Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are a
class of drugs that in addition to providing analgesic,
antipyretic, and anti-inflammatory effects, also possess
chemopreventive actions against the development of a number of
cancers in both animal models and humans (1,2). Even
though the molecular mechanism of this anti-neoplastic effect is
not completely understood, there has been increasing interest in
the chemopreventive activity of NSAIDs due to their demonstrated
ability to reduce the incidence and severity of various cancers
based upon clinical outcome studies (3–5). In
particular, colorectal cancer incidence rates are reduced in
persons who consume daily aspirin or ibuprofen (6–8). We
previously reported that aspirin and a novel aspirin derivative
which is associated with phosphatidylcholine (Aspirin-PC) to
provide protection of the gastrointestinal (GI) tract against
aspirin-induced injury, are both effective cancer-preventing agents
in an animal model of colon cancer (9). That model consists of using the colon
carcinogen azoxymethane (AOM) along with the colon inflammatory
agent dextran sodium sulfate (DSS) to produce colitis-associated
pre-neoplastic aberrant crypts in the colon (10) that are blocked by aspirin or
Aspirin-PC treatments. While this chemically-induced colon cancer
model provides good evidence of chemopreventive activity, and has
been used by others for screening chemopreventive agents (11–13), it is
not the sole model for testing anticancer agents. In order to
further test the potential chemopreventive activity of Aspirin-PC,
we decided to use another animal model, which directly tests the
ability of drugs to inhibit the growth of cancer cells in
vivo. In this model, tissue culture-grown murine colon cancer
cells (MC-26) will be inoculated into the mouse splenic capsule and
allowed to grow for 4 weeks prior to collection of splenic (primary
tumor) and hepatic (metastatic) tissues for analysis of cancer
nodule growth (14). Not only does
this model allow for screening of cancer growth and metastatic
spread, but it has the added advantage that mouse cells are used in
the mouse (syngeneic) and no immunosuppression is required. In
addition, MC-26 cells in culture can be used to study the ability
of test drugs to inhibit cancer cell growth. Previous investigators
showed that the NSAID ibuprofen is effective at blocking cancer
growth in this model (15).
Indomethacin is another NSAID that has previously
been reported to have anti-neoplastic activity at low doses in both
rodents (16,17) and humans (18,19).
Accordingly, we performed in vitro studies to compare the
growth-inhibitory effect of the PC-associated aspirin and
indomethacin, vs. unmodified NSAIDs on MC-26 colon cancer cells.
Also, these drugs were tested in the in vivo MC-26 mouse
model system.
Materials and methods
Test drugs
For cell culture, aspirin was purchased from Rhodia
and indomethacin was from Spectrum Chemical (Gardena, CA, USA). For
the animal study, aspirin (uncoated) was purchased from Walgreens
(Deerfield, IL, USA). Aspirin-PC and Indomethacin-PC were prepared
as described below for the cell culture and animal studies.
We used established procedures to prepare our
PC-associated test drug formulations for cell culture and
intragastric dosing (20,21). For cell culture, the Aspirin-PC stock
solution was prepared as described previously (9). Briefly, the aspirin was firstly
dissolved in the serum-free culture medium at 10 mmol/l and then
combined with an equimolar amount of purified soy
phosphatidylcholine/PC (S-100; Lipoid LLC, Newark, NJ, USA), which
was previously dissolved in chloroform and then blown dry under
nitrogen. The tubes were then sonicated at room temperature in a
bath-type sonicator for 20 min until a homogenous suspension was
obtained (Fig. 1 for the chemical
structures of aspirin, indomethacin and soy PC). In the animal
experiments, we used different procedures preparing Aspirin-PC as
described previously (9,22). To make Indomethacin-PC stock for both
cell culture and animal study, 8 gram of indomethacin (acid form)
and 16 gram of Lipoid S-100 were subsequently dissolved into 60 ml
of Acetone (Thermo Fisher Scientific, Inc., Fair Lawn, NJ, USA) in
a 500-ml flat bottom round flask in 40°C water bath. Then the flask
was connected to a rota-vaporator and vacuum-processed for 14–16 h
to remove the acetone. Finally, the Indomethacin-PC was collected
in a brown glass jar and kept at 4°C. To prepare the
Indomethacin-PC solution for oral administration, the drug was
weighed, and deionized distilled water added to a glass vial to the
desired concentration and sonicated for 20 min at room
temperature.
Cell culture
Murine colon cancer cells (MC-26) were obtained from
the NIH National Cancer Institute. The cell line was cultured in
suggested growth medium with 10% fetal bovine serum (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). Tests for mycoplasma were negative
and were conducted with the MycoAlert Mycoplasma Detection Kit from
Lonza (Rockland, ME, USA). This cell line is known to express COX-2
(14).
MC-26 cells were preincubated with the drugs at a
concentration range from 0 to 1.0 mmol/l (aspirin/Aspirin-PC) or 0
to 50 µmol/l (indomethacin/Indomethacin-PC) for 15 min to promote
optimal exposure to our test-drugs, prior to pipetting the cells
onto 48-well plates at a density of 2×103 cells/well,
and cultured at 37°C in a mixture of 5% CO2 and 95% air.
