Introduction
Leukemic cells and normal hematopoietic tissues are
generally more radiosensitive compared with other tissue types
(1–3).
One method of leukemia treatment, the depletion of leukemia cells
by ionizing radiation (IR), exploits this characteristic to restore
a normal hematopoietic system (4,5). However,
IR may induce the production of radioresistant cells, as radiation
induces genetic mutation. The presence of radioresistant cells may
result in a poor prognosis for radiation therapy (6). The properties of radioresistant human
HL60 acute promyelocytic leukemia (APL) (Res-HL60) cells, a cell
line established as an APL model, have been previously reported
(6,7).
It was observed that high-cluster of differentiation
(CD)38-expressing cells were present among the Res-HL60 cells.
However, to the best of our knowledge, the properties of the cell
population exhibiting high CD38 expression have never been
investigated. If the association between CD38 expression and
radioresistance can be determined, key factors and countermeasures
against the production of radioresistant cells in radiation therapy
may be identified.
In the present study, the cell viability and the
expression of CD38 mRNA were evaluated in Res-HL60 APL cells.
Materials and methods
Reagents
Cell culture medium (RPMI-1640) and
penicillin/streptomycin were purchased from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Fetal bovine serum (FBS) was
purchased from Japan BioSerum Co., Ltd. (Hiroshima, Japan).
Phycoerythrin (PE)-conjugated antihuman CD38 (cat. no. IM1832U)
monoclonal antibodies (mAbs) and PE-cyanin-5-forochrome tandem
(PC5)-conjugated antihuman CD45 (cat. no. IM2653) mAbs were
purchased from Beckman Coulter, Inc. (Brea, CA, USA). The
quantification of viable cells was performed using 0.5% trypan blue
liquid solution (Nacalai Tesque, Inc., Kyoto, Japan). In order to
analyze gene expression, the Qiagen RNeasy mini kit (Qiagen, Inc.,
Valencia, CA, USA), the iScript cDNA synthesis kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and the iQ SYBR Green
Supermix (Bio-Rad Laboratories Inc.) were used.
Irradiation
X-ray irradiation (150 kVp, 20 mA, with 0.5 mm
aluminum and 0.3 mm copper filters) was performed using an X-ray
generator (MBR-1520R-3; Hitachi, Ltd., Tokyo, Japan) with a
distance of 45 cm between the focus and the target. The dose was
monitored using a thimble ionization chamber placed next to the
sample during the irradiation. The dose rate was ~1 Gy/min, for a
total dose of 4 Gy.
Cell preparation and cell culture
Human APL wild-type (Wt) HL60 cells was purchased
from RIKEN BioResource Center (Tsukuba, Japan). The Res-HL60 cell
line was previously established by subjecting Wt-HL60 cells to 4 Gy
X-irradiation/week for 4 weeks (6,7). Wt-HL60
and Res-HL60 cells were maintained in RPMI-1640 medium supplemented
with 10% heat-inactivated FBS and 1% penicillin/streptomycin in a
humidified atmosphere at 37°C under 5% CO2.
Flow cytometry
Isolations of CD38+CD45+ cells
and CD38−CD45+ cells and morphological
analysis were performed using fluorescence-activated cell sorting
(FACS) with the Aria SORP (BD Biosciences, Franklin Lakes, NJ,
USA). The analysis software was used BD FACSDiva v.8.0.1 (BD
Biosciences). Samples containing 2×105 cells were
incubated under saturated concentrations (500 ng/ml) of the
relevant mAbs for 30 min at 4°C, followed by washing and flow
cytometric analysis. Isotype-matched mAbs of anti-CD38
(PE-conjugated anti-mouse IgG1 mAb; cat. no. A07796; 500 ng/ml;
Beckman Coulter, Inc.) and anti-CD45 (PC5-conjugated anti-mouse
IgG1 mAb; cat. no. A07798; 200 ng/ml; Beckman Coulter, Inc.) were
used as negative controls. The threshold values of mean
fluorescence intensity for negative-, low-, medium- and
high-expression groups for CD38 antigen were defined as <0.7,
0.7–2, 2–10 and >10, respectively.
Clonogenic surviving fraction
X-irradiated Wt-HL60 and Res-HL60 cells were
cultured using the plasma clot technique, as previously described
(8), at 37°C in a humidified
atmosphere containing 5% CO2. On the seventh day from
the start of irradiation, colonies consisting of >50 cells were
counted under an inverted optical microscope.
Total RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The cells were harvested after 2 days, and total RNA
was extracted using the RNeasy kit and quantified using a NanoDrop
system (Thermo Fisher Scientific, Inc., Wilmington, DE, USA). The
total RNA quality was confirmed using a 2100 Bioanalyzer (Agilent
Technologies Inc., Santa Clara, CA, USA), and first-strand cDNA
were synthesized using the iScript cDNA Synthesis kit according to
the manufacturer's protocol. The expression of mRNA was then
evaluated using qPCR with the iQ SYBR Green Supermix (Bio-Rad
Laboratories, Inc.) and a SmartCycler® II (Takara Bio
Inc., Otsu, Japan) with the following thermocycler conditions: 95°C
for 170 sec, followed by 40 cycles of 95°C for 10 sec, 65°C for 20
sec and 72°C for 30 sec. Relative levels of CD38, CD45 and
CCAAT/enhancer-binding protein α (CEBPA) was determined using the
2−ΔΔCq method (9) in cells
subjected to 4 Gy X-irradiation after 24 h of no irradiation,
subsequent to normalization with the housekeeping gene GAPDH. The
oligonucleotide primer sets used for RT-qPCR were purchased from
Hokkaido System Science Co., Ltd. (Sapporo, Japan; Table I).
 | Table I.National Center for Biotechnology
Information gene accession numbers and sequences of synthetic human
oligonucleotide polymerase chain reaction primers. |
Table I.
