Introduction
Ovarian cancer (OC) is the most lethal gynecological
malignancy (1). For epithelial OC
(EOC), the prognosis for premenopausal women with early-stage EOC
is favorable (2). In the past few
decades, patients with EOC were treated with the latest
chemotherapeutic drugs and surgical techniques, but the 5-year
survival rate was still ≤40% (3). A
previous study reported that EOC demonstrates genomic instability
(4). During treatment, numerous
patients with EOC have recurrence and become resistant to
chemotherapy, indicating that new treatment strategies are required
(5,6).
Therefore, numerous studies have been performed to identify
effective therapeutic agents and their associated mechanisms of
action (6,7).
Salidroside, a p-hydroxyphenethyl-β-D-glucoside (or
phenylpropanoid glycoside), is one of the major active ingredients
extracted from Rhodiola rosea and has a long history of use
in Chinese medicine (8–11). Salidroside has primarily been used as
a brain tonic, a roborant or headache relief agent (8,12).
Recently, salidroside has been studied in experimental animals for
its protective effects against hypoxia, cold, radiation and heavy
physical exercise (11). It has been
demonstrated that salidroside has various pharmacological
properties (13), including antiaging
(14), anticancer (15), anti-inflammation, hepatoprotective and
antioxidative effects (16).
The aim of the current study was to investigate the
effects of salidroside on OC, and to determine whether it may be a
new therapeutic candidate in the treatment of OC.
Materials and methods
Cell culture
Human ovarian cancer cell lines, SKOV3 and A2780
from the American Type Culture Collection (Manassas, VA, USA), were
cultured in RPMI 1640 medium (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA) containing 10% (v/v) heat-inactivated fetal bovine
serum (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at 37°C in an incubator containing humidified air with 5%
(v/v) CO2.
Cell viability assay
SKOV3 and A2780 cells were seeded in 96-well plates
at 5×103 cells/well and treated with salidroside at
different concentrations (0, 50, 100, 500, 1,000 or 2,000 µmol/l)
for 48 h, at 37°C in an incubator containing humidified air with 5%
(v/v) CO2. A total of 10 µl MTT (5 mg/ml) was added to
each well and incubated in the dark at 37°C for 4 h. The
supernatant was removed and replaced with 150 µl dimethyl
sulfoxide. The plates were oscillated for 10 min and the absorbance
was measured at 490 nm.
Acridine orange/ethidium bromide
(AO/EB) staining
SKOV3 and A2780 cells in the logarithmic growth
phase were deposited in 2×103 cells each well of a
96-well plate. After 24 h, cells were subsequently treated with
salidroside (0 or 1,000 µmol/l) for 48 h at 37°C in an incubator
containing humidified air with 5% (v/v) CO2, stained
with AO (100 µg/ml in PBS) and EB (100 µg/ml in PBS) at room
temperature and analyzed by fluorescence microscopy at ×200
magnification (17). Five fields were
randomly selected for study. Cell apoptosis (%) was defined as the
number of apoptotic cells divided by the total number of cells
(18,19).
Antibodies and western blotting
SKOV3 and A2780 cells [treated with salidroside
(1,000 µmol/l; salidroside group) or PBS (control group) for 48 h
at 37°C in an incubator containing humidified air with 5% (v/v)
CO2] were collected and lysed in ice-cold
radioimmunoprecipitation assay buffer (Roche Diagnostics, Basel,
Switzerland). The protein concentration of the lysates was measured
using a bicinchoninic acid Protein Assay kit (Pierce; Thermo Fisher
Scientific, Inc.) according to the protocol of the manufacturer.
Cell lysates were separated by 10% SDS-polyacrylamide gel
electrophoresis and electrotransferred to nitrocellulose membranes
(Pall Corporation, Port Washington, NY, USA). The membranes were
blocked using 5% skim milk then incubated with primary antibodies
against β-actin (cat. no. 3700; 1:2,000 dilution; Cell Signaling
Technology, Inc., Danvers, MA, USA), p53 (cat. no. 2524; 1:500
dilution; Cell Signaling Technology, Inc.), p21
Cip1/Waf1 (cat. no. 610233; 1:500 dilution; BD
Biosciences, Franklin Lakes, NJ, USA), p16INK4a (cat.
