Introduction
Epigenetic alterations are important in maintaining
genomic stability. DNA methylation is the most common epigenetic
modification, and is involved in various biological processes,
including tumorigenesis, when aberrant DNA methylation occurring in
promoter CpG islands can lead to gain of function in oncogenes and
loss of function in tumor suppressor genes (1–4). The
mechanism by which DNA hypomethylation contributes to tumorigenesis
has been proposed to involve chromosomal instability (5,6),
derepression of imprinted genes (7),
and retrotransposon activation (8,9). Long
interspersed element-1 (LINE-1) is a repetitive retrotransposon and
a major constituent of interspersed DNA repeats. As it constitutes
approximately 17% of the human genome, LINE-1 methylation is used
as a surrogate marker of global DNA methylation (10). LINE-1 hypomethylation has been
observed in several types of cancer, including gastric cancer (GC)
(11–14). However, the mechanism by which LINE-1
methylation is regulated remains undefined.
Ubiquitin-like with PHD and ring-finger protein 1
(UHRF1) is a modular protein containing several functional domains.
The SET and RING finger-associated domain plays an essential role
in DNA methylation, transferring methylation patterns to daughter
cells through the recruitment of DNA methyltransferase 1 (DNMT1) to
newly synthesized strands during DNA replication (9,15–17). UHRF1 is overexpressed in several
cancers (9,18–20).
However, the relationship between LINE-1 hypomethylation and UHRF1
expression has not been reported in GC. Here, we have investigated
LINE-1 methylation status and UHRF1 mRNA expression in GC tissues
compared to matched adjacent normal tissues. In addition, we
explored whether LINE-1 methylation is related with UHRF1
expression as well as clinicopathological features including age,
gender, tumor location, Lauren's histologic classification, tumor
differentiation, tumor stage, and accompanying atrophy and
intestinal metaplasia.
Materials and methods
Ethics statement
The present study protocol was reviewed and approved
by the Institutional Review Board of Chungnam National University
Hospital approved the study protocol (IRB no. 2014-10-031). All
patients enrolled in this study provided their written informed
consent for tissue collection and use.