National Center for Biotechnology
Information gene accession numbers and sequences of synthetic human
oligonucleotide polymerase chain reaction primers.
| Primer name | Accession number | Sequence | Size, bp |
|---|
| CD38 forward | NM_001775 |
5′-CAGCAACAACCCTGTTTCAGT-3′ | 21 |
| CD38 reverse |
|
5′-CCATTGAGCATCACATGGAC-3′ | 20 |
| CD45 forward | NM_002838 |
5′-CCAATGCAAAACTCAACCCTA-3′ | 21 |
| CD45 reverse |
|
5′-CCTCTCTCCTGGGACATCTG-3′ | 20 |
| CEBPA forward | NM_004364 | 5′-
CAGCATTGCCTAGGAACACGAA-3′ | 22 |
| CEBPA reverse |
|
5′-CACAGAGGCCAGATACAAGTGTTGA-3′ | 25 |
| GAPDH forward | NM_002046 |
5′-ACACCCTGGCCTACGCTAAAGAC-3′ | 23 |
| GAPDH reverse |
|
5′-AGCCCAAGCATCAAAGATGGAG-3′ | 22 |
Statistical analysis
Statistical analysis was performed using
OriginLab® Pro Version 9.0 for Windows (OriginLab,
Northampton, MA, USA). Two-way factorial analysis of variance
followed by Tukey's range post-hoc test was used to assess the
statistical significance of differences between the control and
experimental groups. Data from two groups were analyzed using an
unpaired Student's t-test. Data are presented as the mean ±
standard error of the mean. A total of 4 individual experiments
were performed. P<0.05 was considered to indicate a
statistically significant difference.
Results
Analysis of cell viability in
CD38+ cells
To clarify the morphological character of Res-HL60
cells, Res-HL60 cells were analyzed using FACS in a previous study;
smaller cells with lower granularity were observed in Res-HL60
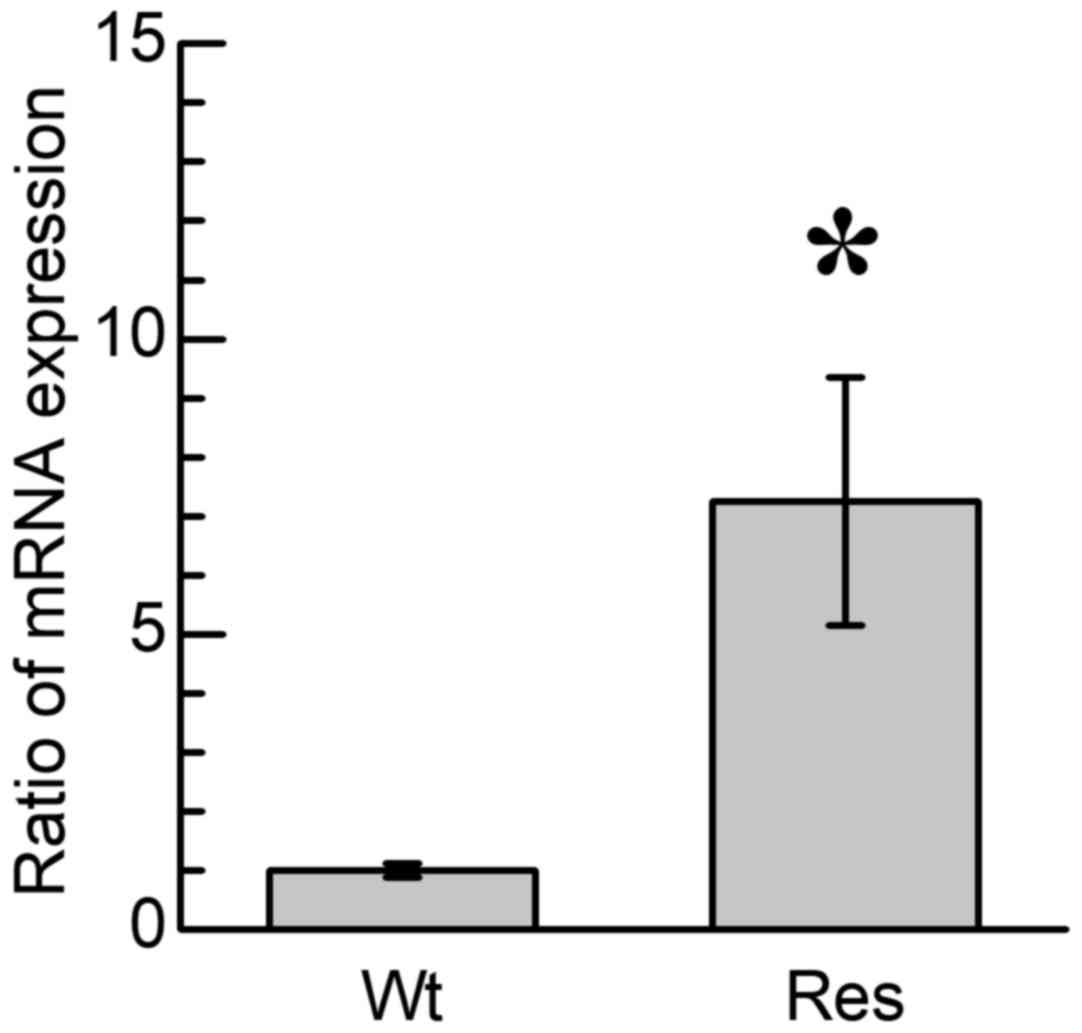
cells in comparison with Wt-HL60 cells (6). In addition, a significantly higher
expression of CD38 protein on the surface of Res-HL60 cells
compared with Wt-HL60 cells was observed (6), which concurs with the results of the
present study, in which CD38 mRNA expression was significantly
increased in the Res-HL60 cells compared with the Wt-HL60 cells
(P<0.05; Fig. 1). In previous
studies a higher rate of cell proliferation was also observed
(6,7).
