Introduction
Breast cancer is a significant worldwide health
problem in women and accounts for 12% of all cancers with 1.7
million newly diagnosed cases and 6% of all cancer deaths globally
in 2012 (https://www.cdc.gov/cancer/international/statistics-htm.
Retrieved on March 30, 2017). Fortunately, decades-long advancement
in breast cancer treatment and prevention lead to dramatic
improvement of breast cancer patient's survival. According to St
Gallen Conference 2013, breast cancer can be molecularly classified
into different subtypes, including luminal A (ER and PR-positive,
low rate of Ki-67 and HER2-negative) and luminal B, luminal B1,
HER2-negative (ER-positive, PR <10% or negative, high rate of
Ki-67), luminal B2 HER2-positive (ER-positive and PR-negative),
HER2-positive non-luminal (ER and PR-negative) and basal-like (ER,
PR and HER2-negative) (1). The
receptors-positive breast cancer is usually cured with
anti-receptor therapy (e.g., tamoxifen treatment for ER+ breast
cancer, while Trastuzumab for HER2+ breast cancer
(2–4).
The basal-like subtype, also known as triple negative breast cancer
(TNBC), is commonly negative for ER, PR and HER2. However, TNBC,
represents 15% of all breast cancer, often occurs in young women
and 80–90% of TNBC is an aggressive invasive ductal carcinoma
(5), while 15–30% of TNBC patients
will eventually develop tumor brain metastasis (6). To date, there is no effective therapy
for TNBC and poor survival is common in TNBC compared to other
breast cancer subtypes. Thus, effective therapy that prolongs or
improves survival of TNBC patients is needed.
Lenalidomide is a derivative of thalidomide, which
is an immunomodulatory drug. Lenalidomide has been reported to
possesses anti-angiogenic and immunomodulatory effects (7–10) and was
approved by the US FDA as the first-line therapy in multiple
myeloma (11). Recent clinical trials
have demonstrated the antitumor effect of lenalidomide in different
solid tumors, such as hepatocellular cancer (12) and colorectal cancer (13). In addition to the anti-angiogenic and
immunomodulatory properties, lenalidomide has been shown to have
cytotoxic effect in tumor cells (13). In clinical trials, lenalidomide was
either used as a single agent or in combination with other
chemotherapeutic drugs in many solid tumors; for example, Said
et al showed that lenalidomide in combination with
5-fluorouracil, leucovorin, and oxaliplatin was able to control
advanced colorectal cancer (13),
while Safran et al assessed the activity and efficacy of
lenalidomide on advanced hepatocellular cancer that was previously
treated with sorafenib (14), where
six out of 40 patients (15%) achieved a partial response, including
two patients (5%) had stable disease for more than 32 months
(14). These data indicate that
lenalidomide could be more effective in combination with
chemotherapeutic drugs, such as gemcitabine or docetaxel (12,15).
Furthermore, Brosseau et al (16) demonstrated that lenalidomide was able
to inhibit proliferation of MDA-MB-231 cells through the
restoration of vitamin D sensitive phenotype. Wu et al
(12) also showed that combination of
lenalidomide with gemcitabine improved survival of pancreatic
cancer patients. Thus, in the present study, we will investigate
the therapeutic effect of lenalidomide on TNBC cells in combination
with cisplatin, a frequently used chemotherapeutic drug for TNBC.
The concentration of drug required to reduce cell viability by 50%
(IC50) was calculated using Compusyn software (ComboSyn,
Inc., Paramus, NJ, USA) (17). We
expected that lenalidomide could reduce of cisplatin, reducing
cisplatin IC50 value, inducing tumor cells to undergo
apoptosis, and inhibiting angiogenesis in triple-negative breast
cancer MDA-MB-231 cells in vitro.
Materials and methods
Reagents and cell culture
Lenalidomide was purchased from the AMQUAR
Corporation (cat. no. EY0006; Denver, CO, USA), while cisplatin was
obtained from MCE Corporation (cat. no. HY-17394; Dublin, CA, USA).
Lenalidomide was dissolved in dimethyl sulfoxide (DMSO) at 10 mM
stock solution, stored at −20°C, and used within a week. Cisplatin
was dissolved in distilled water at 10 mM stock solution and stored
at 4°C.
A human triple-negative breast cancer line
MDA-MB-231 was obtained from the Chinese Academy of Sciences
(Shanghai, China) and maintained in Dulbecco's modified Eagle's
medium (Thermo-Fisher, Waltham, MA, USA) supplemented with 10%
fetal bovine serum and 1% penicillin-streptomycin (both from Gibco,
Gaithersburg, MD, USA) in a humidified incubator with 5%
CO2 at 37°C.