Patients and tissue samples
GC and matched adjacent normal specimens were
obtained from 95 patients who underwent a gastrectomy at Chungnam
National University Hospital between February 2010 and July 2015.
The mean age of the patients (65 males and 30 females) was
65.4±11.0 (Table I). Adjacent normal
mucosa was obtained at least 5.0 cm from the tumor margin. The
biospecimens and data used for this study were provide by the
Biobank of the Chungnam National University Hospital, a member of
the Korea Biobank Network.
 | Table I.Association between LINE-1 methylation
and clinicopathological features. |
Table I.
Association between LINE-1 methylation
and clinicopathological features.
|
|
| LINE-1 methylation
(%) |
|
|---|
|
|
|
|
|
|---|
| Feature | N (%) | Mean | SEM | P-value |
|---|
| Age, years (mean ±
SD) | 65.4±11.0 |
|
|
|
|
<65 | 40 (52.6) | 69.5 | 1.6 | 0.695 |
|
≥65 | 45 (47.4) | 69.1 | 1.2 |
|
| Sex |
|
|
|
|
|
Male | 65 (68.4) | 68.8 | 1.2 | 0.366 |
|
Female | 30 (31.6) | 70.4 | 1.6 |
|
| Tumor location |
|
|
|
|
|
Upper | 27 (28.4) | 71.6 | 1.6 | 0.056 |
|
Lower | 68 (71.6) | 68.4 | 1.2 |
|
| Background
atrophy |
|
|
|
|
| No | 68 (71.6) | 69.5 | 1.2 | 0.514 |
|
Yes | 27 (28.4) | 68.7 | 1.7 |
|
| Background
intestinal metaplasia |
|
|
|
|
| No | 12 (12.6) | 74.0 | 1.6 | 0.042 |
|
Yes | 83 (87.4) | 68.6 | 1.1 |
|
| Tumor
differentiation |
|
|
|
|
|
Differentiated | 47 (49.5) | 66.7 | 1.6 | 0.034 |
|
Undifferentiated | 48 (50.5) | 71.8 | 0.9 |
|
| Lauren's histologic
classification |
|
|
|
|
|
Intestinal | 67 (70.6) | 67.6 | 1.3 | 0.014 |
|
Diffuse | 14 (14.7) | 72.9 | 1.6 |
|
|
Mixed | 14 (14.7) | 73.7 | 1.6 |
|
| T
classification |
|
|
|
|
|
T1/T2 | 29 (30.5) | 71.1 | 1.6 | 0.290 |
|
T3/T4 | 66 (69.5) | 68.5 | 1.2 |
|
| N
classification |
|
|
|
|
| N0 | 29 (30.5) | 69.9 | 1.5 | 0.796 |
| N1 | 24 (25.3) | 68.2 | 2.3 |
|
| N2 | 17 (17.9) | 68.1 | 2.4 |
|
| N3 | 25 (26.3) | 70.3 | 1.9 |
|
| Tumor stage |
|
|
|
|
| I
(A+B) | 20 (21.1) | 69.0 | 2.0 | 0.111 |
| II
(A+B) | 20 (21.1) | 73.0 | 1.5 |
|
| III
(A+B+C) | 46 (48.4) | 68.0 | 1.6 |
|
| IV | 9 (9.5) | 73.0 | 2.4 |
|
DNA and bisulfite treatment
Genomic DNA (gDNA) was extracted using a DNeasy
Blood & Tissue kit (Qiagen, Valencia, CA, USA) according to the
manufacturer's instructions. DNA quality (A260/280 and A260/230)