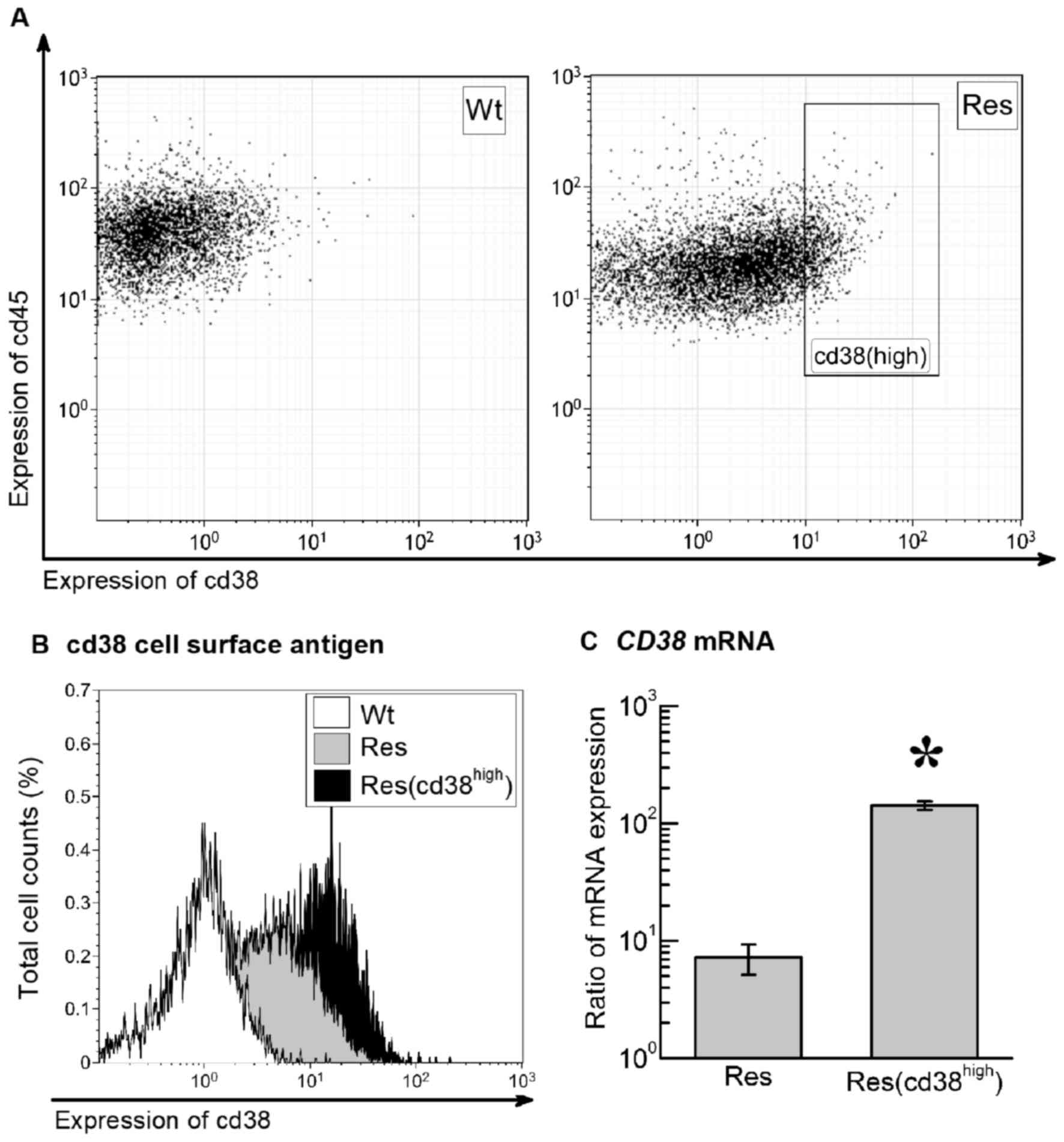
The CD45 leukocyte-specific antigen was expressed in
all Wt-HL60 and Res-HL60 cells. The CD38+ cell
proportion of Wt-HL60 and Res-HL60 cells was 35.2 and 67.9%
respectively (Fig. 2A and B). On the
basis of these data, to confirm whether radiosensitivity is
associated with CD38 expression level, the fraction with the
highest 15% expression of the CD38 antigen in Res-HL60 cells
(CD38high) were isolated using FACS (Fig. 2B). RT-qPCR was used to confirm the
high CD38 expression; the CD38 mRNA expression in the
Res-HL60-CD38high cells was ~37-fold higher than the
