|
|
|
55< | 18 (58.1) | 30 (57.7) |
|
|
Isolation and amplification of γδ T
cells and culture of HCC cell lines
PBMCs were isolated from the fresh peripheral blood
of patients and healthy donors using Ficoll density gradient to
centrifuge at 453 × g for 15 min at room temperature (GE
Healthcare, Chicago, IL, USA). As described previously (5), in order to amplify γδ T cells from fresh
PBMCs (mean viability: 94.4%), 5 µM zoledronate (Zometa; Novartis
International AG, Basel, Switzerland) was added to GT-T551 medium
(Takara Bio, Inc., Otsu, Japan) supplemented with 10%
heat-inactivated autologous plasma, 80 U/ml gentamicin and 1,000
IU/ml recombinant human IL-2 (Proleukin®; Chiron
Therapeutics, Suresnes, France) at the onset of cultivation. Every
3 days, 10 ml GT-T551 and 1,000 IU/ml IL-2 were added to the
cultures. After 12–14 days, γδ T cells were harvested (mean
viability, 96.83±6.81%) which were cultured at 37°C in a 5%
CO2 humidified incubator during this period. The human
HCC cell lines HuH7, PLC, and SMMC-7721 supplied by Shanghai
Institutes for Biological Sciences (Chinese Academy of Sciences,
Shanghai, China) were cultured at 37°C in a 5% CO2
humidified incubator.
Flow cytometry
Prior to and following amplification, normal mouse
serum (cat. no. S-I-000004, EarthOx Life Sciences, Millbrae, CA,
USA) was diluted using PBS (1:50 dilution; cat. no. 10010023,
eBioscience; Thermo Fisher Scientific, Inc. Waltham, MA, USA) and
mixed with cells for 1 min at room temperature in order to block
non-specific binding. Following this, cells were stained (either
intracellularly or on the surface) at 4°C in dark with
fluorochrome-conjugated monoclonal antibodies for 20 min in order
to analyze the proportion, phenotype, tumor-killing capacity and
cytokine secretion of Tregs and γδ T17 cells.
Anti-NKG2D-fluorescein isothiocyanate-FITC (cat. no. 11-5878-41),
anti-cluster of differentiation (CD)3-phycoerythrin (PE)-cyanine
(Cy)5, (cat. no. 15-0038-42), anti-CD27-PE-Cy7, (cat. no.
25-0279-41), anti-TNF-α-FITC (cat. no. 11-7349-82), anti-forkhead
box P3 (FoxP3)-PE (cat. no. 12-4777-42) and anti-IL-17A-PE
antibodies (cat. no. 14-7179-82) were purchased from eBioscience;
Thermo Fisher Scientific, Inc.; anti-Vγ9TCR-PE (cat. no. 555733),
anti-perforin-FITC (cat. no. 556577), anti-granzyme B-FITC (cat.
no. 560211) and anti-CD107a-FITC (cat. no. 555800) antibodies were
purchased from BD Biosciences (Franklin Lakes, NJ, USA); and
anti-IFN-γ-FITC (cat. no. IM2716U), anti-T cell receptor (TCR)
-pan-γδ-FITC (cat. no. IM1571U), anti-CD45-proprotein convertase
subtilisin/kexin type (PC) 7 (cat. no. IM3548U), anti-CD25-PC5
(cat. no. IM2646U), anti-CD4-FITC (cat. no. 6603862) and
anti-CD45RA-FITC (cat. no. IM0584U) antibodies were purchased from
Beckman Coulter, Inc. (Brea, CA, USA). The dilutions used for
different experiments are detailed in the relevant protocols. Prior
to staining for CD107a, cells were stimulated using phorbol
12-myristate 13-acetate (50 ng/ml) and ionomycin (500 ng/ml) for
4–6 h in incubator at 37°C. Immunofluorescence was determined using
a Cytomics FC500 flow cytometer with CXP software (version 2.1;
Beckman Coulter, Inc.).
ELISA
Culture supernatants from γδ T cells were collected
on days 3, 7, 10 and 14. The IL-17A content in the supernatants
were determined using a direct ELISA. Briefly, 200 µl 0.25% gelatin
(Sigma Aldrich; Merck KGaA, Darmstadt, Germany) was added to each
well, and the plates were incubated for 2 h at room temperature.
Then each well of a 96-well plate was coated with 50 ml
supernatants from patients with HCC or healthy donor cells
overnight at 4°C. Following washing with PBS with Tween-20 (PBST;
Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China), 50 µl primary anti-IL-17A antibodies were diluted by a
factor of 1:100 and added to the wells. The plates were then
incubated for 1 h at room temperature and washed with PBST to
remove excess primary antibodies. A 50 µl volume of horseradish
peroxidase (HRP) -labeled secondary antibody (rabbit anti-mouse
IgG; cat. no. 61-6520; eBioscience-Thermo Fisher Scientific, Inc.)
was added to the wells and plates were further incubated for 45 min
at 37°C. Excess secondary antibodies were removed and HRP enzyme
activity was determined by adding o-phenylenediamine for
o-phenylenediamine dihydrochloride reaction at room temperature for
20–30 min in darkness, which was terminated by adding 1 M
H2SO4 after 10 min at room temperature. The
concentration of IL-17A was calculated using CurveExpert 1.4
software (Hyams Development; https://www.curveexpert.net/).