The cells were then cultured in the above growth medium in the
presence and absence of the test drug formulations for 8 days with
one medium change on the 4th day, at which time the culture medium
was collected into 1.5 ml Eppendorf tubes and centrifuged at high
speed for 10 min. Then the supernatant was collected for
prostaglandin (PGE2) assay as a measure of COX-2
activity. Cells on day 8 were used for the MTT
[3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide]
assay as a measure of cell number as outlined below.
MTT assay
MTT (purchased from Sigma-Aldrich; Merck KGaA) was
added to the culture media of cells at a final concentration of 0.5
mg/ml for 4 h. The purple formazan product was then extracted into
a solvent (90% isopropanol, 0.2% sodium dodecyl sulfate, and 0.01
mol/l HCl) which was then collected from the wells and read at an
absorbance of 570 nm, as previously described (22).
Animal study
Young adult (20–24 g) male BALB/c mice were supplied
by Harlan Laboratories, Inc. (Envigo, Indianapolis, IN, USA) and
housed in the Center for Laboratory Animal Medicine and Care
(CLAMC) facility at The University of Texas Health Science Center
at Houston (UTHealth). Mice were maintained in accordance and
compliance with policies approved by the Animal Welfare Committee
(AWC), the Institutional Animal Care and Use Committee (IACUC) for
The University of Texas Health Science Center at Houston
(UTHealth). This facility is approved by the PHS and AAALAC.
On day one, mice were anesthetized and subjected to
a laparotomy under isoflurane anesthesia as previously described
(14,15,23), in
order to inoculate their splenic capsules with 2×105
cells/ml, 0.1 ml per mouse. Following the method of Yao et
al cited above, immediately after cancer cell implantation, the
mice were randomly divided into five treatment groups and treated
once daily orally with vehicle (saline), aspirin (20 mg/kg),
Aspirin-PC (20 mg ASA+20 mg PC/kg), indomethacin (2 mg/kg), or
Indomethacin-PC (2 mg indomethacin + 4 mg PC/kg) and this treatment
was continued daily for 28 days. A non-cancer group was also
included as control. Thereafter, the mice were sacrificed and
tissues were collected for analysis of tumor growth (spleen
weight), possible GI injury due to NSAID (hematocrit), and
metastatic cancer cell spread (liver weight and nodule number),
plus fecal hemoglobin was assessed for evidence of GI bleeding,
serum levels of Thromboxane B2 (TXB2) were
assayed as a measure of NSAID pharmacologic action (inhibition of
platelet COX-1 activity), and spleen tissue was assayed for
PGE2 as a measure of COX-2 activity.
Fecal hemoglobin analysis
Fecal hemoglobin (Hb) was monitored by collecting
the fecal droppings at regular intervals from the bedding and
storing them at −20°C until the day of analysis. The feces were
weighed, and then distilled water was added at a 1:10 feces (g):
Water (ml) ratio. After standing for 1 h, the feces were disrupted
into a homogenous suspension by vortexing for 2 min and then the Hb
analyzed by a previously described method (24).
ELISAs
The animal serum was analyzed by using the
thromboxane B2 EIA kit (Cayman Chemical, Ann Arbor, MI,
USA) according to the manufacturer's specifications. Blood of
individual mice was collected at the end of the experiment under
terminal anesthesia following a protocol for cardiac puncture, and
serum was separated within 1 h following blood collection by
centrifugation at 500 × g for 10 min, and then aliquoted and stored
at −80°C for subsequent testing at a 1/200 dilution.
The MC-26 cell medium collected on day 4 of culture,
and the excised animal spleen tissue were analyzed by using the
Prostaglandin E2 EIA kit (Cayman Chemical) according to
the manufacturer's specifications. Splenic tumor tissues were
homogenized in methanol, followed by SPE (C18) purification as
suggested by the manufacturer's instruction. The final extract was
resuspended in buffer and tested.
Statistics
Statistical analyses were performed using the
statistics application StatView 5.01 (SAS Institute Inc., Cary, NC,
USA). Values are expressed as the mean ± standard error of the
mean, and were evaluated by ANOVA followed by Fisher's PLSD test. A
two-tailed value of P<0.05 was considered to indicate
statistically significant differences.