non-isolated Res-HL60 cells, representing a significant difference
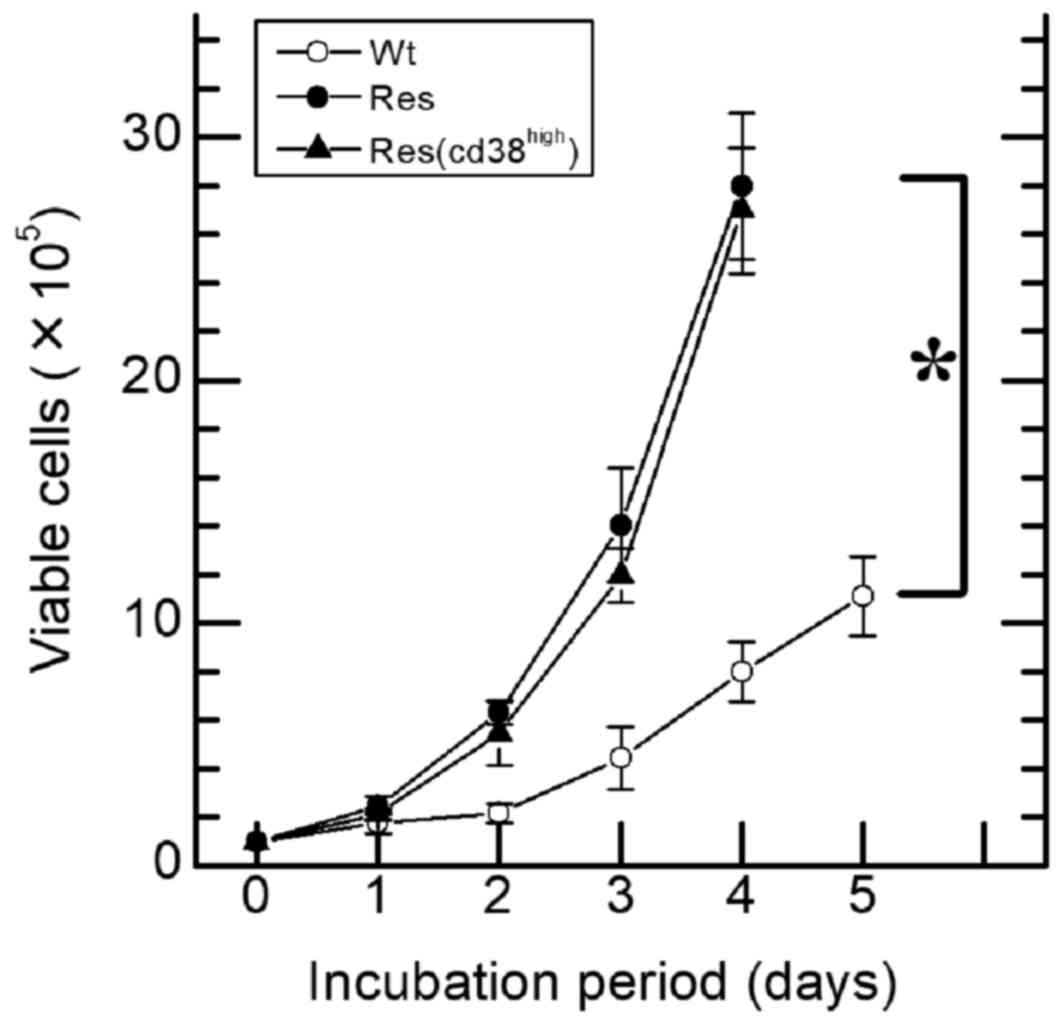
(P<0.05; Fig. 2C). A 4.4±1.3-fold
increase in the number of viable Wt-HL60 cells was observed on day
3 in comparison with day 0 (Fig. 3),
whereas the number of viable Res-HL60 cells underwent a ~14.0-fold
increase by day 3. There was a significant increase in the number
of viable cells between Wt and either Res or
Res-CD38high cells (P<0.01; Fig. 3). However, viability and morphology
(data not shown) did not differ between CD38+/− and
CD38high in the Res-HL60 cells.