Cell viability MTT assay
Cell viability was measured using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) assay. In brief,
MDA-MB-231 cells were seeded into 96-well plates at a density of
5×103 per well and cultured overnight. On the next day,
cells were treated with various concentrations of cisplatin
(0.12–30 µM), lenalidomide (1.25–320 µM) or their combination in
100 µl volumes of growth medium for 72 h at 37°C. After that, 10 µl
of MTT was added to each well and further incubated for 4 h.
Further, the medium was replaced with 150 µl of DMSO in each well
and thoroughly mixed and then the optical absorbance value was
measured at 570 nm using a plate reader (Eppendorf, Hamburg,
Germany). The control cells were treated with 100 µl of
phosphate-buffered saline (PBS) or 1% of DMSO. The IC50
values of cisplatin, lenalidomide, and their combination were
calculated using the Compusyn software version 1.0 (ComboSyn, Inc.,
Paramus, NJ, USA) as described previously (17).
Flow cytometric apoptosis assay
To assess cell apoptosis, MDA-MB-231 cells were
seeded into 6-well plates at a density of 1×105 per
well. After 24 h culture, cells were treated with cisplatin (3 µM),
lenalidomide (1 µM), or their combination for 72 h. Cells were then
collected in ice-cold PBS, and apoptotic cells were detected using
FITC Annexin V-FITC/PI Apoptosis Detection kit (cat. no. 4A;
Biotech Co., Ltd., Beijing, China) according to the manufacturer's
protocol. The stained cells were then measured with a flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Protein extraction and western blot
analysis
MDA-MB-231 cells were seeded into 6-well plates and
they were let grow up to 80% confluency. The medium was replaced
with 3 µM cisplatin, 1 µM lenalidomide or their combination for 72
h. Total cellular protein was then extracted from cells using the
immunoprecipitation assay lysis buffer and protein concentrations
were assessed using bicinchoninic acid (BCA) kit (Cwbiotech,
Beijing, China). Protein samples (20 µg) were heated at 100°C for
10 min and then separated in 10% Tris-Tricine sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel and
transferred onto a polyvinylidenedifluoride (PVDF) membrane (EMD
Millipore, Billerica, MA, USA). For western blotting, the membranes
were blocked for 2 h at the room temperature in 5% bovine serum
albumin (BSA) and then incubated with primary antibodies against
B-cell lymphoma-2 (Bcl-2) (1:1,000), caspase-3 (1:1,000), cleaved
poly-adenosine diphosphate-ribose polymerase (cPARP) (1:1,000)
(Proteintech Group, Inc., Cambridge, MA, USA), phosphorylated and
total extracellular signal-regulated kinase (ERK) (1:1,000; Cell
Signaling Technology, Inc., Shanghai, China), basic fibroblast
growth factor (bFGF) (1:1,000) or vascular endothelial growth
factor (VEGF) (1:1,000) (both from Affinity Biosciences,
Cincinnati, OH, USA) for 12 h at 4°C. On the next day, the
membranes were washed with Tris-based saline-Tween-20 solution
(TBST) for three times and then incubated with a secondary antibody
at a dilution of 1:1,000 (EMD Millipore) for 1 h at room
temperature. Protein bands were visualized using enhanced
chemiluminescence (ECL)-Plus Western blotting detection reagents
(Millipore).
Statistical analysis
All experiments were repeated at least three times
and the data were expressed as mean ± standard deviation. All
statistical analyses were performed by using SPSS 19.0 software
(IBM Corp., Armonk, NY, USA). Student's t-test was used to analyze
the mean difference between two groups, while comparisons of the
means among multiple groups were analyzed using one-way analysis of
variance followed by the post-hoc Student-Newman-Keuls test. The
IC50 and combination index values were calculated using
Compusyn software version 1.0 (ComboSyn, Inc.). A P-value equal to
or <0.05 was considered statistically significant.