In vitro cytotoxicity assay
The in vitro cytotoxicity of γδ T cells from
patients with HCC following amplification was determined using an
MTT assay (Sigma Aldrich; Merck KGaA). Briefly, exponentially
growing target cells (HuH7, PLC and SMMC-7721 cells) were prepared
at a density of 5×103 cells/well and seeded in 96-well
plates with γδ T cells at effector/target ratios of 0:1, 5:1, 10:1
or 20:1. HCC cells and γδ T cells were simultaneously seeded as two
control groups and were incubated at 37°C in an atmosphere
containing 5% CO2 for 48 h. Subsequently, 20 µl MTT (5
mg/ml; Sigma-Aldrich; Merck KGaA) was added to each well, and cells
were cultured at 37°C in incubator for an additional 4 h, and
subsequently 100 µl dimethylsulfoxide (Sigma Aldrich; Merck KGaA)
was added to each well. Cells were shocked for 10 min in the dark
at room temperature, and the optical density (OD) of each well was
determined using a microplate reader at 570 nm. The cytotoxicity
was calculated according to the following formula: Cytotoxicity
(%)=(control OD-experimental OD)/control ODx100%. The assay was
repeated three times.
Statistical analysis
SPSS software (version 17.0; SPSS, Inc., Chicago,
IL, USA) was used for statistical analyses. Data are expressed as
the mean ± standard deviation (SD). Paired or non-paired Student's
t-tests were performed as appropriate. One-way analysis of variance
was used to analyze the differences among three HCC cell lines at
different effector/target ratios. Further comparison of the
differences between two groups was performed using
least-significance difference test or Student-Newman-Keuls.
Univariate analyses were performed using χ2 tests.
Multivariate analyses for factors affecting the quality of
amplification were performed using logarithmic regression analysis.
Spearman's correlation was used to analyze the associations between
α-fetoprotein (AFP) in 10% autologous plasma and the absolute
numbers of γδ T cells following amplification. P<0.05 was
considered to indicate a statistically significant difference.
Results
Proliferation of γδ T cells derived
from patients with HCC and healthy controls
γδ T cells derived from healthy donors and patients
with HCC were cultured in vitro in a humidified atmosphere
at 37°C. Following culture for 240 h, the γδ T cells were amplified
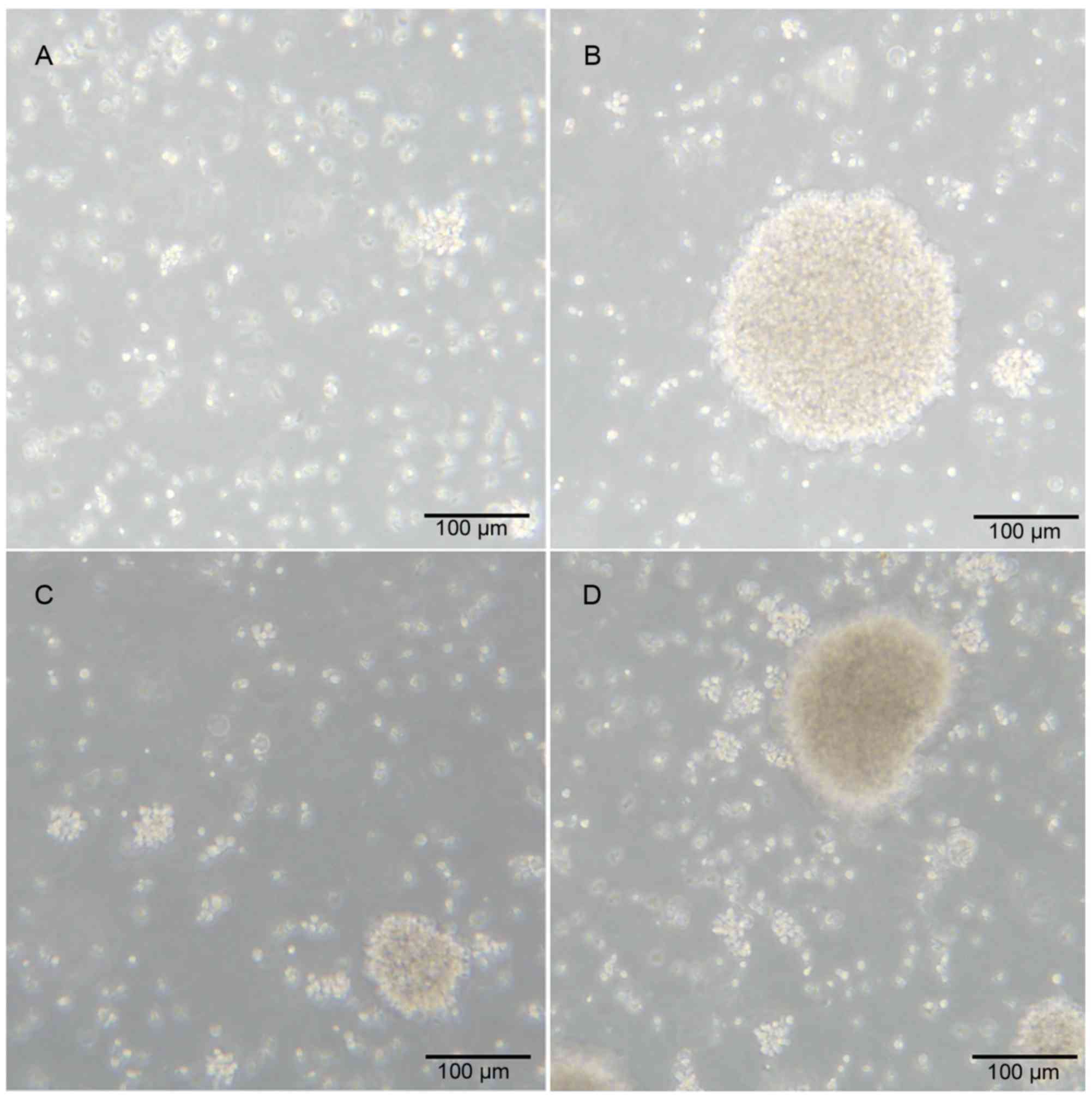
to form a cell mass. The morphology of the cell mass from patients
with HCC and healthy donors were similar (Fig. 1A-D).