Cellular response to exposure to 4 Gy
X-irradiation
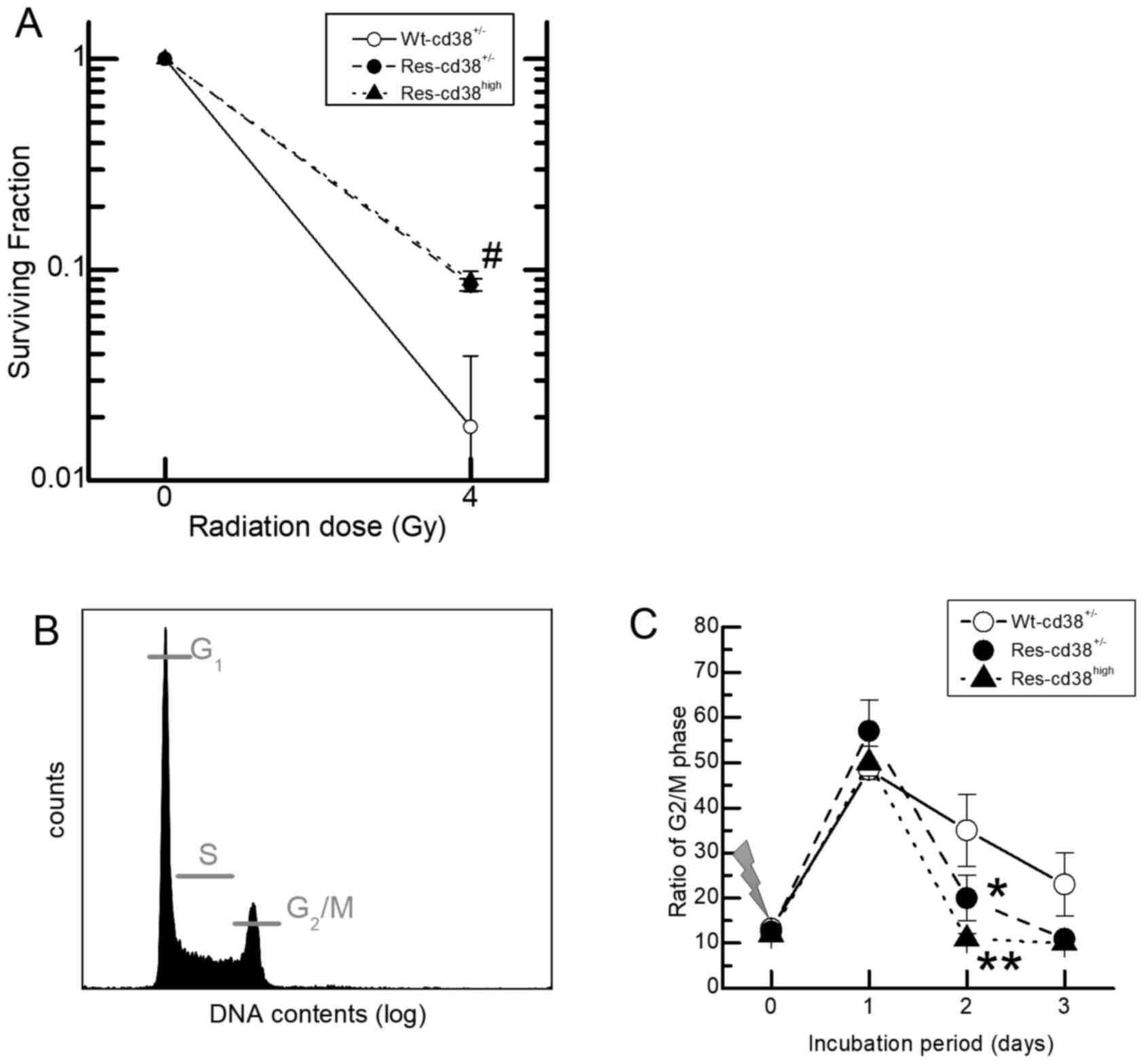
The survival rates of Res-HL60 cells exposed to 4 Gy
X-irradiation were estimated for the CD38-expressing fraction with
a clonogenic survival assay, with the aim of determining the
association between the expression of the CD38 antigen and the
survival rate following radiation exposure. The survival rate for
Wt-HL60 cells exposed to 4 Gy X-irradiation was ~2%, which was
significantly lower than the surviving fraction of Res-HL60 cells,
~10% (P<0.05; Fig. 4A). However,
the Res-CD38high cells exhibited a similar sensitivity
to Res-CD38low/− cells. In order to investigate the
association between the survival rate and the cell cycle
distribution, the DNA content of each cell type was analyzed using
flow cytometry following the exposure to 4 Gy X-irradiation, to
determine the relative fraction of the cell population in
G2/M phase (Fig. 4B). A
higher ratio of cells arrested at G2/M phase on day 1 in
each cell type was observed compared with day 0, which was
relatively decreased on days 2 and 3 (Fig. 4C). The proportion of cells in
G2/M phase on day 2 was reduced in
Res-HL60-CD38high cells in comparison with
Wt-HL60-CD38+/−cells (P<0.01) and
Res-HL60-CD38low/− cells (P<0.05).