was assessed using a Nanodrop 2000 (Thermo Fisher Scientific, Inc.,
Wilmington, DE, USA). The isolated DNA was treated with sodium
bisulfite using an EZ DNA Methylation kit (Zymo Research
Corporation, Irvine, CA, USA) according to the manufacturer's
instructions.
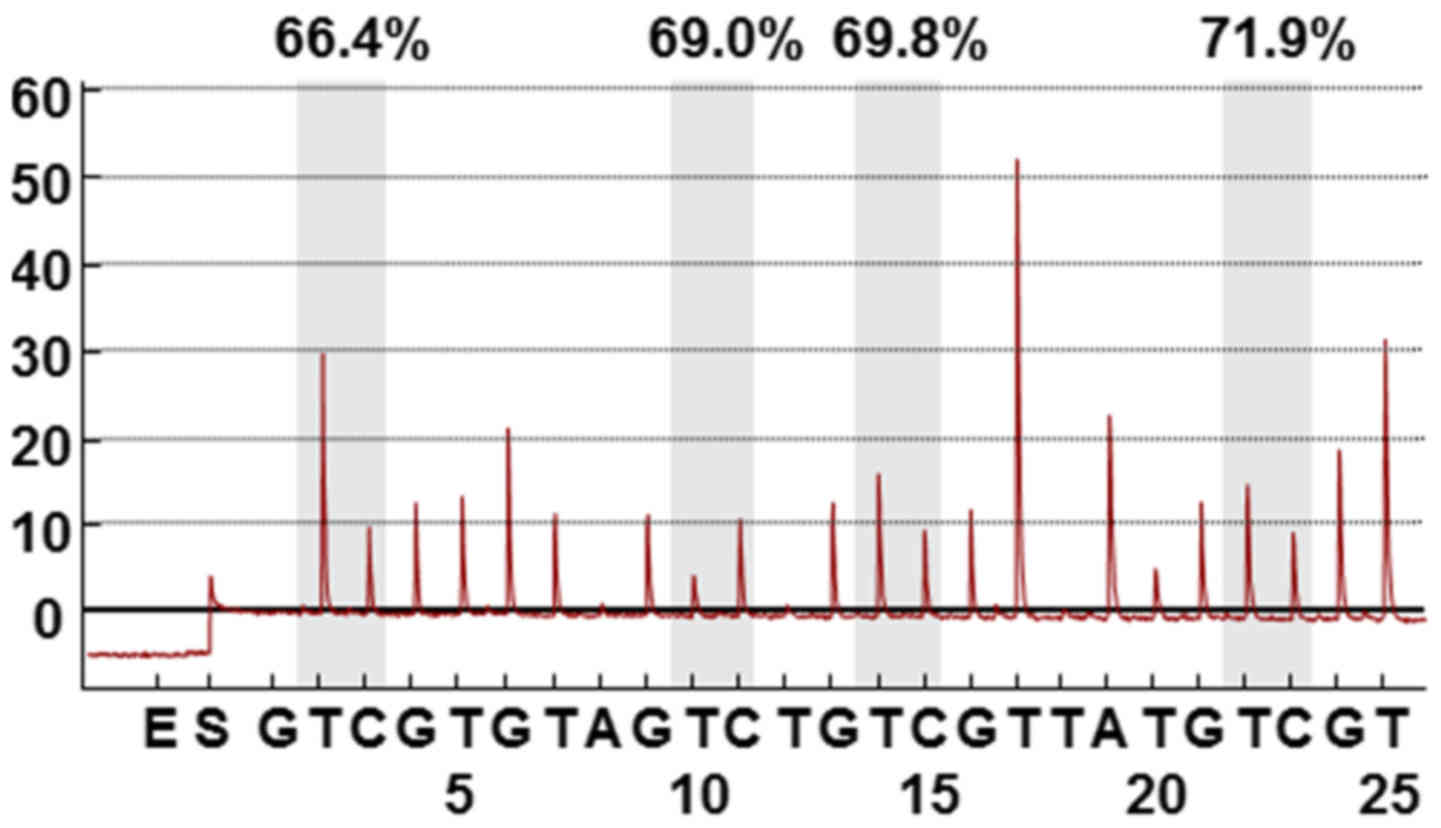
Pyrosequencing
The methylation status of four CpG islands in the
LINE-1 promoter (position 305, 318, 321, and 328 in GenBank
accession no. X58075) was measured by pyrosequencing. A 50 µl
volume PCR was performed using 50 ng of bisulfite-treated gDNA, Taq
DNA polymerase, 50 pmol of the forward
(5′-TTTTGAGTTAGGTGTGGGATATA-3′) and reverse
(5′-biotin-AAAATCAAAAAATTCCCTTTC-3′) primers, and 0.3 µM of
pyrosequencing primer (5′-AGTTAGGTGTGGGATATAGT-3′) with a Veriti
Thermal Cycler (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) using the following PCR conditions: 95°C for 10
min; 45 cycles of 95°C for 30 sec, 50°C for 30 sec, 72°C for 30
sec; and 72°C for 5 min. Pyrosequencing was performed using a
PyroMark Gold Q24 reagent kit and the Pyromark ID system (both from
Qiagen, Germantown, MD, USA). Data were analyzed using PyroMark Q24
2.0.6 software (Qiagen). The methylation level was calculated as
the percent of 5-methylated cytosine divided by the total cytosine,
and represents the mean of the four sites.
Reverse transcription-quantitative
polymerase chain reacvtion (RT-qPCR)
Total RNA was isolated with TRIzol (Thermo Fisher
Scientific, Inc.) and cDNA was synthesized using a SuperScript III
First Strand cDNA synthesis kit (Thermo Fisher Scientific, Inc.),
both according to the manufacturer's instructions. PCR
amplification was performed using cDNA, TaqMan Universal Master Mix
II with UNG (Applied Biosystems; Thermo Fisher Scientific, Inc.),
and a TaqMan Gene Expression Assay kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.); the genes assayed were UHRF1
(Hs00273589_m1) and GAPDH (Hs99999905_m1). Amplification
reactions were performed in triplicate with a StepOne Plus system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) using the
following conditions: 2 min at 50°C, 10 min at 95°C, 40 cycles of
15 sec at 95°C and 1 min at 60°C. The relative mRNA expression was
calculated as the difference in quantification cycle (ΔCq) between
the triplicate mean Cq for UHRF1 and the triplicate mean Cq for
GAPDH from the same sample.