Results
In vitro effects of test drugs on
MC-26 colon cancer cells in culture
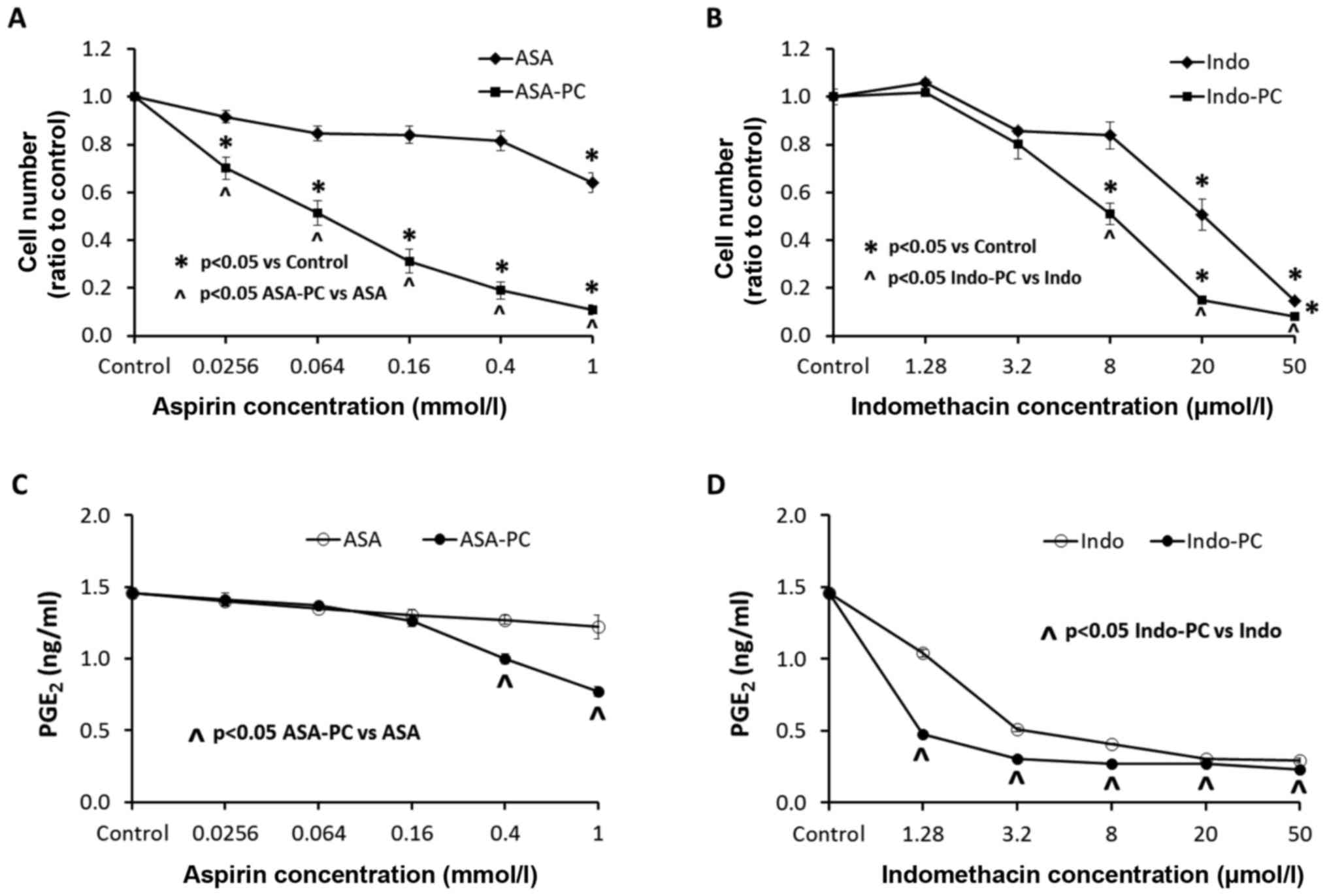
The effects of our test drugs on the growth of MC-26
colon cancer cell line were examined over an 8-day culture period
(Fig. 2A and B). Aspirin alone had an
inhibitory effect on the growth of the cells only at the highest
concentration of 1 mmol/l, while the PC complexed aspirin,
Aspirin-PC, showed significant inhibition at the much lower
concentration of 25 µmol/l (Fig. 2A).
All of the Aspirin-PC concentrations gave significantly lower cell
growth than the comparable doses of aspirin alone. In comparison,
the other tested NSAID, indomethacin, was much more potent than
aspirin with a significant inhibition of the cancer cell growth at
a concentration of 20 µmol/l, and Indomethacin-PC was inhibitory at
an even lower concentration of 8 µmol/l (Fig. 2B). The Indomethacin-PC concentrations
of 8–50 µmol/l were significantly more effective at cell growth
inhibition than comparable doses of indomethacin alone.
The expression of PGE2 in culture medium
(Fig. 2C and D) did not parallel the
effects on cell growth for either NSAID. Aspirin alone (Fig. 2C) had no apparent effect on
PGE2 levels, while Aspirin-PC was significantly
inhibitory at concentrations of 0.4–1 mmol/l, which was higher than
the level that inhibited cell growth. In contrast, indomethacin
alone (Fig. 2D) was a potent
inhibitor of PGE2, even at the lowest concentration
tested. Once again, Indomethacin-PC was even more potent than the
unmodified NSAID, with significantly lower levels of
PGE2 than indomethacin at every concentration. These
concentrations of both indomethacin and Indomethacin-PC that
inhibited PGE2 levels were considerably lower than the
concentrations that affected cell growth.