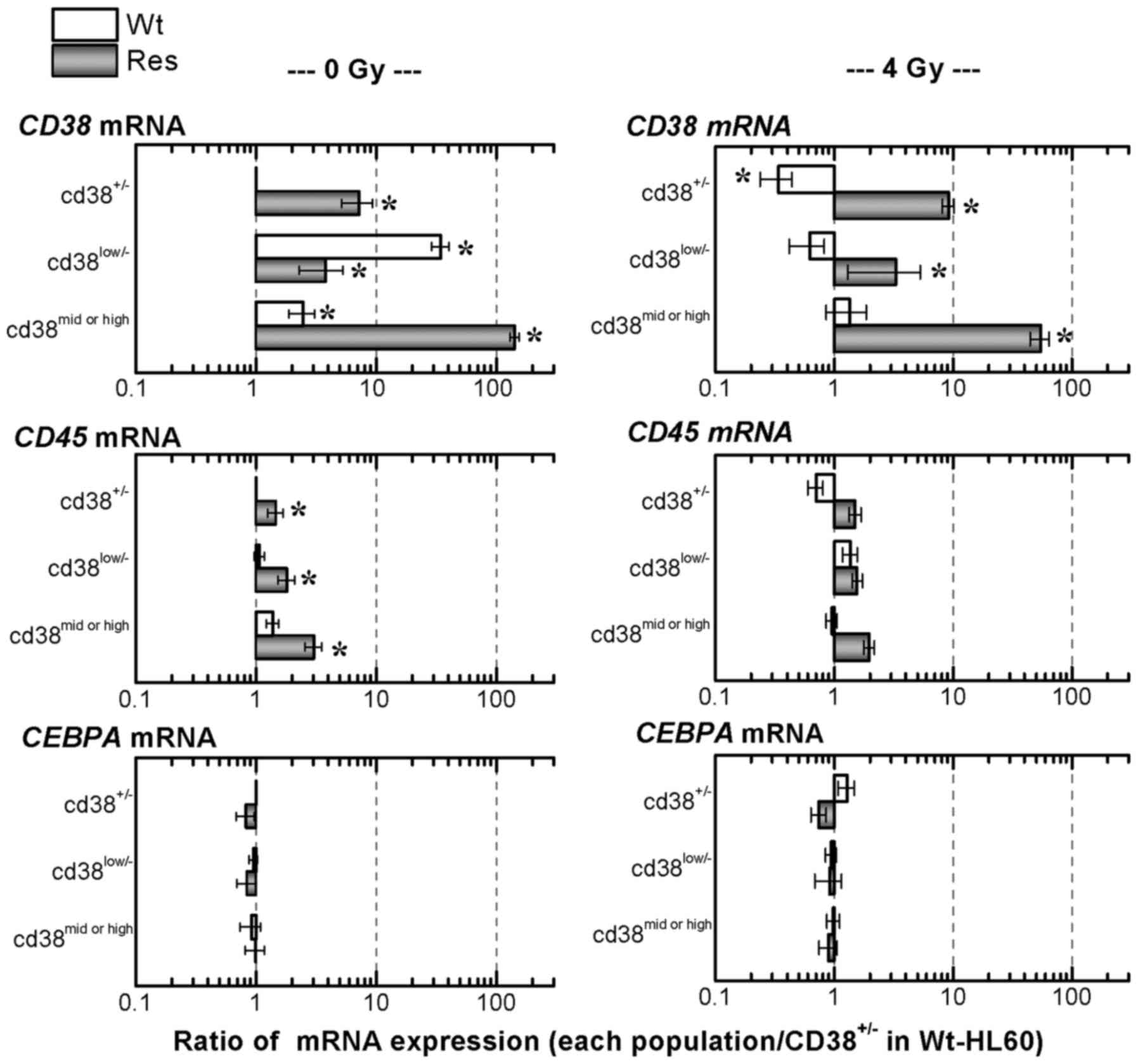
The expression levels of mRNAs associated with
cell-surface antigens were evaluated using RT-qPCR (Fig. 5). The expression of the CD38 mRNA in
CD38low/− and CD38mid/high Wt-HL60 cells
underwent 34.6- and 2.5-fold increases, respectively, in comparison
with unexposed Wt-HL60 cells. In CD38+/−,
CD38low/− and CD38mid/high Res-HL60 cells,
7.2-fold, 3.8-fold and 142.7-fold increases of CD38 mRNA
expression, respectively, were observed compared with unexposed
Wt-HL60 cells; the difference in the CD38 mRNA expression in
Res-HL60-CD38high cells was particularly remarkable.
Additionally, a higher CD45 mRNA expression level in
CD38+/−, CD38low/− and
CD38mid/high Res-HL60 cells was observed. Significant
differences in the CEBPA mRNA expression, a specific marker of
mature white blood cells, were not observed in CD38+/−,
CD38low/− and CD38mid/high Res-HL60 cells
compared with Wt-HL60 cells.
Following the exposure of 4 Gy X-irradiation, the
expression of CD38 mRNA in Res-HL60 cells was significantly
increased in comparison with unexposed Wt-HL60 cells, and the CD38
mRNA expression in Res-CD38mid/high cells was ~50-fold
upregulated (Fig. 5). In contrast,
CD45 and CEBPA mRNA expression were similarly expressed in each
cell type.
Discussion
The present study focused on the behavior of
Res-HL60 APL cells expressing the CD38 cell-surface antigen.
Res-HL60 cells expressing high levels of the CD38 antigen were
identified and quantified. Consistent with a previous report
(7), viability of Res-HL60 cells was
higher compared with Wt-HL60 cells, independent of the expression
of the CD38 antigen (Fig. 3). At the
gene expression level, a higher expression of CD38 mRNA in Res-HL60
cells was observed, particularly in CD38high cells
(Fig. 4). However, no significant
increase or suppression of CEBPA mRNA was observed. These results
reveal that the human radioresistant APL cell line previously
established by repeated exposure to IR continued to express the
CD38 cell-surface antigen and its mRNA; however, this expression
was not associated with the capability for cell viability between
subpopulations of radioresistant cells.