Zoledronate and IL-2 may efficiently
expand the γδ T cells from PBMCs of patients with HCC
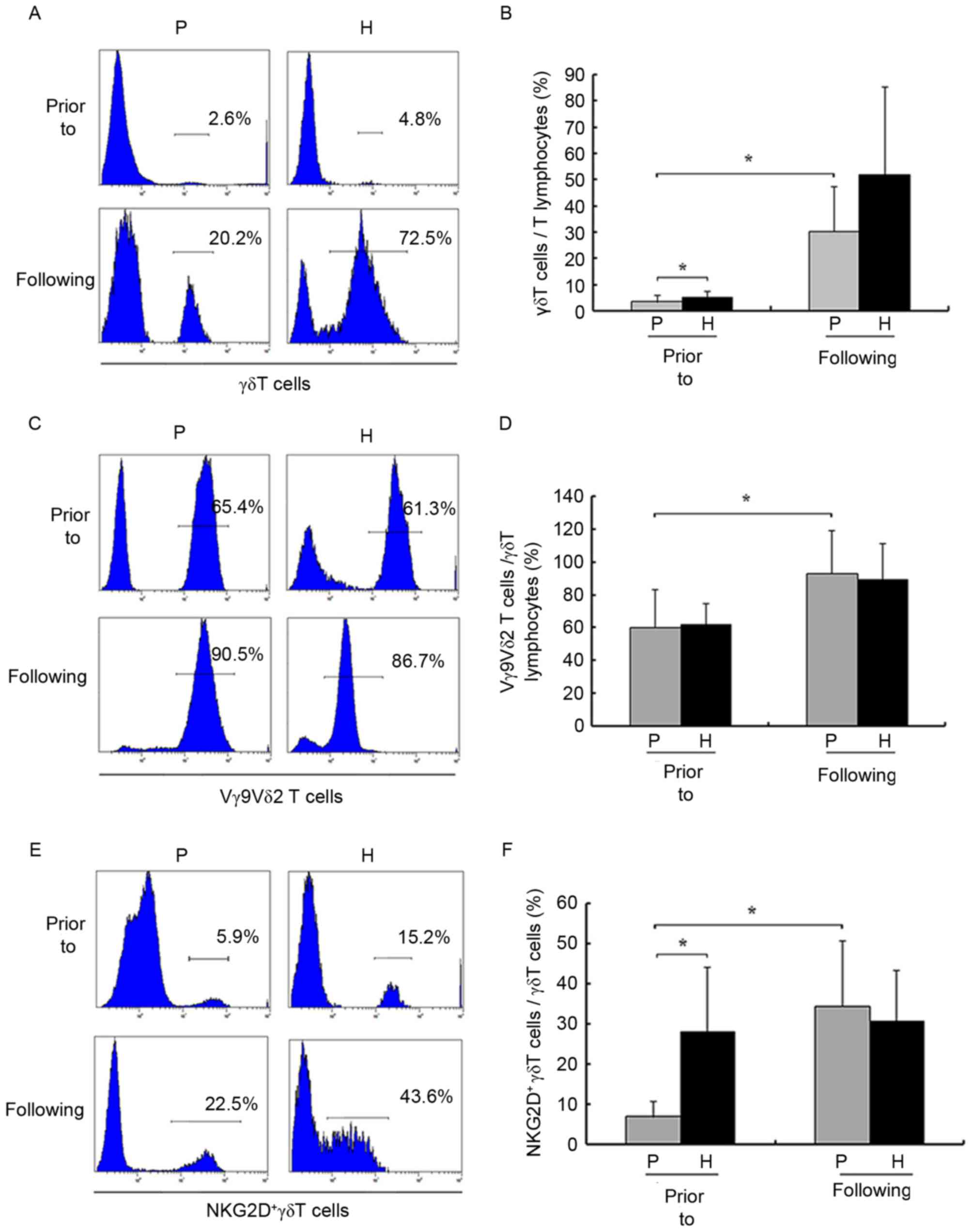
Prior to amplification, the numbers of γδ T cells
from patients with HCC and healthy donors were
(2.12±1.15)×104 and (1.78±0.91)×105,
respectively, and the proportion of γδ T cells out of the total
number of T cells was significantly decreased in patients with HCC
compared with healthy donors (3.32±1.67 vs. 5.06±1.91%,
respectively; P<0.05; Fig. 2A and
B). Prior to and following amplification, the proportion of
Vγ9Vδ2 T cells out of the total number of γδ T cells was not
significantly decreased compared with healthy donors (P>0.05;
Fig. 2C and D). However, following
amplification, the numbers of γδ T cells from patients with HCC and
healthy donors were (1.68±0.92)×107 and
(1.05±0.65)×108, respectively, and the proportion of γδ
T cells out of the total number of T cells (3.32±1.67 vs.
30.27±15.25%, respectively; P<0.05) and Vγ9Vδ2 T cells out of
the total number of γδ T cells (60.26±19.31% vs. 93.14±12.87%,
prior to and following amplification, respectively; P<0.05) were
significantly increased in patients with HCC.
In terms of phenotype, there were also significant
differences in patients with HCC prior to and following
amplification. Following amplification, the proportions and numbers
of Tnaïve (24.88±13.17 vs. 6.52±4.43% prior to and
following amplification, respectively; P<0.05) and
TEMRA (34.18±18.45 vs. 13.38±5.81% prior to and
following amplification, respectively; P<0.05) cells were
significantly decreased. The proportion of TEM cells was
significantly increased following amplification (6.76±4.07 vs.