MC-26 colon cancer cell implantation
mouse study
As described above, the MC-26 colon cancer study in
mice was terminated after four weeks of cancer cell implantation
and animal dosing. This time allowed for greater cancer cell growth
as evidenced by spleen weights in vehicle-treated mice (20 mg/g
body weight), compared to that of the non-cancer control mice (3.3
mg/g body weight). Treatment with indomethacin, Indomethacin-PC or
Aspirin-PC gave clear and significant reductions (P<0.05) in
splenic tumor nodules and spleen weights (Fig. 3A). However, aspirin alone was not
protective in this model at the dose tested. Since a previous
unpublished animal study showed PC alone had no effect on cancer
cell growth in this model, we did not include a PC alone treatment
group in this experiment.
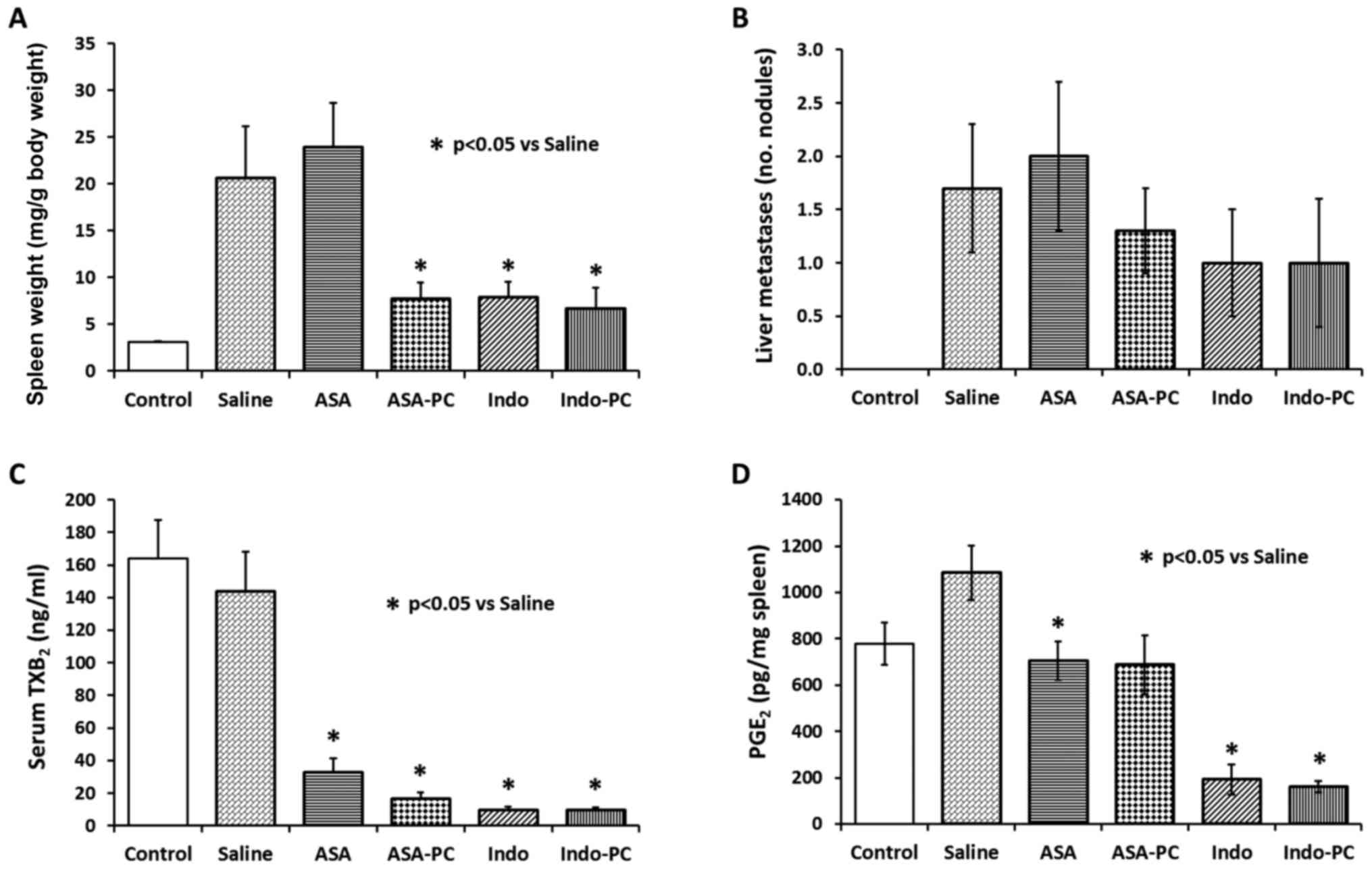 | Figure 3.Effect of test drugs on MC-26 cancer
cell implantation mouse model system. Immediately after cancer cell
inoculation into the splenic capsule, the mice were randomly
grouped and administered with saline (vehicle), aspirin,
Aspirin-PC, indomethacin, or Indomethacin-PC for 28 days, daily at
the following NSAID doses: 20 mg/kg ASA, 20 mg/kg ASA + 20 mg/kg
PC, 2 mg/kg Indo, and 2 mg/kg Indo + 4 mg/kg PC, respectively. A
non-cancer group was also included as control. Values are: (A)
spleen weight; (B) liver metastases; (C) serum TXB2; and
(D) spleen PGE2. *P<0.05 vs. saline group. |
An analysis of liver tissue revealed the presence of
a number of metastatic tumor nodules (Fig. 3B), which tended to be reduced by
treatment with indomethacin, Indomethacin-PC or Aspirin-PC, but not
aspirin alone, similar to the spleen weight results. However, there
were too few liver nodules to see a significant difference and the
liver organs weights did not show differences either (not
shown).