CD38 is a novel multifunctional ectoenzyme that is
widely expressed in cells and tissues, most notably in leukocytes.
The synthesis and hydrolysis of cyclic ADP ribose (cADPR) by CD38
is a marker of human leukocyte differentiation (10), and in the context of hematopoietic
progenitors, CD34+CD38− cells are more basal
than CD34+CD38+ cells (11). CD45 is a major high-molecular-mass
leukocyte cell-surface molecule (12). CEBPA is essential for normal
granulopoiesis, and the dominant-negative mutations of the CEBPA
gene have been identified in a large proportion of the malignant
cells from patients with myeloblastic subtypes of acute myeloid
leukemia (13). The results of the
present study suggest that the Res-HL60 cells that express the CD38
antigen are at a similar differentiation stage to CD38−
cells, given that the expression of CD45 and CEBPA did not
significantly differ between them.
The expression of the CD38 antigen in Res-HL60 cells
did not affect the cell viability (Fig.
3) or clonogenic survival (Fig.
4A). However, the CD38high cells were less likely to
arrest in G2/M phase on day 2 after IR exposure
(Fig. 4C). The accumulation of cells
in G2/M phase is associated with the repair of cellular
damage or the induction of apoptosis (14). It was previously reported that the
phosphorylation of H2A histone family, member X (H2AX), Checkpoint
kinase 1/2 and DNA-dependent protein kinase, catalytic subunit was
suppressed in Res-HL60 cells in comparison with Wt-HL60 cells
(7). Fernandez-Capetillo et al
(15) reported that G2
checkpoint activation was induced by H2AX and tumor suppressor
p53-binding protein 1 phosphorylation, markers of DNA damage. In
the present study, Res-HL60 cells expressing higher levels of the
CD38 antigen exposed to X-irradiation accumulated in
G2/M phase on day 2–3 at a lesser rate to other cell
groups, suggesting that CD38 expression in Res-HL60 cells
influences the G2/M checkpoint of the cell cycle
directly or indirectly.
CD38/cADPR signaling is an important metabolic
pathway for insulin secretion following glucose stimulation to
intracellular ATP, and activates a range of cell behaviors
(16,17). Thus, we hypothesize that the
radiosensitivity in HL60 cells with a higher expression of CD38 is
associated with the intracellular energy metabolism, including that
of glucose-ATP, and does not induce differentiation. The exposure
of Res-HL60 cells to 4 Gy X-irradiation induced a higher expression
of CD38 mRNA compared with non-exposed cells, with notably higher
expression in the CD38high cell fraction (Fig. 5). The constant expression of CD38 in
Res-HL60 cells may be the result of a mutation inducing CD38 mRNA
expression following repeated exposure to ionizing radiation.
X-irradiation, which is low-energy transfer radiation, produces
reactive oxygen species (ROS), which cause DNA damage (18). Tessitore et al (19) reported that the microRNA associated
with DNA repair are necessary for cancer maintenance. Thus,
transcription factors directly and/or indirectly regulating the
promoter region for CD38 should be identified (20). In a hematological malignancy model,
Yalçintepe et al (21)
reported that CD38 participates in the mechanism of drug resistance
to chemotherapy. There is a possibility that CD38/cADPR signaling
protects from various extracellular stresses to promote
radioresistance or anti-cancer drug resistance in leukemia. We
hypothesize that CD38 mRNA expression protects the cell from
DNA-damaging ROS, as are induced by X-irradiation. However, the
present study had limitations, including that the interaction
between CD38/cADPR signaling, the CD38 gene network and cell damage
by ROS was not evaluated.
Given that the X-irradiation exposure of
CD38high Res-HL60 cells did not influence their
differentiation level, the suppression of the monocyte lineage
induction by Res-HL60 cells, may not directly involve CD45 and
CEBPA mRNA (6). There is little
information about radioresistant leukemia in clinical literature.
The results of the present study may suggest countermeasures
against radioresistant leukemia.
In conclusion, the accumulation of the CD38 protein
in radioresistant APL, induced by the constant expression of CD38
mRNA following X-irradiation, is a characteristic response of the
radioresistant-surviving fraction; however, the accumulated volume
of CD38 protein was not observed to influence the extent of
radioresistant behavior.
Acknowledgements
The present study was supported by KAKENHI,
Grant-in-Aid for Scientific Research (C; General; grant no.,
16K10339 SM) and the Grant for Hirosaki University Young
Institutional Research (2013–2014).