63.16±11.16% prior to and following amplification, respectively;
P<0.05). As presented in Fig. 2E and
F, prior to amplification, γδ T cells were generally positive
for NKG2D in healthy donors (6.93±2.89 vs. 27.93±13.48% for
patients and healthy donors, respectively; P<0.05). Following
amplification, numbers of NKG2D+ γδ T cells were
significantly increased compared with healthy donors.
Amplification capacity of γδ T cells
is correlated with the clinicopathological characteristics of
patients
Notably, γδ T cells from all patients did not expand
equally as well. Therefore, the aim of the present study was to
elucidate the factors underlying this phenomenon. The results of
the univariate analysis, presented in Table I, demonstrate that the quality of
amplification was significantly associated with clinical stage,
levels of AFP and albumin, duration of disease (DOD), size and
number of tumors, numbers of Tregs and γδ T17 cells and levels of
IL-17A. The results of the multivariate analysis revealed that the
levels of AFP and the proportions of Tregs and γδ T17 cells were
independent factors associated with low-quality amplification,
whereas DOD was an independent factor associated with high-quality
amplification (Table II). There was
no correlation between AFP in 10% autologous plasma and the
amplification ability of γδ T cells (rs=−0.396;
P=0.379), indicating that exogenous AFP did not affect the
amplification of γδ T cells in vitro.
 | Table II.Multivariate analyses of the quality
of amplification associated with clinicopathological
characteristics and a number of suppressive factors. |
Table II.
Multivariate analyses of the quality
of amplification associated with clinicopathological
characteristics and a number of suppressive factors.
|
|
|
| 95% confidence
interval |
|---|
|
|
|
|
|
|---|
| Variables | P-value | OR | Lower | Upper |
|---|
| AFP | 0.041 | 3.734 | 1.112 | 15.801 |
| DOD | 0.030 | 0.041 | 0.002 | 0.729 |
| Tregs | 0.006 | 4.808 | 2.915 | 17.357 |
| γδ T17 cells | 0.023 | 2.479 | 1.415 | 11.089 |
These data indicated that amplification with
zoledronate and IL-2 may increase the proportion of γδ T cells and
promote the effective phenotype. However, the amplification ability
was not the same in all patients, varying depending on the
clinicopathological characteristics of the patients with HCC and
the presence of specific suppressive factors.
Secretion and cytotoxic activity of γδ
T cells
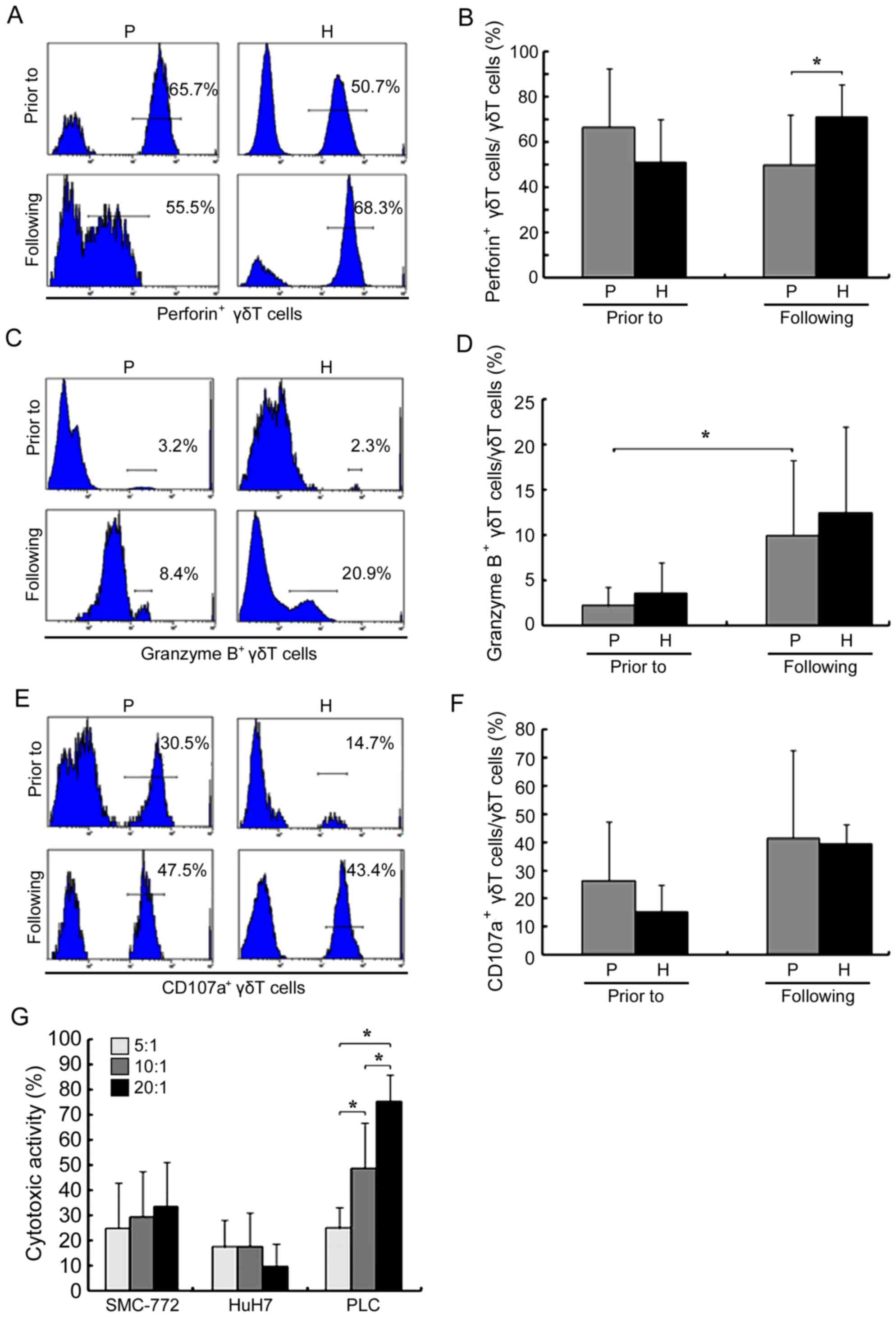
Prior to amplification, the proportion of
perforin+ γδ T cells in patients with HCC was not
significantly decreased compared with healthy donors (P>0.05;
Fig. 3A and B). However, following
amplification, the proportion in patients with HCC decreased
(66.61±20.87 vs. 49.97±15.97% prior to and following amplification,
respectively; P<0.05), becoming significantly lower when
compared with healthy donors (71.25±14.06%; P<0.05). The
proportion of granzyme B+ γδ T cells in patients with
HCC significantly increased following amplification (2.17±1.62 vs.