no. sc-53392; Santa Cruz Biotechnology, Santa Cruz, CA, USA),
Bcl-2-associated X protein (Bax) (cat. no. 5020; 1:1,000 dilution;
Cell Signaling Technology, Inc.), Bcl-2-associated death promoter
(Bad) (cat. no. 9268; 1:500 dilution; Cell Signaling Technology,
Inc.), and p-Bad (cat. no. 9291; 1:500 dilution; Cell Signaling
Technology, Inc.), Bcl-2 (cat. no. 15071; 1:1,000 dilution; Cell
Signaling Technology, Inc.), apoptosis-inducing factor (AIF) (cat.
no. 5318; 1:1,000 dilution; Cell Signaling Technology, Inc.) and
X-linked inhibitor of apoptosis (XIAP) (cat. no. 14334; 1:1,000
dilution; Cell Signaling Technology, Inc.) at 4°C overnight. The
membranes were incubated with rabbit (cat. no. 7054; 1:5,000
dilution; Cell Signaling Technology, Inc.) or mouse (cat. no. 7056;
1:5,000 dilution; Cell Signaling Technology, Inc.) secondary
antibodies at room temperature for 1.5 h. Immunoreactive bands were
visualized using an enhanced chemiluminescence reagent (GE
Healthcare, Chicago, IL, USA). Intensities of immunoreactive bands
were determined by densitometric analysis using ImageJ software
(version 1.61; National Institutes of Health, Bethesda, MD, USA)
and normalized against β-actin.
Caspase-3 activity assay
A caspase-3 activity assay kit (cat no. C1115;
Beyotime Institute of Biotechnology, Haimen, China) was used to
test the activity of caspase-3 following treatment with salidroside
(0 or 1,000 µmol/l) for 48 h in ovarian cancer (SKOV3 and A2780)
cells. Caspase activity was expressed as a percentage of the
control.
Statistical analysis
Data are presented as the mean ± standard error of
the mean of three independent experiments. Statistical analysis was
conducted using SPSS 19.0 software (IBM Corp., Armonk, NY, USA) and
illustrated using GraphPad Prism 5.0 (GraphPad Software, Inc., La
Jolla, CA, USA). Statistical significance was determined using
Student's t-test to compare two groups or analysis of variance with
Tukey's post hoc test to compare multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
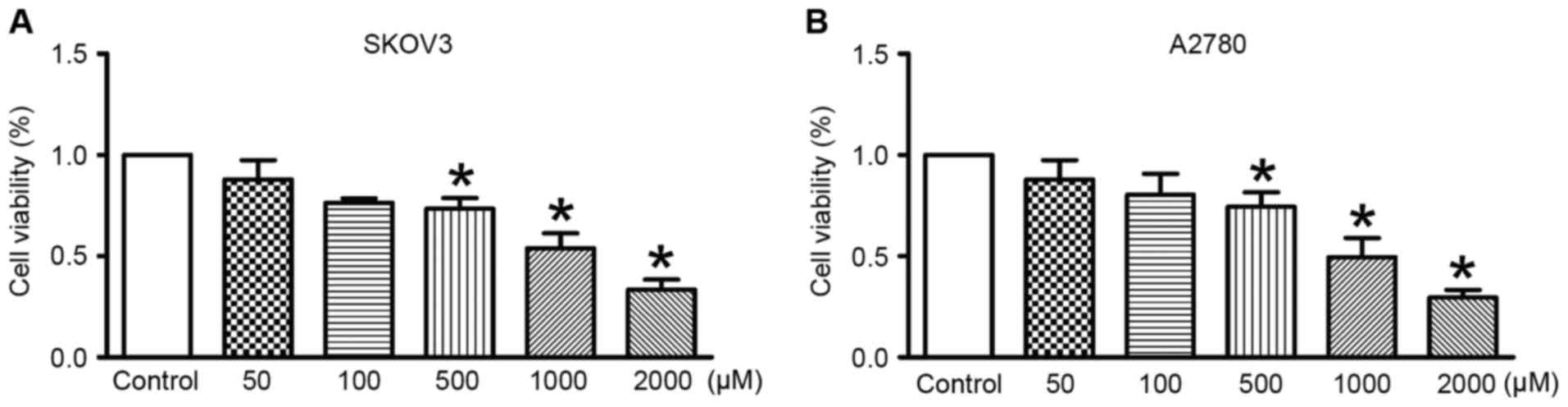
Assessment of OC cell viability after
treatment with salidroside
Across different concentrations (500, 1,000 or 2,000
µmol/l) of salidroside treatment, cell viability was significantly
inhibited compared with the control (Fig.
1). The data revealed that salidroside treatment at 1,000
µmol/l for 48 h inhibited viability of SKOV3 and A2780 cells by
~50%. For this reason, in subsequent experiments 1,000 µmol/l was
used as salidroside treatment.