Results
Lenalidomide enhanced the cytotoxic
effect of cisplatin on MDA-MB-231 cells
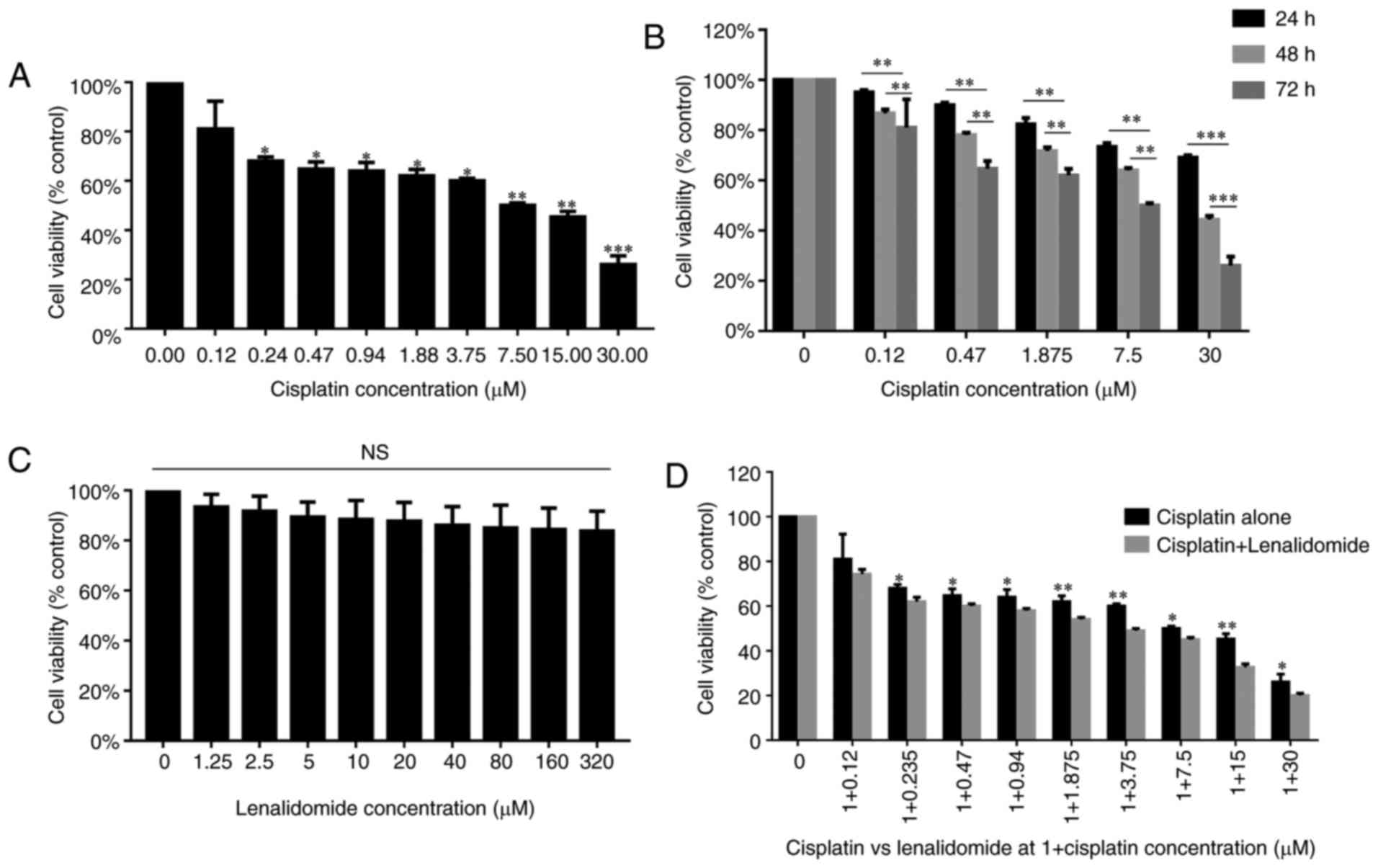
We first assessed the effects of lenalidomide and
cisplatin combination on MDA-MB-231 cell viability using the MTT
sassy. Our results showed that cisplatin had a dramatic effect on
the viability of MDA-MB-231 cells with an IC50 of 7.8 µM
(Fig. 1A); in addition, MDA-MB-231
cells viability was decreased with increasing the incubation time
from 24 to 72 h. At 72 h, MDA-MB-231 cell viability was decreased
to the minimum (Fig. 1B). However,
lenalidomide treatment alone had a minimal effect on MDA-MB-231
cell viability, up to 320 µM (Fig.
1C). The maximum concentration of lenalidomide that could be
safely used in patients is 1 µM; thus, we combined lenalidomide at
1 µM with nine concentrations of cisplatin in MDA-MB-231 cells. As
a result, cell viability was dramatically reduced in combined group
as compared to cisplatin alone (Fig.
1D). The effect of drug combination was assessed by Compusyn
software. IC50 is the major evaluation index in Compusyn
software. The IC50 of cisplatin was reduced to 3.0 µM in
combined treatment group. Therefore, this concentration was further
applied in apoptosis experiments.