9.96±6.22% prior to and following amplification, respectively;
P<0.05; Fig. 3C and D); however,
there was no significant difference when compared with that in
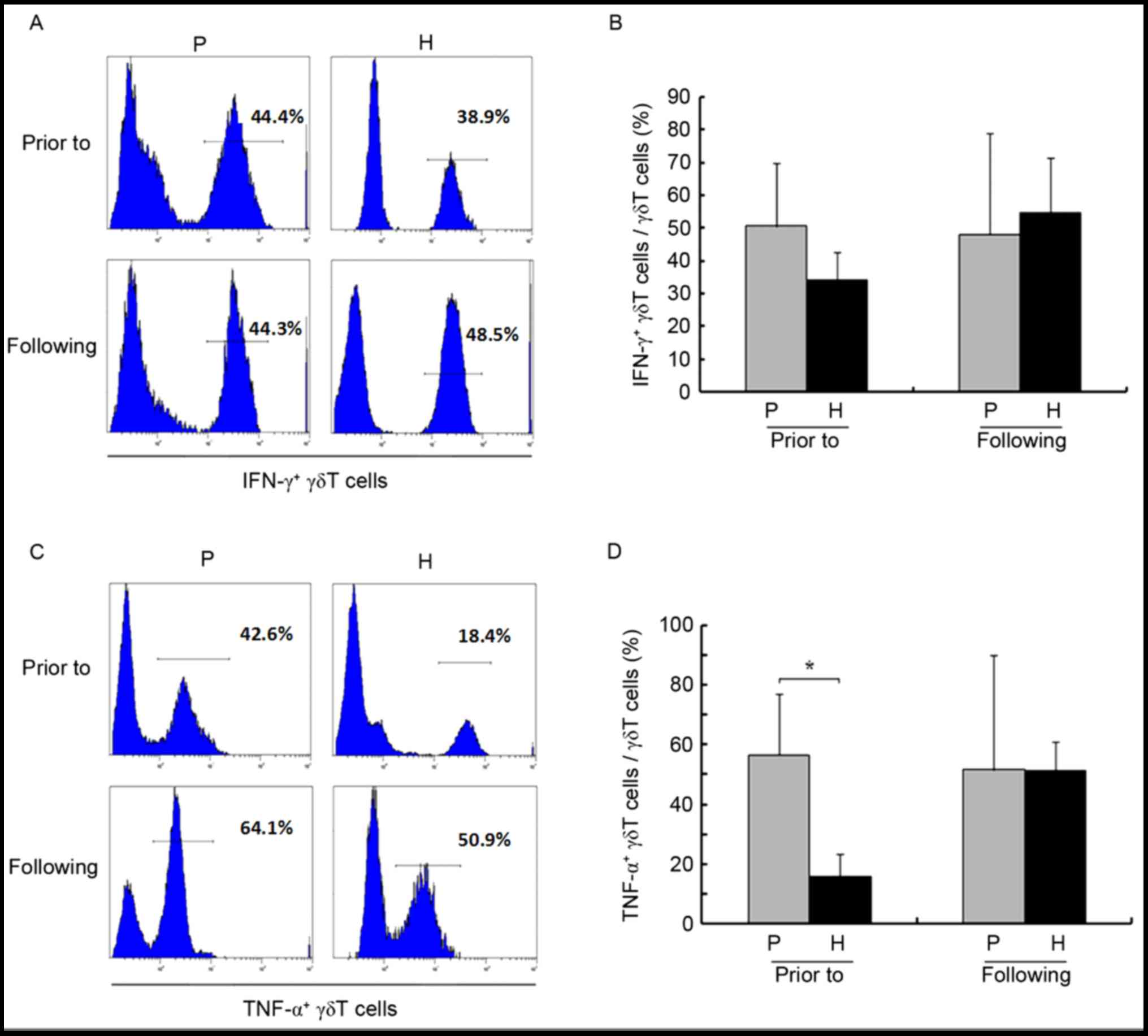
healthy donors (P>0.05). Amplification also did not
significantly affect CD107a (26.41±15.66 vs. 41.52±26.17% prior to
and following amplification, respectively; P>0.05) and IFN-γ
(50.61±15.25 vs. 48.07±25.10% prior to and following amplification,
respectively; P>0.05; Figs. 3E and
F, 4A and B). In addition, prior
to amplification, the proportion of TNF-α+ γδ T cells
was higher in patients compared with healthy controls (56.70±16.43
vs. 15.74±5.71%, respectively; P<0.05; Fig. 4C and D), and amplification had almost
no effect on this parameter (56.70±16.43 vs. 51.62±30.67% prior to
and following amplification, respectively; P>0.05).