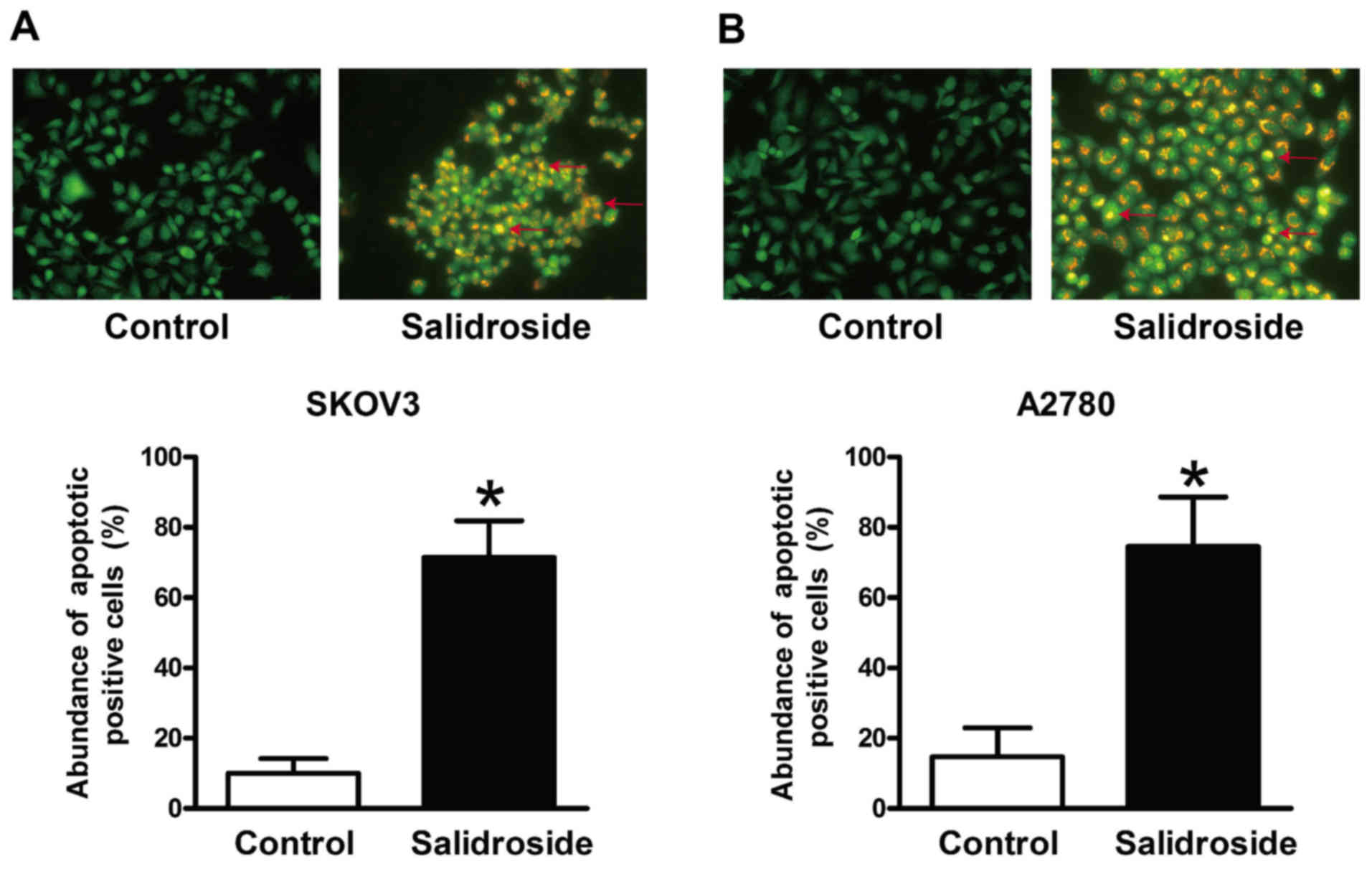
Salidroside induces apoptosis in SKOV3
and A2780 cells
Cell viability is a balance between cell
proliferation and apoptosis (20).
AO/EB staining revealed that salidroside significantly increased
the rate of apoptosis in SKOV3 (Fig.
2A) and A2780 cells (Fig.
2B).