Combination of lenalidomide and
cisplatin increased cell apoptosis in MDA-MB-231 cells
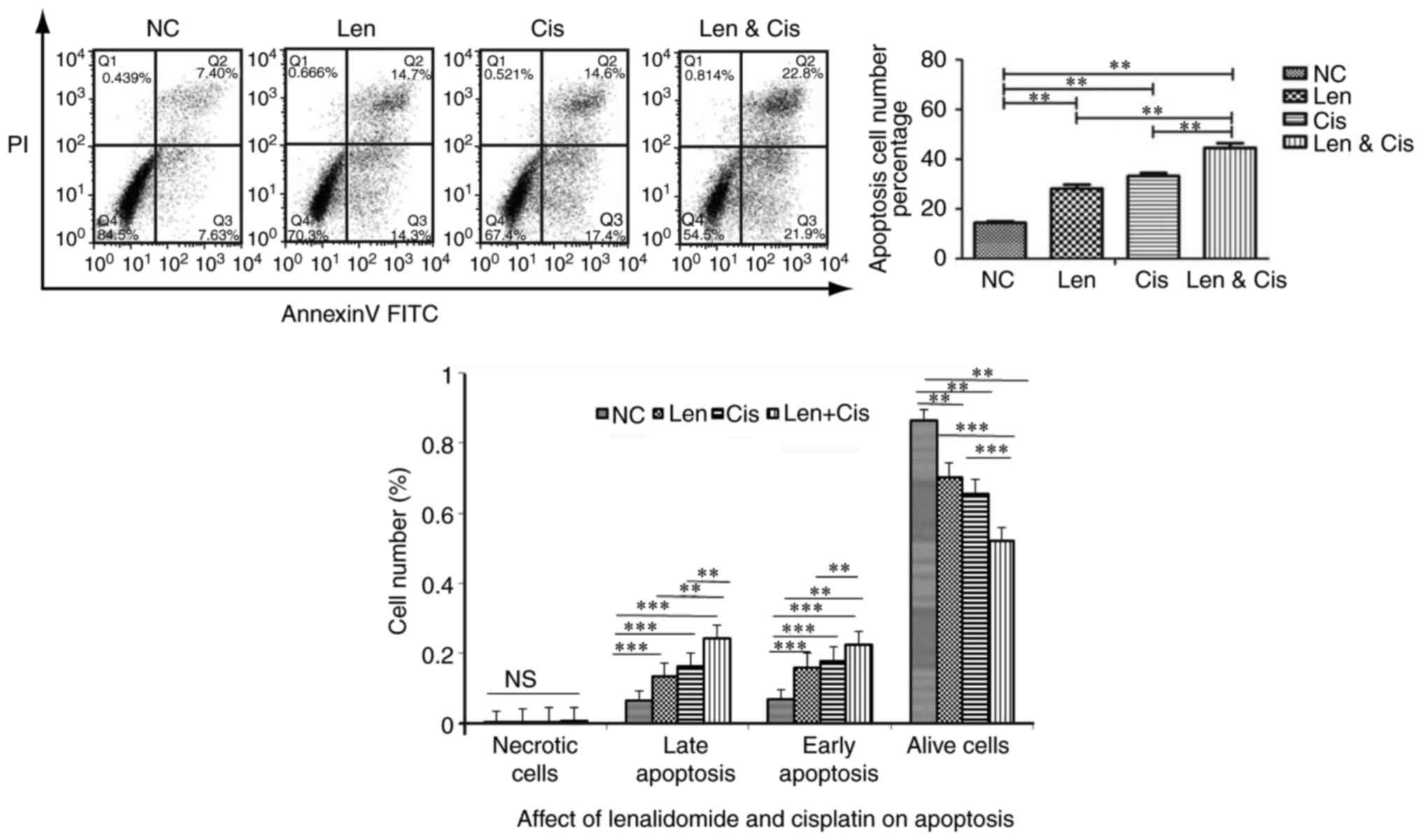
We then treated MDA-MB-231 cells with 1 µM
lenalidomide, 3.0 µM cisplatin, or combination and cell apoptosis
was measured using FITC Annexin V-FITC/PI apoptosis detection kit.
Our results demonstrated that lenalidomide and cisplatin alone
induced cell apoptosis (P<0.05). In addition, their combination
significantly increased cell apoptosis by 1.60 and 1.38-folds
compared to lenalidomide and cisplatin single drug treatment
(P<0.01) (Fig. 2). The combination
treatment increased the rate of early apoptosis by 1.41 and
1.27-folds as well as the rate of later apoptosis by 1.80 and
1.51-folds compared to lenalidomide and cisplatin treatments,
respectively. This suggested that lenalidomide and cisplatin
combination reduces MDA-MB-231 cell viability through the induction
of cell apoptosis.