The results of the MTT assay, as presented in
Fig. 3G, revealed that γδ T cells
exerted significant cytotoxic effects on four HCC cell lines at
differing effector/target ratios. In addition, for the PLC cells,
cytotoxicity was significantly increased when the effector/target
ratio was increased.
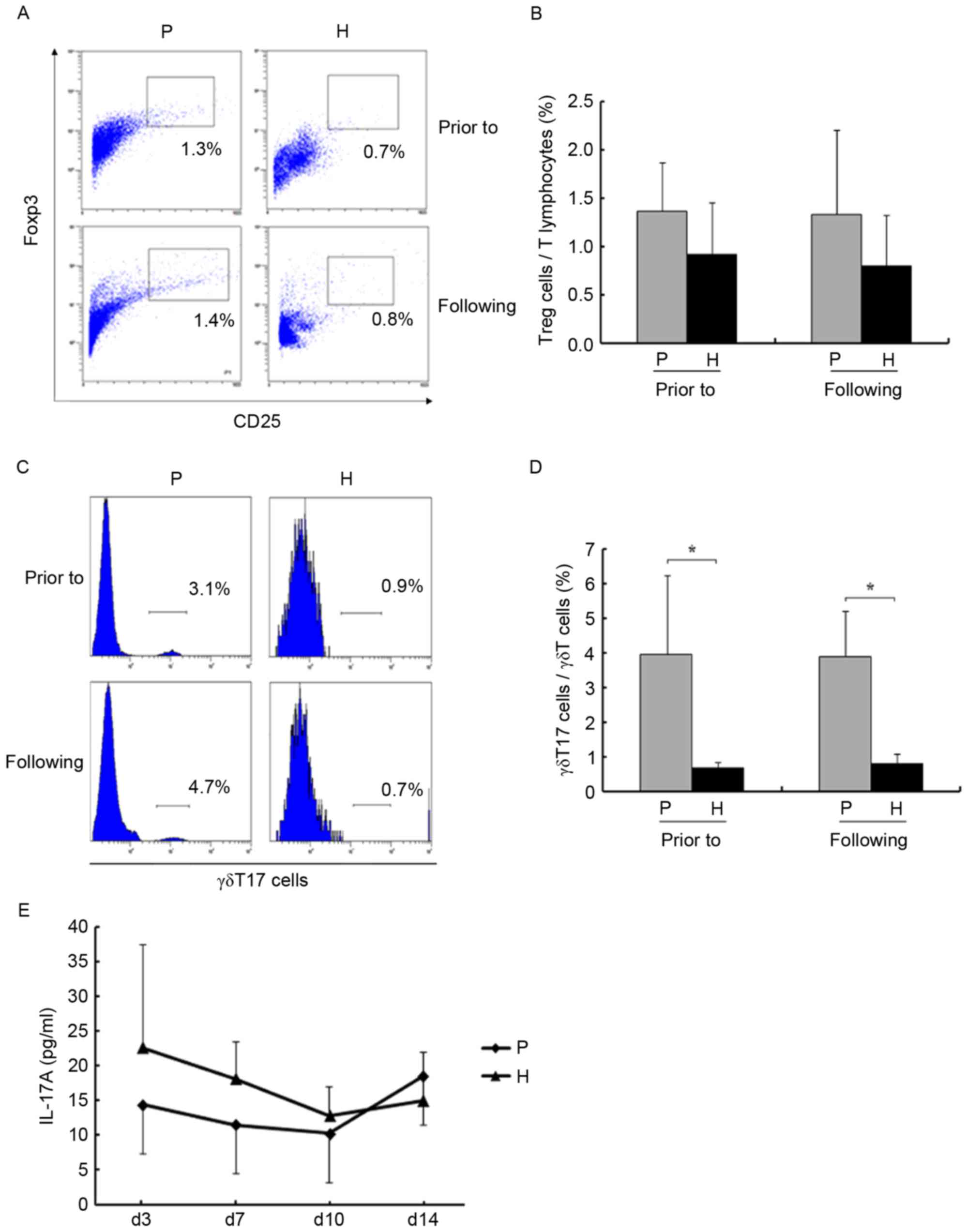
γδ T17 cells, Tregs and IL-17A were
not altered during amplification
Immunosuppressive cells and factors were examined
during amplification. Amplification had almost no effect on the
levels of Tregs and γδ T17 cells (P>0.05; Fig. 5A-D). The levels of IL-17A in the
supernatants were assessed using an ELISA. As presented in Fig. 5E, the levels were not significantly
altered on days 3, 7, 10 and 14 (P>0.05).
Discussion
Previously, numerous immunotherapeutic methods have
been developed in an attempt to induce tumor-specific adaptive
immune responses. Adaptive immunotherapy with γδ T cells represents
a novel, safe and effective approach to inducing immunological and
clinical responses (22–24). However, few studies have examined
these parameters in HCC. In the present study, it was concluded
that circulating γδ T cells in patients with HCC expanded by the
use of zoledronate and IL-2 in vitro, and may lyse HCC cells
effectively, without increasing immunosuppressive factors during
amplification. Additionally, the amplification ability of γδ T
cells was associated with the clinicopathological features of
patients with HCC.
A γδ T-cell proliferation of at ≥70% was considered
the threshold for therapy (25). A
real-time cell analyzer may be used for monitoring the absolute
cell numbers and cytotoxicity of circulating γδ T cells from
patients with cancer, in order to provide a more comprehensive
assessment for personalized tumor treatment (26). In the present study, the absolute
numbers and proportion of γδ T cells in patients with HCC increased
significantly following amplification; however, this was not
consistently observed in all patients, and this effect may be
associated with various clinicopathological characteristics and
suppressive factors. It was revealed that the quality of
amplification was negatively associated with the serum AFP level,
proportion of γδ T17 cells and proportion of Tregs, but positively
associated with the DOD. These results suggested that optimized
immunotherapy of γδ T cells in patients with HCC should be
individualized.