Assessments of GI bleeding showed no differences
between treatment groups, with hematocrits in a normal range of
0.43 to 0.47 and fecal hemoglobin also showing minimal alterations
(0.62 to 0.88 mg Hb/g feces) (Table
I).
 | Table I.Measures of gastric bleeding in
mice. |
Table I.
Measures of gastric bleeding in
mice.
| Treatment
group | Hematocrit | Fecal hemoglobin
(mg/g feces) |
|---|
| Control | 0.43±0.02 | 0.62±0.04 |
| Saline | 0.44±0.02 | 0.64±0.03 |
| ASA | 0.45±0.02 | 0.85±0.07 |
| ASA-PC | 0.47±0.01 | 0.88±0.06 |
| Indo | 0.44±0.04 | 0.61±0.04 |
| Indo-PC | 0.44±0.02 | 0.79±0.05 |
To verify that the NSAIDs used in this study were
pharmacologically active, serum was analyzed for COX-1 activity by
measurement of TXB2 formed from platelets during blood
clotting. Fig. 3C shows 80–90%
inhibition of TXB2 by all treatments, including aspirin
alone, supporting the NSAIDs' ability to inhibit prostaglandin
formation, a primary action of this class of drugs. It was noted
that there was no difference between TXB2 levels in
non-cancer controls and vehicle (cancer) controls, suggesting there
are no cancer-driven differences in platelet counts and/or
activity. This lack of a difference was confirmed by platelet
counts in a sampling of animals where measured values were between
366 to 634×103/µl.
Spleen tissue levels of PGE2 in
Saline-treated cancer controls (Fig.
3D) were elevated over non-cancer Controls (~35%), but not
significantly so, by the infiltration of cancer cells, as the
Saline group was not different from Control (P=0.0708). There were
apparent reductions of PGE2 by all test NSAIDs, with
indomethacin and Indomethacin-PC inducing the greatest inhibition
(~84%). Aspirin and Aspirin-PC gave similar reductions of
PGE2 (~36% vs. saline-treated controls), although
Aspirin-PC just missed the level of significance (P=0.0545).
Discussion
As briefly mentioned earlier, aspirin and related
NSAIDs have been demonstrated to possess chemopreventive/anticancer
activity against colorectal cancers and a number of other cancers,
reducing both the incidence and cancer-related mortality (1,2). Most of
this clinical evidence is based upon outcome studies, demonstrating
a link between NSAID consumption and risk of developing cancer
(3–8).
However, there have been several published prospective studies
demonstrating chemopreventive efficacy of aspirin and celecoxib and
colorectal cancer (25–27), as well as a pilot clinical study
demonstrating that indomethacin-treatment can significantly
increase length of survival of patients with advanced cancer
(19).
Previous in vitro testing of NSAIDs and
PC-NSAIDs in our laboratory has shown that aspirin and ibuprofen
are effective at inhibiting the growth of the human colon cancer
cell line SW480 which involves inhibition of DNA synthesis
(28). Both PC-NSAIDs were more
effective than the unmodified NSAID. Aspirin and Aspirin-PC were
also shown to be effective against MC-26 and Caco-2 (human colon
cancer) cell lines when cultured in the presence of washed
platelets which involves epithelial-mesenchymal transition (EMT)
(9). We also reported that
indomethacin and Indomethacin-PC (21), but not aspirin or ibuprofen +/− PC
(28), can promote apoptosis. Others
have described a variety of actions to explain the anti-neoplastic
actions of NSAIDs, many involving COX inhibition (1,2).
Our in vitro studies show that both of the
PC-NSAIDs were more potent than their parent NSAID at inhibiting
cancer cell growth. This direct action of the Aspirin-PC and
Indomethacin-PC is an important distinction and supports their
further development for chemoprevention of colon cancer. However,
our attempt to explain a possible mechanism related to COX
inhibitory activity was not consistent for both NSAIDs. Aspirin-PC
suppressed cell growth at a much lower concentration than that at
which it inhibited PGE2 produced by COX (Fig. 2C vs. A). In contrast, Indomethacin-PC
inhibited COX at a lower concentration than it needed to inhibit
cell growth (Fig. 2D vs. B). Thus,
the action of Indomethacin-PC, but not Aspirin-PC, could be
explained, only in part, by COX inhibition.