Changes in protein expression after
lenalidomide, cisplatin, or their combination treatment of TNBC
cells in vitro
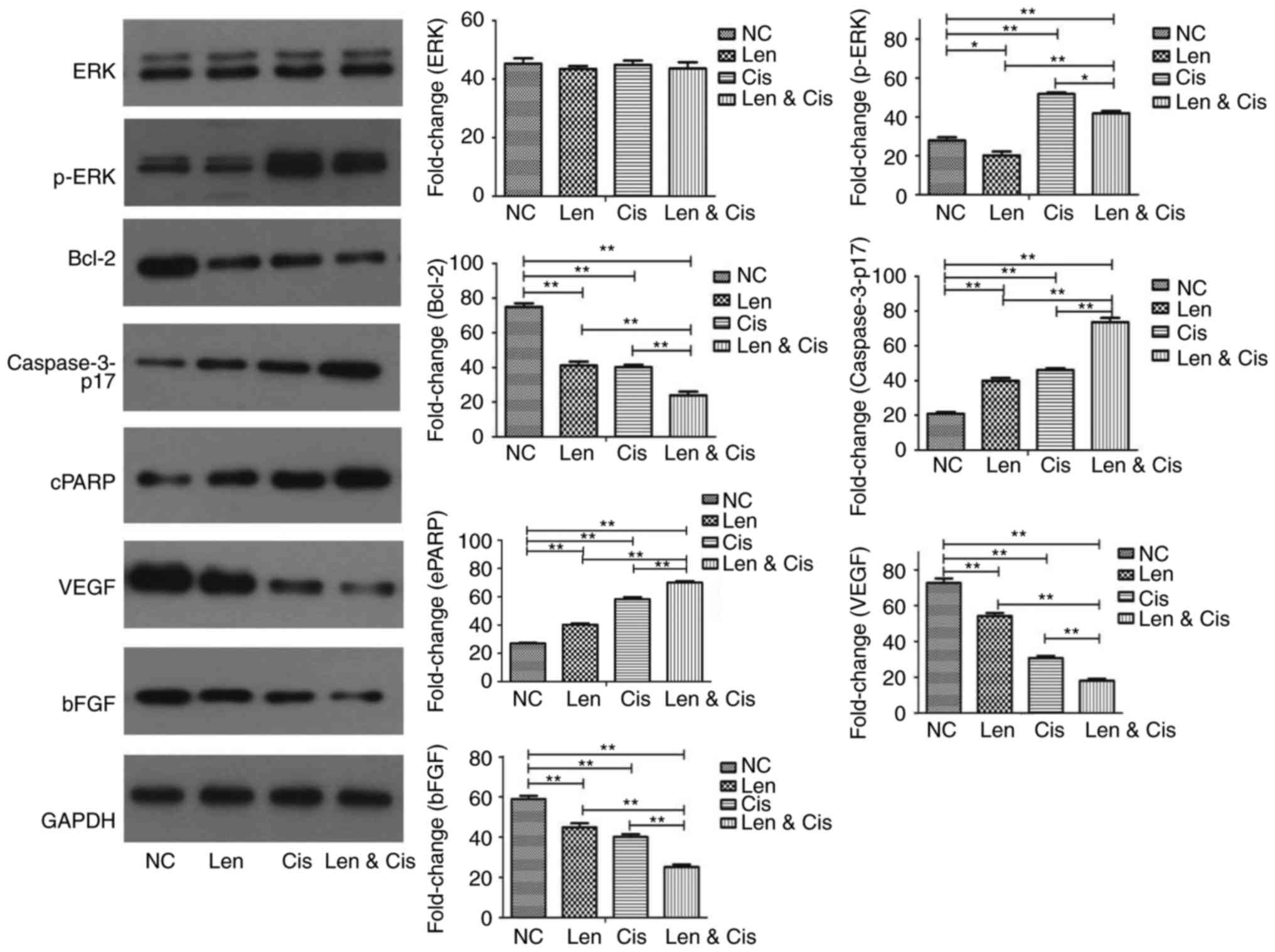
We further assessed the modulation in protein
expression in MDA-MB-231 cells upon treating with l lenalidomide
and cisplatin combination. We found that lenalidomide treatment
alone was able to reduce the level of phosphorylated (p)-ERK, which
is known to regulate cell proliferation, in MDA-MB-231 cells,
whereas p-ERK level was induced by cisplatin treatment alone
(Fig. 3). Their combination
significantly reduced the p-ERK level in MDA-MB-231 cells compared
to cisplatin alone. Furthermore, lenalidomide and cisplatin alone
downregulated the expression of anti-apoptotic protein Bcl-2 but
upregulated expression of pro-apoptotic protein caspase-3 and PARP
in MDA-MB-231 cells (Fig. 3).
However, the combination further inhibited Bcl-2 expression and
upregulated caspase-3 and cleaved PARP expression compared to
single treatment (Fig. 3).
Lenalidomide plays a key role in tumor progression
through the inhibition of angiogenesis. VEGF and bFGF are the most
potent mediators in angiogenesis in human cells. We, therefore,
assessed their levels in MDA-MB-231 cells after treating with
lenalidomide, cisplatin or combination. Our data showed that
lenalidomide or cisplatin alone was able to reduce the levels of
VEGF and bFGF proteins in MDA-MB-231 cells, which were further
reduced with the combination (Fig.