Salidroside activates pro-apoptotic
signaling pathways
To explore the functional mechanisms of salidroside,
the expression of Bax, Bcl-2, Bad, p-Bad, AIF and XIAP proteins was
investigated by western blotting in SKOV3 (Fig. 3A-E) and A2780 (Fig. 3F-J) cells. The results indicated that
treatment with salidroside significantly upregulated the ratio of
Bax/Bcl-2 and the expression of Bad and AIF, but significantly
downregulated p-Bad and XIAP expression in SKOV3 and A2789 cells.
Meanwhile, caspase-3 activity was significantly increased by
salidroside in SKOV3 (2.1-fold increase) and A2780 (2.5-fold
increase) cells.
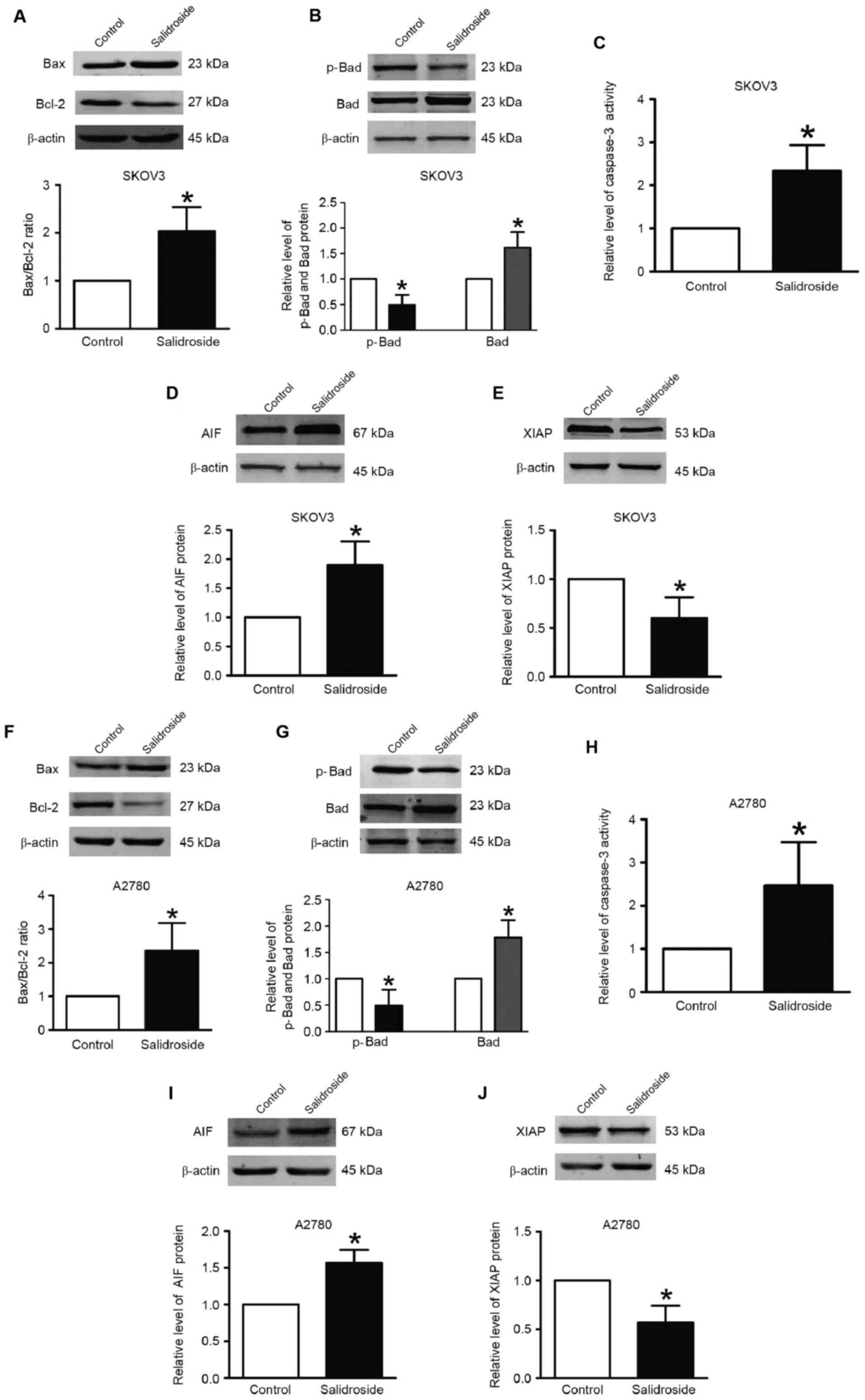 | Figure 3.Salidroside alters p-Bad, Bad, Bax,
Bcl-2, AIF and XIAP expression, and promotes caspase-3 activation.