The in vivo chemopreventive/anti-cancer
effects of NSAIDs likely involve even more complicated mechanisms
than seen with in vitro work. There are numerous reports to
support a role for COX-2 overexpression in solid cancers, and
specific COX-2 inhibitors have found use clinically in some of
those cancers (29). Because MC-26
cells possess COX-2, they have been used in the MC-26 animal model
to test the anticancer activity of specific COX-2 inhibitor drugs
such as NS-398 (23) and rofecoxib
(14), both of which displayed
significant chemopreventive activity. In addition, our laboratory
has proposed that blood platelets (which possess COX-1), which are
elevated in some cancers such as ovarian cancer, may provide a
means for cancer cells to migrate and invade distant organs
(22). In an AOM/DSS mouse colon
cancer model, we previously showed an increased number of
circulating platelets that was reduced following aspirin or
Aspirin-PC treatment (9). However, in
the current cancer cell implantation animal model there was no
indication of elevated platelet counts in the cancer controls.
Consistent with this data, there was no increase in TXB2
between non-cancer controls and MC-26 injected controls.
Nevertheless, all of our test NSAIDs were very effective in
significantly reducing thromboxane levels by >80%. However,
COX-independent mechanisms have also been proposed to explain the
anticancer actions of NSAIDs (30),
and investigations into a role for microRNAs may offer a means to
understand these mechanisms (31).
Testing of aspirin and indomethacin compounds in the
MC-26 model revealed that aspirin alone at the dose tested was not
effective at limiting cancer cell growth in the spleen, while
Aspirin-PC provided significant reductions in spleen weight.
Further, indomethacin alone showed a significant effect that was
equaled by Indomethacin-PC. This result with indomethacin is
consistent with a report that indomethacin in the drinking water
was able to suppress tumor growth with the MC-26 model (32). No previous reports of aspirin use in
this model were found. Thus, both of these PC-associated NSAIDs
gave clear protection against cancer cell growth in the spleen.
Regarding metastatic spread to the liver in this model, there was
considerable variability seen in controls, so that the small
reductions seen with the PC-NSAIDs were not significant, although
the effects were consistent with the splenic size reductions.
Measures of GI bleeding in the MC-26 colon cancer
model including hematocrit and fecal hemoglobin did not reveal any
signs of adverse effects at the doses of drugs used. The drugs were
all administered orally for 28 days, which was enough time for GI
bleeding to be manifest, but none occurred that we could
detect.
The doses of drugs used here were sufficient to see
anticancer activity and COX-1 inhibition (inhibition of
TXB2 formation), indicating they were pharmacologically
active doses. However, we cannot attribute the chemopreventive
action solely to COX-1 inhibition, as aspirin alone demonstrated
that property, but did not display anti-cancer activity in the
syngeneic colon cancer mouse model employed in the current
study.
Similarly, the anticancer actions may be related
partly but not fully, to COX-2 inhibition (ie, anti-inflammatory
dose) based on current knowledge of dose effects. The human
equivalent to the mouse aspirin dose of 20 mg/kg is 96 mg for a 60
kg person, or about the dosage of a baby aspirin (81–100 mg). This
mouse dose of aspirin/Aspirin-PC was able to minimally inhibit
COX-2 to some extent (see Fig 3D),
but not fully, which is consistent with our previous reports that
30 and 40 mg/kg reduced GI prostaglandin E2
(PGE2) by 66 and 80%, respectively (24,33). Yet
only Aspirin-PC, and not aspirin, was effective at preventing
growth of cancer cells in vivo.
The human equivalent to the mouse indomethacin dose
of 2 mg/kg is 9.6 mg for a 60 kg person, which is well below the
maximum recommended daily (anti-inflammatory) dose of 150–200 mg
per day for treatment of gout or bursitis. However, the mouse dose
of indomethacin in our study was able to almost fully inhibit COX-2
as seen from the data presented in Fig.
3D, where splenic levels of PGE2 were reduced by
>80% in mice treated with either indomethacin or
Indomethacin-PC. We cannot rule out the possibility that
indomethacin or Indomethacin-PC may have a COX-2 inhibitory
component as part of their anticancer mechanism.
It is notable that all of these animal drug doses
were effective at lower levels than are generally associated with
their use for pain and inflammation in man. While it is possible
that anti-inflammatory doses of NSAIDs may differ between mouse and
human, it is also possible that non-COX mechanisms are involved
with this cancer model. This possibility is also supported by the
finding that a COX-2 prostaglandin (ie, PGE2) was not
elevated over control in cancer tissues tested with the MC-26
model. This finding underscores that animal models represent
various aspects of human cancer, and that multiple models are
needed to elucidate a more complete picture of anticancer activity.