Statistical analysis
Data are presented as the mean ± standard error of
the mean (SEM). Two group comparisons were performed using a Paired
Samples t-test. The Wilcoxon test was used to compare LINE-1
methylation or UHRF1 mRNA expression between tumors and adjacent
normal tissues. Mann-Whitney U and Kruskal-Wallis tests were used
to evaluate associations between LINE-1 methylation and
clinicopathological parameters. The Spearman correlation test was
used to evaluate correlation between LINE-1 methylation and UHRF1
mRNA expression in tumor tissues. A Two-sided P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using SPSS (SPSS, Inc.,
Chicago, IL, USA), version 22.0 for Windows.
Results
Correlation between LINE-1 methylation
and UHRF1 expression in GC
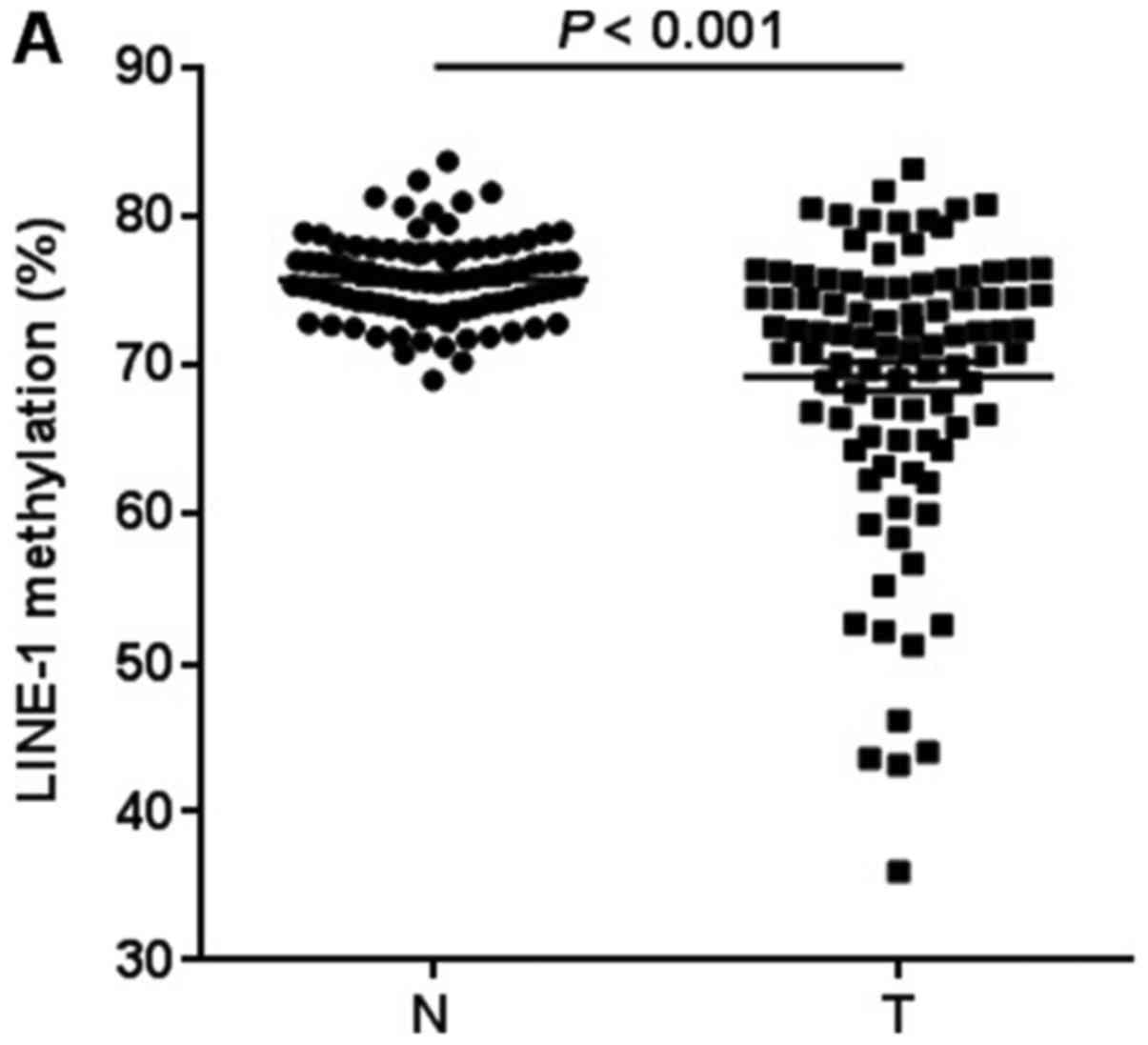
We first quantified LINE-1 methylation in 95 GC
tissues and matched adjacent normal tissues by pyrosequencing
analysis (Fig. 1). LINE-1 was
significantly hypomethylated in GC tissues compared with matched
controls (75.8±0.3 vs. 69.3±1.0%, P<0.001; Fig. 2A). We next measured UHRF1 mRNA
expression in 95 GC tissues and matched adjacent normal tissues by
RT-qPCR analysis. UHRF1 was highly expressed in GC tissues compared
to adjacent normal tissues (P=0.001; Fig.
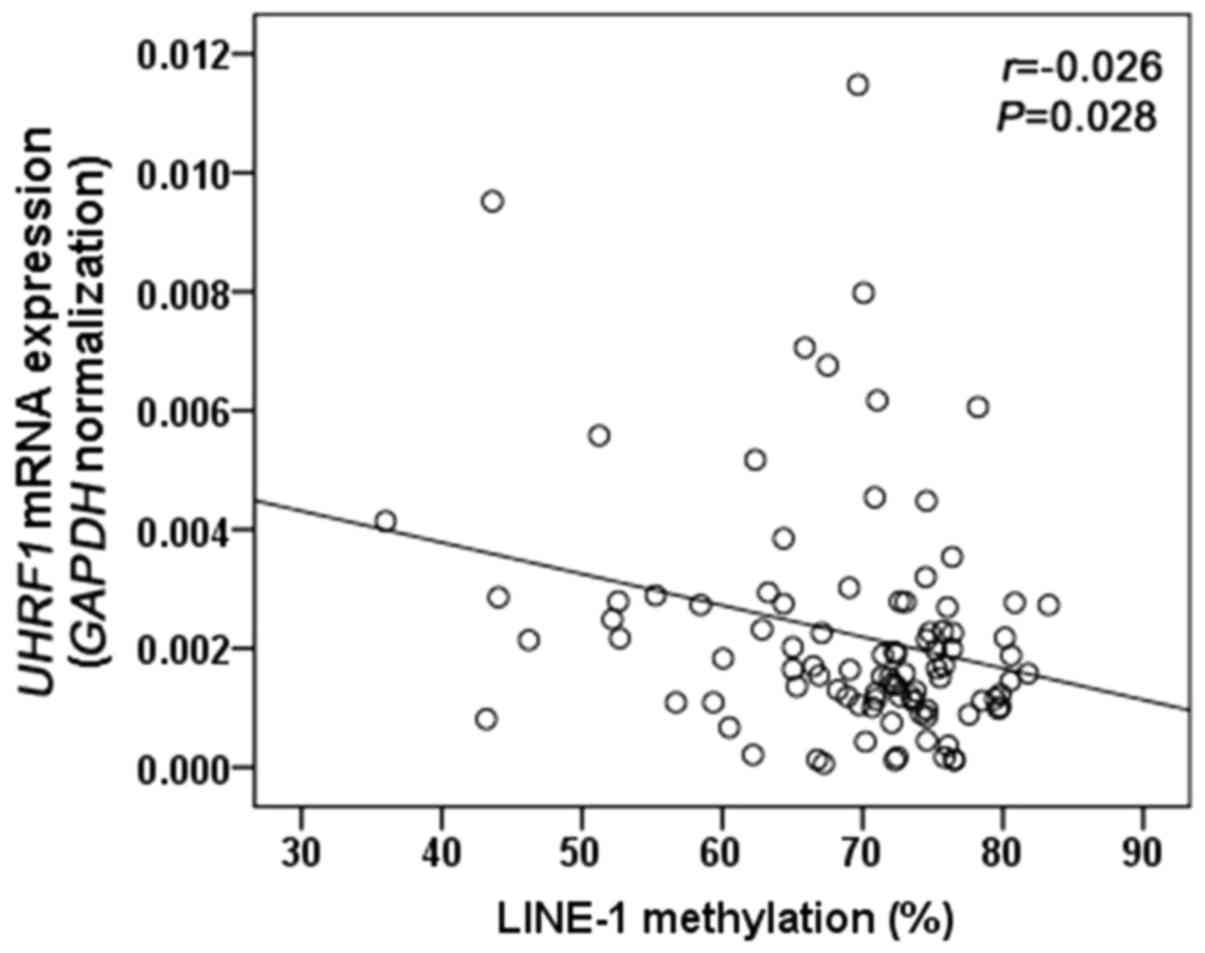