In order to further explore the feasibility and
efficacy of immunotherapy, the phenotype, secretion and
cytotoxicity of Vγ9Vδ2 T cells were examined. Encouragingly, the
results of the present study suggested that there was substantial
differentiation of Vγ9Vδ2 T cells towards the effective phenotype
of secretion and lysis following amplification, which was
consistent with other studies (24,27).
Previous studies have revealed that activated Vγ9Vδ2 T cells are a
primary source of IFN-γ and TNF-α, which have direct cytotoxic
activity against tumor cells and indirect cytotoxic activity via
the stimulation of macrophages and dendritic cells (28–30). In
the present study, although the proportions of IFN-γ+
and TNF-α+ γδ T cells were not significantly altered,
the absolute numbers and proportions of TEM cells were
significantly increased following amplification. It was revealed
that the secretion of γδ T cells was increased following
amplification. Collectively, the results of the present study
revealed that the cytotoxic activity of γδ T cells was also
increased following amplification.
Immunosuppressive factors are the main obstacles for
the anticancer immunity effects of γδ T cells in vivo. The
accumulation of Tregs in a number of tumors mediate tumor-promoting
effects through the suppression of antitumor immunity (31). Furthermore, IL-17A has been revealed
to promote metastasis and is associated with a poor prognosis in
patients with HCC (32).
Immunosuppressive cells and factors should not be expanded during
the amplification of effective cells. To the best of our knowledge,
the present study is the first to explore the changes in Tregs, γδ
T17 cells and IL-17A during the amplification of circulating γδ T
cells in patients with HCC in vitro. The results of the
present study revealed that these immunosuppressive cells and
factors were not increased following amplification, which suggested
that γδ T cells expanded by zoledronate and IL-2 in vitro
may be safe for immunotherapy in patients with HCC.
A number of studies have demonstrated that Tregs
express immune checkpoint proteins, including programmed cell
death-1 (PD-1) and cytotoxic T lymphocyte-associated antigen-4
(CTLA-4) (33,34), and impair the function of
HCC-infiltrating γδ T cells (18).
Additionally, activated T cells upregulate CTLA-4 and PD-1, which
act to increase T-cell responses, and antibody blockade of immune
checkpoints enhances T-cell responses (35). Adoptive γδ T-cell immunotherapy
combined with checkpoint inhibitors may be a promising therapeutic
strategy for the treatment of HCC.
In summary, circulating γδ T cells from patients
with HCC expanded using zoledronate and IL-2 in vitro may be
used for immunotherapy in patients with HCC without increasing
immunosuppressive factors. However, this immunotherapy should be
individualized according to the specific clinicopathological
features of the patients.
Acknowledgements
The present study was supported by the Scientific
and Technological Project of Shanxi Province (grant no.
130313021–17).
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Braza MS and Klein B: Anti-tumour
immunotherapy with Vγ9Vδ2 T lymphocytes: From the bench to the
bedside. Br J Haematol. 160:123–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dhar S and Chiplunkar SV: Lysis of
aminobisphosphonate-sensitized MCF-7 breast tumor cells by Vγ9Vδ2 T
cells. Cancer Immun. 10:102010.PubMed/NCBI
|
|
4
|
Wu YL, Ding YP, Tanaka Y, Shen LW, Wei CH,
Minato N and Zhang W: γδ T cells and their potential for
immunotherapy. Int J Biol Sci. 10:119–135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bouet-Toussaint F, Cabillic F, Toutirais
O, Le Gallo M, Thomas de la Pintière C, Daniel P, Genetet N,
Meunier B, Dupont-Bierre E, Boudjema K and Catros V: Vgamma9Vdelta2
T cell-mediated recognition of human solid tumors. Potential for
immunotherapy of hepatocellular and colorectal carcinomas. Cancer
Immunol Immunother. 57:531–539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Toutirais O, Cabillic F, Le Friec G, Salot
S, Loyer P, Le Gallo M, Desille M, de La Pintière CT, Daniel P,
Bouet F and Catros V: DNAX accessory molecule-1 (CD226) promotes
human hepatocellular carcinoma cell lysis by Vgamma9Vdelta2 T
cells. Eur J Immunol. 39:1361–1368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cabillic F, Toutirais O, Lavoué V, de La
Pintière CT, Daniel P, Rioux-Leclerc N, Turlin B, Mönkkönen H,
Mönkkönen J, Boudjema K, et al: Aminobisphosphonate-pretreated
dendritic cells trigger successful Vgamma9Vdelta2 T cell
amplification for immunotherapy in advanced cancer patients. Cancer
Immunol Immunother. 59:1611–1619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kondo M, Sakuta K, Noguchi A, Ariyoshi N,
Sato K, Sato S, Sato K, Hosoi A, Nakajima J, Yoshida Y, et al:
Zoledronate facilitates large-scale ex vivo expansion of functional
gammadelta T cells from cancer patients for use in adoptive
immunotherapy. Cytotherapy. 10:842–856. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nicol AJ, Tokuyama H, Mattarollo SR, Hagi
T, Suzuki K, Yokokawa K and Nieda M: Clinical evaluation of
autologous gamma delta T cell-based immunotherapy for metastatic
solid tumours. Br J Cancer. 105:778–786. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rincon-Orozco B, Kunzmann V, Wrobel P,
Kabelitz D, Steinle A and Herrmann T: Activation of V gamma 9V
delta 2 T cells by NKG2D. J Immunol. 175:2144–2151. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pang DJ, Neves JF, Sumaria N and
Pennington DJ: Understanding the complexity of γδ T-cell subsets in
mouse and human. Immunology. 136:283–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bauer S, Groh V, Wu J, Steinle A, Phillips
JH, Lanier LL and Spies T: Activation of NK cells and T cells by
NKG2D, a receptor for stress-inducible MICA. Science. 285:727–729.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kunzmann V and Wilhelm M: Anti-lymphoma
effect of gammadelta T cells. Leuk Lymphoma. 46:671–680. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Todaro M, D'Asaro M, Caccamo N, Iovino F,
Francipane MG, Meraviglia S, Orlando V, La Mendola C, Gulotta G,
Salerno A, et al: Efficient killing of human colon cancer stem
cells by gammadelta T lymphocytes. J Immunol. 182:7287–7296. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE
and Ansell SM: Attenuation of CD8(+) T-cell function by
CD4(+)CD25(+) regulatory T cells in B-cell non-Hodgkin's lymphoma.