While the mechanistic basis of the anti-neoplastic action of
PC-NSAIDs remains to be fully elucidated, it is clear that
PC-NSAIDs, notably Aspirin-PC and Indomethacin-PC may provide an
effective and potentially GI-safer alternative for colon cancer
chemoprevention and possibly treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by NIH grants (grant
nos. R03 CA171613 and R41 CA171408).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
LML directed the full project, reviewed the results
and wrote the paper with EJD. TP was responsible for all the animal
experiments, including animal surgery, animal dosing and collecting
the tissue and blood samples following euthanasia and measuring the
fecal hemoglobin. DF did all the cell culture studies, prepared
PC-NSAIDs for the in vitro studies and performed thromboxane
and prostaglandin analyses by ELISA. EJD prepared MC-26 cells to be
injected into the mice, prepared the PC-associated drugs for animal
studies, directed the animal studies, analyzed the data and was the
lead writer of the paper.
Ethics approval and consent to
participate
Mice were maintained in accordance and compliance
with policies approved by the Animal Welfare Committee, the
Institutional Animal Care and Use Committee (IACUC) for The
University of Texas Health Science Center at Houston (UTHealth),
meeting NIH Guide for the Care and Use of Laboratory Animals. The
institution's animal facility is approved by the PHS and
AAALAC.
Consent for publication
Not applicable.
Competing interests
LML is a co-founder and shareholder in PLx Pharma
Inc., which is developing PC-NSAIDs for commercial use. The
remaining co-authors have no competing interests to report.
Glossary
Abbreviations
Abbreviations:
|
NSAID
|
non-steroidal anti-inflammatory
drug
|
|
PC
|
phosphatidylcholine
|
|
GI
|
gastrointestinal
|
|
AOM
|
azoxymethane
|
|
DSS
|
dextran sodium sulfate
|
|
TXB2
|
thromboxane B2
|
|
COX
|
cyclooxygenase
|
|
PGE2
|
prostaglandin E2
|
References
|
1
|
Harris RE, Beebe-Donk J, Doss H and Burr
Doss D: Aspirin, ibuprofen, and other non-steroidal
anti-inflammatory drugs in cancer prevention: A critical review of
non-selective COX-2 blockade (review). Oncol R. 13:559–583.
2005.
|
|
2
|
Umar A, Steele VE, Menter DG and Hawk ET:
Mechanisms of nonsteroidal anti-inflammatory drugs in cancer
prevention. Semin Oncol. 43:65–77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rothwell PM, Fowkes FG, Belch JF, Ogawa H,
Warlow CP and Meade TW: Effect of daily aspirin on long-term risk
of death due to cancer: Analysis of individual patient data from
randomised trials. Lancet. 377:31–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rothwell PM, Wilson M, Elwin CE, Norrving
B, Algra A, Warlow CP and Meade TW: Long-term effect of aspirin on
colorectal cancer incidence and mortality: 20-year follow-up of
five randomised trials. Lancet. 376:1741–1750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rothwell PM, Wilson M, Price JF, Belch JF,
Meade TW and Mehta Z: Effect of daily aspirin on risk of cancer
metastasis: A study of incident cancers during randomised
controlled trials. Lancet. 379:1591–1601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan AT, Giovannucci EL, Meyerhardt JA,
Schernhammer ES, Wu K and Fuchs CS: Aspirin dose and duration of
use and risk of colorectal cancer in men. Gastroenterology.
134:21–28. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fajardo AM and Piazza GA: Chemoprevention
in gastrointestinal physiology and disease. Anti-inflammatory
approaches for colorectal cancer chemoprevention. Am J Physiol
Gastrointest Liver Physiol. 309:G59–G70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matos P and Jordan P: Beyond
COX-inhibition: ‘Side-effects’ of ibuprofen on neoplastic
development and progression. Curr Pharm Des. 21:2978–2982. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lichtenberger LM, Fang D, Bick RJ,
Poindexter BJ, Phan T, Bergeron AL, Pradhan S, Dial EJ and Vijayan
KV: Unlocking Aspirin's chemopreventive activity: Role of
irreversibly inhibiting platelet cyclooxygenase-1. Cancer Prev Res
(Phila). 10:142–152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parang B, Barrett CW and Williams CS:
AOM/DSS model of colitis-associated cancer. Methods Mol Biol.
1422:297–307. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Byun SY, Kim DB and Kim E: Curcumin
ameliorates the tumor-enhancing effects of a high-protein diet in
an azoxymethane-induced mouse model of colon carcinogenesis. Nutr
Res. 35:726–735. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamaguchi M, Takai S, Hosono A and Seki T:
Bovine milk-derived alpha-lactalbumin inhibits colon inflammation
and carcinogenesis in azoxymethane and dextran sodium
sulfate-treated mice. Biosci Biotechnol Biochem. 78:672–679. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tian Y, Ye Y, Gao W, Chen H, Song T, Wang
D, Mao X and Ren C: Aspirin promotes apoptosis in a murine model of
colorectal cancer by mechanisms involving downregulation of
IL-6-STAT3 signaling pathway. Int J Colorectal Dis. 26:13–22. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yao M, Kargman S, Lam EC, Kelly CR, Zheng
Y, Luk P, Kwong E, Evans JF and Wolfe MM: Inhibition of
cyclooxygenase-2 by rofecoxib attenuates the growth and metastatic
potential of colorectal carcinoma in mice. Cancer Res. 63:586–592.