3).
Discussion
To date, surgery, chemotherapy, and radiotherapy are
the main therapeutic strategies in TNBC. However, a considerable
number of TNBC patients are diagnosed at advanced stages of the
disease, leading to curable surgery inaccessible because TNBC
occurs more often in young women and is prone to early metastasis.
Thus, present selections for TNBC treatment are limited and novel
therapeutic strategies could help medical oncologists to cure TNBC
or improve and prolong survival rates in TNBC patients. The present
study assessed the in vitro antitumor activity of
lenalidomide and cisplatin combination in TNBC cells. Our data
showed that cisplatin alone showed inhibitory effect on MDA-MB-231
cell viability, whereas lenalidomide alone had only minimal effect,
even at very high concentrations that are far beyond the clinically
achievable dose in humans (16).
Moreover, cell apoptosis was significantly induced in MDA-MB-231
cells upon treating with lenalidomide and cisplatin combination.
Due to the potential significance of lenalidomide and cisplatin
combination in MDA-MB-231, it was also necessary to explore the
mechanism of their action in TNBC cells. We found that the
combination dramatically reduced the levels of p-ERK, VEGF, bFGF
and Bcl-2 proteins (P<0.05), and upregulated caspase-3 activity
and cleaved PARP expression. This study, therefore, provides
preliminary data to support lenalidomide combination with cisplatin
in TNBC treatment.
As a single agent, cisplatin is one of the common
chemotherapeutic drugs used in various human cancers, especially
TNBC (18) and has been shown to
inhibit tumor cell proliferation. In the present study, cisplatin
significantly reduced MDA-MB-231 cell viability at an
IC50 value of 7.8 µM. On the other hand, it is
controversy and debatable whether lenalidomide can be used to
control solid tumors (14,16), although lenalidomide significantly
inhibited growth of the chronic lymphocytic leukemia cells
(19). In the present study, we
further confirmed that lenalidomide alone had only a minimal effect
on MDA-MB-231 cell viability, even at a very high dose of 320 µM,
far beyond the clinically achievable concentration in humans
(20). Thus, it is suggested that
lenalidomide alone does not have much anti-proliferative activity
in solid tumors, especially in TNBC cells. However, the combination
of cisplatin with lenalidomide (at 1 µM) showed a synergetic
inhibitory effect on MDA-MB-231 cells as compared to cisplatin
alone. Furthermore, cisplatin IC50 value was reduced
from 7.8 µM to 3 µM when combined with 1 µM lenalidomide. To
achieve the same inhibitory effect on MDA-MB-231 cell, cisplatin
dose was significantly reduced when combined with lenalidomide and,
thus, cisplatin side effects were also reduced.
Furthermore, we examined the ability of cisplatin,
lenalidomide, and their combination in the induction of MDA-MB-231
cell apoptosis in vitro. Czarnomysy et al (21) reported that cisplatin at
concentrations 25, 50 and 100 µM was found to induce apoptosis of
MDA-MB-231 cells after 24 h incubation. In the present study, we
investigated whether cisplatin at a lower concentration 3 µM could
induce MDA-MB-231 cell apoptosis. It is supportive that
lenalidomide at the 1 µM dose was also able to induce apoptosis of
MAD-MB-231 cells or other cancer cell lines (16,22).
However, to date, there was no study reporting the effect of
combined lenalidomide with cisplatin on TNBC cells. In the present
study, we investigated the significant increase in apoptosis after
combined treatment, compared with cisplatin and lenalidomide alone.
However, further investigation using different TNBC cell lines is
required to confirm our current data.
In addition, the present study also illustrated the
underlying molecular events on lenalidomide and cisplatin
modulation of different cell growth, apoptosis and
angiogenesis-related proteins. For example, the ERK1/2 pathway is
the classic cell growth signaling of the mitogen-activated protein
kinase (MAPK) pathway. Activated ERK1/2 will promote cell survival
and proliferation by phosphorylating many nuclear transcription
factors (23). The present study
showed that lenalidomide alone or in combination with cisplatin
could reduce p-ERK levels in TNBC cells, although cisplatin alone
induced p-ERK level. Indeed, Fryer et al (24) also reported that lenalidomide
significantly reduced level of p-ERK protein in pancreatic cancer
cell lines, while Chen et al (25) demonstrated that cisplatin had no
effect on p-ERK protein expression in hepatocellular carcinoma
cells. Furthermore, it is well established that a decrease in cell
apoptosis could contribute to human carcinogenesis (26). The B-cell/leukemia-2 (Bcl-2), cleaved
PARP and caspase-3 proteins were reported as the key proteins in
regulating cell apoptosis. Decreased Bcl-2 or increased cleaved
PARP and caspase-3 will promote cell apoptosis (27). In the present study, we found that
lenalidomide and cisplatin induces caspases-3 activity and cleaved
PARP expression, while reduces Bcl-2 expression. Our findings are
consistent with a previous study (28). However, this study does have some
limitations; for example, we only analyzed the expression of
caspase-3 and cleaved poly-adenosine diphosphate-ribose polymerase
(cPARP) and whether this combination may affect other
apoptosis-related genes is not clear and needs further analysis. In
addition, inhibition of tumor angiogenesis was one of the main
mechanisms by which lenalidomide mediate its effect in multiple
myeloma (7). Lenalidomide is a potent
inhibitor of VEGF and bFGF (29),
which is confirmed by the present study. VEGF and bFGF are the core
factors in promoting tumor angiogenesis.