Western blotting was used to detect protein expression. Relative
protein expression was normalized to β-actin. Caspase-3 activity
was measured using a commercial assay kit. (A) Bax and Bcl-2
expression, (B) Bad and p-Bad expression, (C) caspase-3 activity,
(D) AIF expression and (E) XIAP expression in SKOV3 cells treated
with salidroside. (F) Bax and Bcl-2 expression, (G) Bad and p-Bad
expression, (H) caspase-3 activity, (I) AIF expression and (J) XIAP
expression in A2780 cells treated with salidroside. Data are
expressed as the mean ± standard error of the mean; n=3 independent
experiments for each group. *P<0.05 vs. control. Bax,
Bcl-2-associated X protein; Bad, Bcl-2-associated death promoter;
AIF, apoptosis-inducing factor; XIAP, X-linked inhibitor of
apoptosis. |
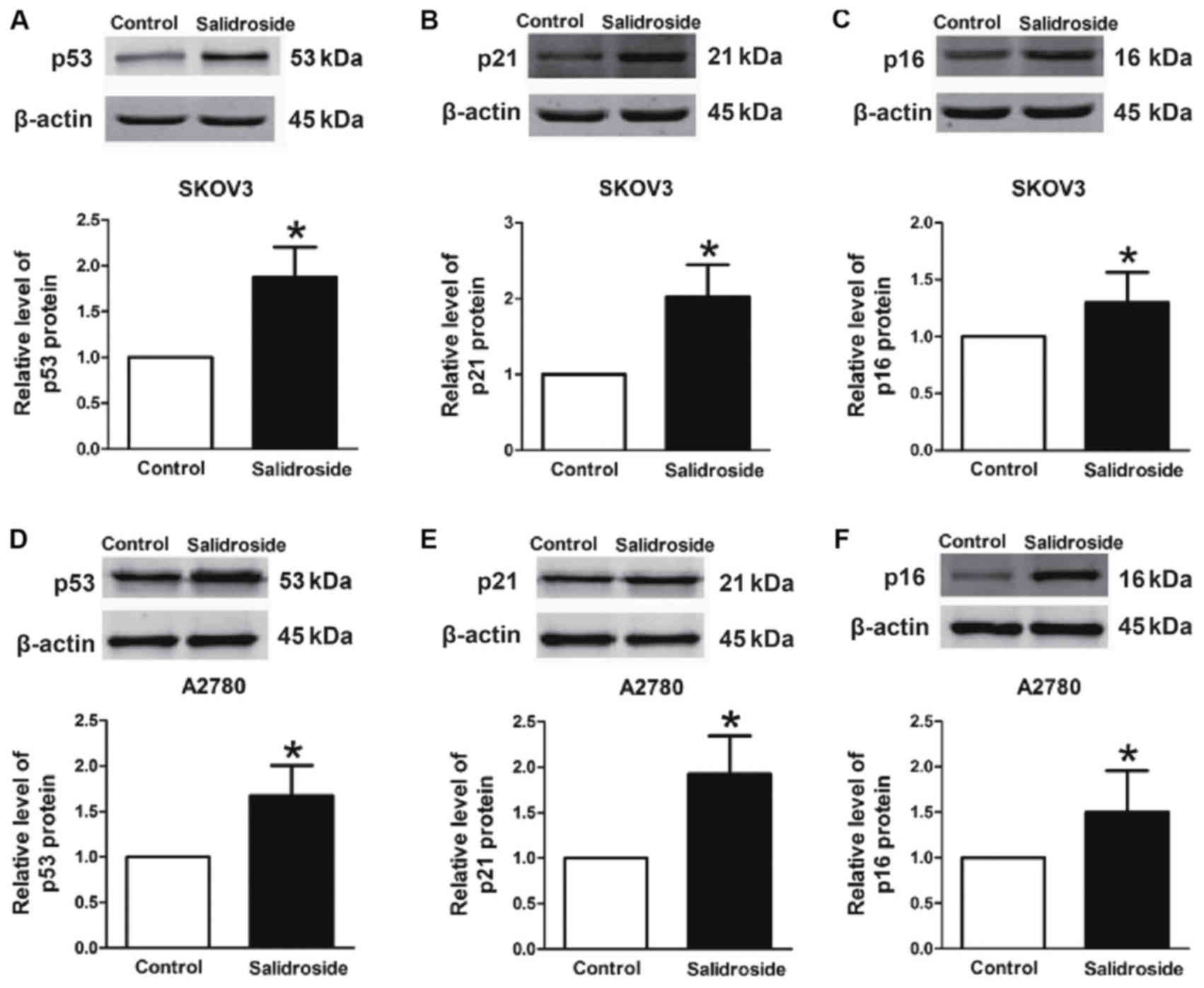
Salidroside activates p53 signaling
pathways
To define whether p53 signaling was involved in
salidroside-induced apoptosis, the protein levels of p53,
p21Cip1/Waf1 and p16INK4a were evaluated.