References
|
1
|
Ariyoshi K, Takabatake T, Shinagawa M,
Kadono K, Daino K, Imaoka T, Kakinuma S, Nishimura M and Shimada Y:
Age dependence of hematopoietic progenitor survival and chemokine
family gene induction after gamma irradiation in bone marrow tissue
in C3H/He mice. Radiat Res. 181:302–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Monzen S, Yoshino H, Kasai-Eguchi K and
Kashiwakura I: Characteristics of myeloid differentiation and
maturation pathway derived from human hematopoietic stem cells
exposed to different linear energy transfer radiation types. PLoS
One. 8:e593852013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haro KJ, Scott AC and Scheinberg DA:
Mechanisms of resistance to high and low linear energy transfer
radiation in myeloid leukemia cells. Blood. 120:2087–2097. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Berranger E, Cousien A, Petit A,
Peffault de Latour R, Galambrun C, Bertrand Y, Salmon A, Rialland
F, Rohrlich PS, Vannier JP, et al: Impact on long-term OS of
conditioning regimen in allogeneic BMT for children with AML in
first CR: TBI+CY versus BU+CY: A report from the Société Française
de Greffe de Moelle et de Thérapie Cellulaire. Bone Marrow
Transplant. 49:382–388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Willemze AJ, Geskus RB, Noordijk EM, Kal
HB, Egeler RM and Vossen JM: HLA-identical haematopoietic stem cell
transplantation for acute leukemia in children: less relapse with
higher biologically effective dose of TBI. Bone Marrow Transplant.
40:319–327. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Monzen S, Takimura K, Kashiwakura I and
Hosokawa Y: Acute promyelocytic leukemia mutated to radioresistance
suppressed monocyte lineage differentiation by phorbol 12-myristate
13-acetate. Leuk Res. 37:1162–1169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hazawa M, Hosokawa Y, Monzen S, Yoshino H
and Kashiwakura I: Regulation of DNA damage response and cell cycle
in radiation-resistant HL60 myeloid leukemia cells. Oncol Rep.
28:55–61. 2012.PubMed/NCBI
|
|
8
|
Kashiwakura I, Kuwabara M, Inanami O,
Murakami M, Hayase Y, Takahashi TA and Takagi Y: Radiation
sensitivity of megakaryocyte colony-forming cells in human
placental and umbilical cord blood. Radiat Res. 153:144–152. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan JS, Wang D and Stewart CN Jr:
Statistical methods for efficiency adjusted real-time PCR
quantification. Biotechnol J. 3:112–123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takasawa S, Tohgo A, Noguchi N, Koguma T,
Nata K, Sugimoto T, Yonekura H and Okamoto H: Synthesis and
hydrolysis of cyclic ADP-ribose by human leukocyte antigen CD38 and
inhibition of the hydrolysis by ATP. J Biol Chem. 268:26052–26054.
1993.PubMed/NCBI
|
|
11
|
Petzer AL, Hogge DE, Landsdorp PM, Reid DS
and Eaves CJ: Self-renewal of primitive human hematopoietic cells
(long-term-culture-initiating cells) in vitro and their expansion
in defined medium. Proc Natl Acad Sci USA. 93:pp. 1470–1474. 1996;
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Charbonneau H, Tonks NK, Walsh KA and
Fischer EH: The leukocyte common antigen (CD45): A putative
receptor-linked protein tyrosine phosphatase. Proc Natl Acad Sci
USA. 85:pp. 7182–7186. 1988; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pabst T, Mueller BU, Harakawa N, Schoch C,
Haferlach T, Behre G, Hiddemann W, Zhang DE and Tenen DG: AML1-ETO
downregulates the granulocytic differentiation factor C/EBPalpha in
t(8;21) myeloid leukemia. Nat Med. 7:444–451. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamamori T, Yasui H, Yamazumi M, Wada Y,
Nakamura Y, Nakamura H and Inanami O: Ionizing radiation induces
mitochondrial reactive oxygen species production accompanied by
upregulation of mitochondrial electron transport chain function and
mitochondrial content under control of the cell cycle checkpoint.
Free Radic Biol Med. 53:260–270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fernandez-Capetillo O, Chen HT, Celeste A,
Ward I, Romanienko PJ, Morales JC, Naka K, Xia Z, Camerini-Otero
RD, Motoyama N, et al: DNA damage-induced G2-M checkpoint
activation by histone H2AX and 53BP1. Nat Cell Biol. 4:993–997.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takasawa S, Nata K, Yonekura H and Okamoto
H: Cyclic ADP-ribose in insulin secretion from pancreatic beta
cells. Science. 259:370–373. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guedes AG, Rude EP and Kannan MS:
Potential role of the CD38/cADPR signaling pathway as an underlying
mechanism of the effects of medetomidine on insulin and glucose
homeostasis. Vet Anaesth Analg. 40:512–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamaguchi M and Kashiwakura I: Role of
reactive oxygen species in the radiation response of human
hematopoietic stem/progenitor cells. PLoS One. 8:e705032013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tessitore A, Cicciarelli G, Del Vecchio F,
Gaggiano A, Verzella D, Fischietti M, Vecchiotti D, Capece D,
Zazzeroni F and Alesse E: MicroRNAs in the DNA damage/repair
network and cancer. Int J Genomics. 2014:8202482014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun L, Iqbal J, Zaidi S, Zhu LL, Zhang X,
Peng Y, Moonga BS and Zaidi M: Structure and functional regulation
of the CD38 promoter. Biochem Biophys Res Commun. 341:804–809.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yalçintepe L, Halis E and Ulku S: Effect
of CD38 on the multidrug resistance of human chronic myelogenous
leukemia K562 cells to doxorubicin. Oncol Lett. 11:2290–2296. 2016.
View Article : Google Scholar : PubMed/NCBI
|