Cancer Res. 66:10145–10152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mempel TR, Pittet MJ, Khazaie K, Weninger
W, Weissleder R, von Boehmer H and von Andrian UH: Regulatory T
cells reversibly suppress cytotoxic T cell function independent of
effector differentiation. Immunity. 25:129–141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B,
Zhang Z, Yang H, Zhang H, Zhou C, et al: Increased regulatory T
cells correlate with CD8 T-cell impairment and poor survival in
hepatocellular carcinoma patients. Gastroenterology. 132:2328–2339.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yi Y, Hong WH, Wang JX, Cai XY, Li YW,
Zhou J, Cheng YF, Jin JJ, Fan J and Qiu SJ: The functional
impairment of HCC-infiltrating γδ T cells, partially mediated by
regulatory T cells in a TGFβ- and IL-10-dependent manner. J
Hepatol. 58:977–983. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, Wang
Z, Wang C, Zhang Z and Xia W: γδT17 cells promote the accumulation
and expansion of myeloid-derived suppressor cells in human
colorectal cancer. Immunity. 40:785–800. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma S, Cheng Q, Cai Y, Gong H, Wu Y, Yu X,
Shi L, Wu D, Dong C and Liu H: IL-17A produced by γδ T cells
promotes tumor growth in hepatocellular carcinoma. Cancer Res.
74:1969–1982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Llovet JM, Di Bisceglie AM, Bruix J,
Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M,
Talwalkar J, et al: Design and endpoints of clinical trials in
hepatocellular carcinoma. J Natl Cancer Inst. 100:698–711. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wilhelm M, Kunzmann V, Eckstein S, Reimer
P, Weissinger F, Ruediger T and Tony HP: Gammadelta T cells for
immune therapy of patients with lymphoid malignancies. Blood.
102:200–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dieli F, Vermijlen D, Fulfaro F, Caccamo
N, Meraviglia S, Cicero G, Roberts A, Buccheri S, D'Asaro M, Gebbia
N, et al: Targeting human{gamma}delta} T cells with zoledronate and
interleukin-2 for immunotherapy of hormone-refractory prostate
cancer. Cancer Res. 67:7450–7457. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Santini D, Martini F, Fratto ME, Galluzzo
S, Vincenzi B, Agrati C, Turchi F, Piacentini P, Rocci L, Manavalan
JS, et al: In vivo effects of zoledronic acid on peripheral T
lymphocytes in early breast cancer patients. Cancer Immunol
Immunother. 58:31–38. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bennouna J, Bompas E, Neidhardt EM,
Rolland F, Philip I, Galéa C, Salot S, Saiagh S, Audrain M, Rimbert
M, et al: Phase-I study of innacell gammadelta, an autologous
cell-therapy product highly enriched in gamma9delta2 T lymphocytes,
in combination with IL-2, in patients with metastatic renal cell
carcinoma. Cancer Immunol Immunother. 57:1599–1609. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oberg HH, Kellner C, Peipp M, Sebens S,
Adam-Klages S, Gramatzki M, Kabelitz D and Wesch D: Monitoring
circulating γδ T cells in cancer patients to optimize γδ T
cell-based immunotherapy. Front Immunol. 5:6432014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dieli F, Gebbia N, Poccia F, Caccamo N,
Montesano C, Fulfaro F, Arcara C, Valerio MR, Meraviglia S, Di Sano
C, et al: Induction of gammadelta T-lymphocyte effector functions
by bisphosphonate zoledronic acid in cancer patients in vivo.
Blood. 102:2310–2311. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ismaili J, Olislagers V, Poupot R, Fournie
JJ and Goldman M: Human gammadelta T cells induce dendritic cell
maturation. Clin Immunol. 103:296–302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Conti L, Casetti R, Cardone M, Varano B,
Martino A, Belardelli F, Poccia F and Gessani S: Reciprocal
activating interaction between dendritic cells and
pamidronate-stimulated gammadelta T cells: Role of CD86 and
inflammatory cytokines. J Immunol. 174:252–260. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Devilder MC, Maillet S, Bouyge-Moreau I,
Donnadieu E, Bonneville M and Scotet E: Potentiation of
antigen-stimulated V Gamma 9V delta 2 T cell cytokine production by
immature dendritic cells (DC) and reciprocal effect on DC
maturation. J Immunol. 176:1386–1393. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nishikawa H and Sakaguchi S: Regulatory T
cells in tumor immunity. Int J Cancer. 127:759–767. 2010.PubMed/NCBI
|
|
32
|
Li J, Lau GK, Chen L, Dong SS, Lan HY,
Huang XR, Li Y, Luk JM, Yuan YF and Guan XY: Interleukin 17A
promotes hepatocellular carcinoma metastasis via NF-kB induced
matrix metalloproteinases 2 and 9 expression. PLoS One.
6:e218162011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity and immune correlates
of anti-PD-1 antibody in cancer. N Engl J Med. 366:2443–2454. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|