They were identified to be significantly upregulated in SKOV3
(Fig. 4A-C) and A2780 (Fig. 4D-F) cells after treatment with
salidroside compared with the control. These results indicated that
the p53/p21Cip1/Waf1/p16INK4a pathway serves
a critical function in salidroside-induced apoptosis in SKOV3 and
A2780 cells.
Discussion
In the present study, in vitro experiments
demonstrated that salidroside exerts potent anti-proliferative
effects on SKOV3 and A2780 cells by inducing apoptosis.
Furthermore, the mechanisms underlying the anticancer effects of
salidroside on OC were investigated. The results indicated that
activation of the caspase-3-dependent pathway and the p53 signaling
pathway were involved in mediating these salidroside-induced
effects. Therefore, the present results may provide an experimental
basis for the potential role of salidroside in treating OC.
The current results are consistent with previous
reports demonstrating that salidroside exerts anticancer effects in
breast carcinoma (15), human
fibrosarcoma (21,22) and neuroblastoma (23). The mechanism of action of salidroside
has been reported to involve autophagy (24). However, the effect of salidroside in
OC is not yet fully understood.
The present data suggested that salidroside has
antiproliferative and pro-apoptotic effects on OC cells. It was
revealed that salidroside inhibited the viability of SKOV3 and
A2780 cells. Furthermore, AO/EB staining indicated that salidroside
induced apoptosis in OC cells.
The effect of salidroside on the regulation of gene
expression has been studied previously. Numerous studies have
revealed that Bax, Bcl-2 and caspase-3 are involved in apoptosis in
SKOV3 and A2780 cells (25). Liu
et al (26) identified that
caspase-mediated cleavage of Beclin1 inhibits autophagy and
promotes apoptosis in SKOV3 cells. Furthermore, andrographolide
radiosensitizes human SKOV3 cells (27), and miRNA-149 modulates the
chemosensitivity of A2780 cells by modulating Bax protein
expression (28). In the present
study, it was demonstrated that salidroside could induce Bcl-2 and
Bax expression, and upregulate caspase-3 in SKOV3 and A2780 cells.
In addition, the ratio of Bcl-2/Bax was /decreased, indicating that
salidroside promotes apoptosis in OC. Bad is known to regulate
apoptosis by forming heterodimers with Bax and Bcl-2 (29). XIAP inhibits activation of caspases by
binding to them, preventing apoptosis of tumor cells (30). In the present study, Bad protein was
significantly increased while p-Bad and XIAP levels were
significantly decreased following treatment with salidroside. These
results demonstrate that salidroside could induce apoptosis via
caspase-3-dependent apoptosis signaling in OC (31,32).
Furthermore, p53 can influence apoptosis by regulating Bcl-2
(33). Previous studies have revealed
that >50% of tumors are associated with p53 gene mutation;
wild-type p53 gene therapy has been suggested to strengthen
sensitivity to cisplatin in SKOV3 cells (34–36). In
the present study, it was demonstrated that salidroside has the
ability to induce OC apoptosis. Salidroside also promoted the
expression of p53, p21Cip1/Waf1 and p16 INK4a
expression in SKOV3 and A2780 cells. It was identified that
salidroside promotes the expression of caspase-3 and activation of
the p53/p21Cip1/Waf1/p16INK4a pathway.
In summary, salidroside was demonstrated to reduce
cell viability and promote apoptosis in OC. Furthermore, it was
identified that salidroside activates caspase-3 and the
p53/p21Cip1/Waf1/p16INK4a pathway. Therefore,
salidroside is a promising new approach for the treatment of OC,
but its underlying mechanism needs to be explored further.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vang R, Shih IeM and Kurman RJ: Ovarian
low-grade and high-grade serous carcinoma: Pathogenesis,
clinicopathologic and molecular biologic features, and diagnostic
problems. Adv Anat Pathol. 16:267–282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vaughan S, Coward JI, Bast RC Jr, Berchuck
A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R,
Etemadmoghadam D, et al: Rethinking ovarian cancer: Recommendations
for improving outcomes. Nat Rev Cancer. 11:719–725. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Landen CN Jr, Birrer MJ and Sood AK: Early
events in the pathogenesis of epithelial ovarian cancer. J Clin
Oncol. 26:995–1005. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Friedlander M, Rau J, Lee CK, Meier W,
Lesoin A, Kim JW, Poveda A, Buck M, Scambia G, Shimada M, et al:
Quality of life in patients with advanced epithelial ovarian cancer
(EOC) randomized to maintenance pazopanib or placebo after
first-line chemotherapy in the AGO-OVAR 16 trial. Measuring what
matters-patient centered endpoints in trials of maintenance
therapy. Ann Oncol. Dec 18–2017.Doi: 10.1093/annonc/mdx796.