2003.PubMed/NCBI
|
|
15
|
Yao M, Zhou W, Sangha S, Albert A, Chang
AJ, Liu TC and Wolfe MM: Effects of nonselective cyclooxygenase
inhibition with low-dose ibuprofen on tumor growth, angiogenesis,
metastasis, and survival in a mouse model of colorectal cancer.
Clin Cancer Res. 11:1618–1628. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sandström R, Gelin J and Lundholm K: The
effect of indomethacin on food and water intake, motor activity and
survival in tumour-bearing rats. Eur J Cancer. 26:811–814. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johnson SD and Young MR: Indomethacin
treatment of mice with premalignant oral lesions sustains cytokine
production and slows progression to cancer. Front Immunol.
7:3792016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lundholm K, Daneryd P, Körner U, Hyltander
A and Bosaeus I: Evidence that long-term COX-treatment improves
energy homeostasis and body composition in cancer patients with
progressive cachexia. Int J Oncol. 24:505–512. 2004.PubMed/NCBI
|
|
19
|
Lundholm K, Gelin J, Hyltander A, Lönnroth
C, Sandström R, Svaninger G, Körner U, Gülich M, Kärrefors I, Norli
B, et al: Anti-inflammatory treatment may prolong survival in
undernourished patients with metastatic solid tumors. Cancer Res.
54:5602–5606. 1994.PubMed/NCBI
|
|
20
|
Lichtenberger LM, Barron M and Marathi U:
Association of phosphatidylcholine and NSAIDs as a novel strategy
to reduce gastrointestinal toxicity. Drugs Today (Barc).
45:877–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lim YJ, Phan TM, Dial EJ, Graham DY and
Lichtenberger LM: In vitro and in vivo protection against
indomethacin-induced small intestinal injury by proton pump
inhibitors, acid pump antagonists, or
indomethacin-phosphatidylcholine. Digestion. 86:171–177. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang Y, Lichtenberger LM, Taylor M,
Bottsford-Miller JN, Haemmerle M, Wagner MJ, Lyons Y, Pradeep S, Hu
W, Previs RA, et al: Antitumor and antiangiogenic effects of
Aspirin-PC in ovarian cancer. Mol Cancer Ther. 15:2894–2904. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yao M, Lam EC, Kelly CR, Zhou W and Wolfe
MM: Cyclooxygenase-2 selective inhibition with NS-398 suppresses
proliferation and invasiveness and delays liver metastasis in
colorectal cancer. Br J Cancer. 90:712–719. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lichtenberger LM, Romero JJ and Dial EJ:
Surface phospholipids in gastric injury and protection when a
selective cyclooxygenase-2 inhibitor (Coxib) is used in combination
with aspirin. Br J Pharmacol. 150:913–919. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Burn J, Gerdes AM, Macrae F, Mecklin JP,
Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario L,
et al: Long-term effect of aspirin on cancer risk in carriers of
hereditary colorectal cancer: An analysis from the CAPP2 randomised
controlled trial. Lancet. 378:2081–2087. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Movahedi M, Bishop DT, Macrae F, Mecklin
JP, Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario
L, et al: Obesity, aspirin, and risk of colorectal cancer in
carriers of hereditary colorectal cancer: A prospective
investigation in the CAPP2 study. J Clin Oncol. 33:3591–3597. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Steinbach G, Lynch PM, Phillips RK,
Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y,
Fujimura T, et al: The effect of celecoxib, a cyclooxygenase-2
inhibitor, in familial adenomatous polyposis. N Engl J Med.
342:1946–1952. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dial EJ, Doyen JR and Lichtenberger LM:
Phosphatidylcholine-associated nonsteroidal anti-inflammatory drugs
(NSAIDs) inhibit DNA synthesis and the growth of colon cancer cells
in vitro. Cancer Chemother Pharmacol. 57:295–300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ranger GS: Current concepts in colorectal
cancer prevention with cyclooxygenase inhibitors. Anticancer Res.
34:6277–6282. 2014.PubMed/NCBI
|
|
30
|
Gurpinar E, Grizzle WE and Piazza GA:
NSAIDs inhibit tumorigenesis, but how? Clin Cancer Res.
20:1104–1113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma R, Yi B, Piazza GA and Xi Y:
Mechanistic role of MicroRNA in cancer chemoprevention by
nonsteroidal anti-inflammatory drugs. Curr Pharmacol Rep.
1:154–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tanaka Y, Tanaka T and Ishitsuka H:
Antitumor activity of indomethacin in mice bearing advanced colon
26 carcinoma compared with those with early transplants. Cancer
Res. 49:5935–5939. 1989.PubMed/NCBI
|
|
33
|
Darling RL, Romero JJ, Dial EJ, Akunda JK,
Langenbach R and Lichtenberger LM: The effects of aspirin on
gastric mucosal integrity, surface hydrophobicity, and
prostaglandin metabolism in cyclooxygenase knockout mice.
Gastroenterology. 127:94–104. 2004. View Article : Google Scholar : PubMed/NCBI
|