In conclusion, the combination of lenalidomide and
cisplatin demonstrated a synergetic antitumor effect on MDA-MB-231
cells in vitro. This effect was at least partially due to
induction of tumor cell apoptosis. Moreover, the inhibition of VEGF
and bFGF by lenalidomide could partially contribute to the
synergetic effect of lenalidomide and cisplatin combination,
suggesting the potential strategy of lenalidomide and cisplatin
combination in TNBC treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and XWW conceived and designed the experiments.
LLY, XMW and QHL performed the experiments. LLY and XMW analyzed
the data. LLY and XWW wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
References
|
1
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ: Panel members:
Personalizing the treatment of women with early breast cancer:
Highlights of the St Gallen international expert consensus on the
primary therapy of early breast cancer 2013. Ann Oncol.
24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carlson RW, Allred DC, Anderson BO,
Burstein HJ, Carter WB, Edge SB, Erban JK, Farrar WB, Goldstein LJ,
Gradishar WJ, et al: Breast cancer. Clinical practice guidelines in
oncology. J Natl Compr Canc Netw. 7:122–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Romond EH, Perez EA, Bryant J, Suman VJ,
Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman
PA, et al: Trastuzumab plus adjuvant chemotherapy for operable
HER2-positive breast cancer. N Engl J Med. 353:1673–1684. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burstein HJ, Temin S, Anderson H, Buchholz
TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Rowden D, Solky
AJ, et al: Adjuvant endocrine therapy for women with hormone
receptor-positive breast cancer: American society of clinical
oncology clinical practice guideline focused update. J Clin Oncol.
32:2255–2269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan N, Meng M, Liu C, Feng L, Hou L, Ning
Q, Xin G, Pei L, Gu S, Li X and Zhao X: Clinical characteristics
and prognostic analysis of triple-negative breast cancer patients.
Mol Clin Oncol. 2:245–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anders C and Carey LA: Understanding and
treating triple-negative breast cancer. Oncology (Williston Park).
22:1233–1240, 1243. 2008.PubMed/NCBI
|
|
7
|
McCarthy PL, Owzar K, Hofmeister CC, Hurd
DD, Hassoun H, Richardson PG, Giralt S, Stadtmauer EA, Weisdorf DJ,
Vij R, et al: Lenalidomide after stem-cell transplantation for
multiple myeloma. N Engl J Med. 366:1770–1781. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Teo SK: Properties of thalidomide and its
analogues: Implications for anticancer therapy. AAPS J. 7:E14–E19.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu L, Parton A, Lu L, Adams M, Schafer P
and Bartlett JB: Lenalidomide enhances antibody-dependent cellular
cytotoxicity of solid tumor cells in vitro: Influence of host
immune and tumor markers. Cancer Immunol Immunother. 60:61–73.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qu Z, Jiang C, Wu J and Ding Y:
Lenalidomide induces apoptosis and inhibits angiogenesis via
caspase-3 and VEGF in hepatocellular carcinoma cells. Mol Med Rep.
14:4781–4786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weber DM, Chen C, Niesvizky R, Wang M,
Belch A, Stadtmauer EA, Siegel D, Borrello I, Rajkumar SV,
Chanan-Khan AA, et al: Lenalidomide plus dexamethasone for relapsed
multiple myeloma in North America. N Engl J Med. 357:2133–2142.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu L, Liu W, Galustian C, Schafer P,
Dalgleish AG and Bartlett JB: Effect of lenalidomide on the
antiproliferative effect of gemcitabine against pancreatic tumor
cells and on immune-mediated pancreatic cancer cell death. J Clin
Oncol. 27 Suppl 15:e146352009.
|
|
13
|
Said R, Ye Y, Hong DS, Naing A, Falchook
G, Fu S, Wheler JJ, Piha-Paul S and Tsimberidou AM: Phase I
clinical trial of lenalidomide in combination with 5-fluorouracil,
leucovorin, and oxaliplatin in patients with advanced cancer.