View Article : Google Scholar
|
|
6
|
Paik ES, Kim JH, Kim TJ, Lee JW, Kim BG,
Bae DS and Choi CH: Prognostic significance of normal-sized ovary
in advanced serous epithelial ovarian cancer. J Gynecol Oncol.
29:e132018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Westin SN, Herzog TJ and Coleman RL:
Investigational agents in development for the treatment of ovarian
cancer. Invest New Drugs. 31:213–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jin N, Wu H, Miao Z, Huang Y, Hu Y, Bi X,
Wu D, Qian K, Wang L, Wang C, et al: Network-based
survival-associated module biomarker and its crosstalk with cell
death genes in ovarian cancer. Sci Rep. 5:115662015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Darbinyan V, Kteyan A, Panossian A,
Gabrielian E, Wikman G and Wagner H: Rhodiola rosea in stress
induced fatigue-a double blind cross-over study of a standardized
extract SHR-5 with a repeated low-dose regimen on the mental
performance of healthy physicians during night duty. Phytomedicine.
7:365–371. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spasov AA, Wikman GK, Mandrikov VB,
Mironova IA and Neumoin VV: A double-blind, placebo-controlled
pilot study of the stimulating and adaptogenic effect of Rhodiola
rosea SHR-5 extract on the fatigue of students caused by stress
during an examination period with a repeated low-dose regimen.
Phytomedicine. 7:85–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Panossian A, Wikman G and Sarris J:
Rosenroot (Rhodiola rosea): Traditional use, chemical composition,
pharmacology and clinical efficacy. Phytomedicine. 17:481–493.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mao GX, Xing WM, Wen XL, Jia BB, Yang ZX,
Wang YZ, Jin XQ, Wang GF and Yan J: Salidroside protects against
premature senescence induced by ultraviolet B irradiation in human
dermal fibroblasts. Int J Cosmet Sci. 37:321–328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barhwal K, Das SK, Kumar A, Hota SK and
Srivastava RB: Insulin receptor A and Sirtuin 1 synergistically
improve learning and spatial memory following chronic salidroside
treatment during hypoxia. J Neurochem. 135:332–346. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang X, Zou L, Yu X, Chen M, Guo R, Cai
H, Yao D, Xu X, Chen Y, Ding C, et al: Salidroside attenuates
chronic hypoxia-induced pulmonary hypertension via adenosine A2a
receptor related mitochondria-dependent apoptosis pathway. J Mol
Cell Cardiol. 82:153–166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lai MC, Lin JG, Pai PY, Lai MH, Lin YM,
Yeh YL, Cheng SM, Liu YF, Huang CY and Lee SD: Protective effect of
salidroside on cardiac apoptosis in mice with chronic intermittent
hypoxia. Int J Cardiol. 174:565–573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao G, Shi A, Fan Z and Du Y: Salidroside
inhibits the growth of human breast cancer in vitro and in vivo.
Oncol Rep. 33:2553–2560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang ZR, Wang HF, Zuo TC, Guan LL and Dai
N: Salidroside alleviates oxidative stress in the liver with
non-alcoholic steatohepatitis in rats. BMC Pharmacol Toxicol.
17:162016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McGahon AJ, Martin SJ, Bissonnette RP,
Mahboubi A, Shi Y, Mogil RJ, Nishioka WK and Green DR: The end of
the (cell) line: Methods for the study of apoptosis in vitro.
Methods Cell Biol. 46:153–185. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen H, Takahashi S, Imamura M, Okutani E,
Zhang ZG, Chayama K and Chen BA: Earthworm fibrinolytic enzyme:
Anti-tumor activity on human hepatoma cells in vitro and in vivo.
Chin Med J (Engl). 120:898–904. 2007.PubMed/NCBI
|
|
20
|
Ribble D, Goldstein NB, Norris DA and
Shellman YG: A simple technique for quantifying apoptosis in
96-well plates. BMC Biotechnol. 5:122005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lambert KE, Huang H, Mythreye K and Blobe
GC: The type III transforming growth factor-beta receptor inhibits
proliferation, migration, and adhesion in human myeloma cells. Mol
Biol Cell. 22:1463–1472. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lv C, Huang Y, Liu ZX, Yu D and Bai ZM:
Salidroside reduces renal cell carcinoma proliferation by
inhibiting JAK2/STAT3 signaling. Cancer Biomark. 17:41–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu X, Lin S, Yu D, Qiu S, Zhang X and Mei
R: A preliminary study: The anti-proliferation effect of
salidroside on different human cancer cell lines. Cell Biol
Toxicol. 26:499–507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun C, Wang Z, Zheng Q and Zhang H:
Salidroside inhibits migration and invasion of human fibrosarcoma
HT1080 cells. Phytomedicine. 19:355–363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Yu H, Sun Y, Lin X, Chen B, Tan
C, Cao G and Wang Z: Protective effects of salidroside on hydrogen
peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells.
Eur J Pharmacol. 564:18–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Z, Li X, Simoneau AR, Jafari M and Zi
X: Rhodiola rosea extracts and salidroside decrease the growth of
bladder cancer cell lines via inhibition of the mTOR pathway and
induction of autophagy. Mol Carcinog. 51:257–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li X, Su J, Xia M, Li H, Xu Y, Ma C, Ma L,
Kang J, Yu H, Zhang Z and Sun L: Caspase-mediated cleavage of
Beclin1 inhibits autophagy and promotes apoptosis induced by S1 in
human ovarian cancer SKOV3 cells. Apoptosis. 21:225–238. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang C and Qiu X: Andrographolide
radiosensitizes human ovarian cancer SKOV3 xenografts due to an
enhanced apoptosis and autophagy. Tumour Biol. 36:8359–8365. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhan Y, Xiang F, Wu R, Xu J, Ni Z, Jiang J
and Kang X: MiRNA-149 modulates chemosensitivity of ovarian cancer
A2780 cells to paclitaxel by targeting MyD88. J Ovarian Res.
8:482015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang C, Gu H, Zhang W, Herrmann JL and
Wang M: Testosterone-down-regulated Akt pathway during cardiac
ischemia/reperfusion: A mechanism involving BAD, Bcl-2 and FOXO3a.
J Surg Res. 164:e1–e11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Paulsen M, Ussat S, Jakob M, Scherer G,
Lepenies I, Schütze S, Kabelitz D and Adam-Klages S: Interaction
with XIAP prevents full caspase-3/-7 activation in proliferating
human T lymphocytes. Eur J Immunol. 38:1979–1987. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie Y, Tobin LA, Camps J, Wangsa D, Yang
J, Rao M, Witasp E, Awad KS, Yoo N, Ried T and Kwong KF:
MicroRNA-24 regulates XIAP to reduce the apoptosis threshold in
cancer cells. Oncogene. 32:2442–2451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ikenberg K, Valtcheva N, Brandt S, Zhong
Q, Wong CE, Noske A, Rechsteiner M, Rueschoff JH, Caduff R, Dellas
A, et al: KPNA2 is overexpressed in human and mouse endometrial
cancers and promotes cellular proliferation. J Pathol. 234:239–252.
2014.PubMed/NCBI
|
|
34
|
Li YD, Hong YF, Yusufuaji Y, Tang BP, Zhou
XH, Xu GJ, Li JX, Sun L, Zhang JH, Xin Q, et al: Altered expression
of hyperpolarization-activated cyclic nucleotide-gated channels and
microRNA-1 and −133 in patients with age-associated atrial
fibrillation. Mol Med Rep. 12:3243–3248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gu J, Tang Y, Liu Y, Guo H, Wang Y, Cai L,
Li Y and Wang B: Murine double minute 2 siRNA and wild-type p53
gene therapy enhances sensitivity of the SKOV3/DDP ovarian cancer
cell line to cisplatin chemotherapy in vitro and in vivo. Cancer
Lett. 343:200–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gherman C, Braicu OL, Zanoaga O, Jurj A,
Pileczki V, Maralani M, Drigla F, Braicu C, Budisan L,
Achimas-Cadariu P and Berindan-Neagoe I: Caffeic acid phenethyl
ester activates pro-apoptotic and epithelial-mesenchymal
transition-related genes in ovarian cancer cells A2780 and
A2780cis. Mol Cell Biochem. 413:189–198. 2016. View Article : Google Scholar : PubMed/NCBI
|