Cancer Chemother Pharmacol. 77:575–581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Safran H, Charpentier KP, Kaubisch A,
Mantripragada K, Dubel G, Perez K, Faricy-Anderson K, Miner T, Eng
Y, Victor J, et al: Lenalidomide for second-line treatment of
advanced hepatocellular cancer: A Brown University oncology group
phase II study. Am J Clin Oncol. 38:1–4. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Henry JY, Lu L, Adams M, Meyer B, Bartlett
JB, Dalgleish AG and Galustian C: Lenalidomide enhances the
anti-prostate cancer activity of docetaxel in vitro and in vivo.
Prostate. 72:856–867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brosseau C, Colston K, Dalgleish AG and
Galustian C: The immunomodulatory drug lenalidomide restores a
vitamin D sensitive phenotype to the vitamin D resistant breast
cancer cell line MDA-MB-231 through inhibition of BCL-2: Potential
for breast cancer therapeutics. Apoptosis. 17:164–173. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jovanović B, Mayer IA, Mayer EL, Abramson
VG, Bardia A, Sanders M, Kuba MG, Estrada MV, Beeler JS, Shaver TM,
et al: A randomized phase II neoadjuvant study of cisplatin,
paclitaxel with or without everolimus in patients with stage II/III
triple-negative breast cancer (TNBC): Responses and long-term
outcome correlated with increased frequency of DNA damage response
gene mutations, TNBC subtype, AR status, and Ki67. Clin Cancer Res.
23:4035–4045. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fecteau JF, Corral LG, Ghia EM, Gaidarova
S, Futalan D, Bharati IS, Cathers B, Schwaederlé M, Cui B,
Lopez-Girona A, et al: Lenalidomide inhibits the proliferation of
CLL cells via a cereblon/p21(WAF1/Cip1)-dependent mechanism
independent of functional p53. Blood. 124:1637–1644. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bridoux F, Chen N, Moreau S, Arnulf B,
Moumas E, Abraham J, Desport E, Jaccard A and Fermand JP:
Pharmacokinetics, safety, and efficacy of lenalidomide plus
dexamethasone in patients with multiple myeloma and renal
impairment. Cancer Chemother Pharmacol. 78:173–182. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Czarnomysy R, Surażyński A, Popławska B,
Rysiak E, Pawłowska N, Czajkowska A, Bielawski K and Bielawska A:
Synergistic action of cisplatin and echistatin in MDA-MB-231 breast
cancer cells. Mol Cell Biochem. 427:13–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin Z, Qing K, Ouyang Y, Liu Z, Wang W, Li
X, Xu Z and Li J: Low dose of lenalidmide and PI3K/mTOR inhibitor
trigger synergistic cytoxicity in activated B cell-like subtype of
diffuse large B cell lymphoma. J Exp Clin Cancer Res. 35:522016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Bi T, Wang G, Dai W, Wu G, Qian L,
Gao Q and Shen G: Lupeol inhibits proliferation and induces
apoptosis of human pancreatic cancer PCNA-1 cells through AKT/ERK
pathways. Naunyn Schmiedebergs Arch Pharmacol. 388:295–304. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fryer RA, Barlett B, Galustian C and
Dalgleish AG: Mechanisms underlying gemcitabine resistance in
pancreatic cancer and sensitisation by the iMiD™ lenalidomide.
Anticancer Res. 31:3747–3756. 2011.PubMed/NCBI
|
|
25
|
Chen FS, Cui YZ, Luo RC, Wu J and Zhang H:
Coadministration of sorafenib and cisplatin inhibits proliferation
of hepatocellular carcinoma HepG2 cells in vitro. Nan Fang Yi Ke Da
Xue Xue Bao. 28:1684–1687. 2008.(In Chinese). PubMed/NCBI
|
|
26
|
Bold RJ, Termuhlen PM and McConkey DJ:
Apoptosis, cancer and cancer therapy. Surg Oncol. 6:133–142. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu J, Cai Y, Li M, Zhang Y, Li H and Tan
Z: Oxymatrine promotes S-phase arrest and inhibits cell
proliferation of human breast cancer cells in vitro through
mitochondria-mediated apoptosis. Biol Pharm Bull. 40:1232–1239.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Munugalavadla V, Mariathasan S, Slaga D,
Du C, Berry L, Del Rosario G, Yan Y, Boe M, Sun L, Friedman LS, et
al: The PI3K inhibitor GDC-0941 combines with existing clinical
regimens for superior activity in multiple myeloma. Oncogene.
33:316–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang XW, Ma LM, Zhao XQ and Ruan LH:
Clinical curative efficacy of lenalidomide combined with
chemotherapy for acute leukemia and its impact on VEGF. Zhongguo
Shi Yan Xue Ye Xue Za Zhi. 24:702–706. 2016.(In Chinese).
PubMed/NCBI
|