2B). To evaluate whether UHRF1 overexpression is related to
LINE-1 hypomethylation, we analyzed the correlation between UHRF1
mRNA expression and LINE-1 methylation in GC tissues. LINE-1
methylation was inversely correlated with UHRF1 mRNA expression
(Spearman r=−0.026, P=0.028; Fig.
3).
Association between LINE-1 methylation
and clinicopathological features
We also evaluated possible correlations between
LINE-1 methylation and clinicopathological features of the GCs. The
level of LINE-1 methylation was significantly related to the
Lauren's histologic classification, tumor differentiation, and the
presence of background intestinal metaplasia (P=0.014, P=0.042, and
P=0.034, respectively; Table I).
However, there were no significant associations between LINE-1
methylation and other clinicopathological features of GC.
Discussion
Pyrosequencing is a reliable assay to measure LINE-1
methylation (21,22). In this study, we investigated LINE-1
methylation and UHRF1 mRNA expression in GC tissues and matched
controls using pyrosequencing and RT-qPCR, respectively. LINE-1
hypomethylation and UHRF1 overexpression were observed in GC
tissues compared to adjacent normal tissues (Fig. 2A and B).
Several studies have reported LINE-1 hypomethylation
and UHRF1 overexpression in various cancers, including GC (9,11–14,18–20,23,24).
However, there have been no reports demonstrating that LINE-1
hypomethylation is correlated with UHRF1 overexpression in GC.
Here, we show that LINE-1 hypomethylation is inversely correlated
with UHRF1 overexpression, indicating that LINE-1 methylation may
be regulated by UHRF1, which need to be confirmed molecular
mechanism by further studies.
Accumulating evidence suggests that UHRF1 regulates
gene expression through epigenetic mechanisms including DNA
methylation, histone methylation (25), histone deacetylation (26), and possibly histone ubiquitination
(27). A previous study demonstrated
that plasmid-mediated UHRF1 overexpression delocalized and
destabilized zebrafish Dnmt1, causing DNA methylation (18). In addition, they demonstrated that
oncogenic UHRF1 overexpression induces global DNA hypomethylation,
causing hepatocellular carcinoma in zebrafish (18). The relationship between UHRF1
expression and LINE-1 methylation has been investigated in
esophageal cancer (19). Consistent
with our results, UHRF1 mRNA expression was negatively associated
with LINE-1 methylation in esophageal cancer tissues (19); however, the mechanism by which UHRF1
overexpression causes global DNA hypomethylation remains to be
fully elucidated. Mechanisms including DNMT1 delocalization and
destabilization (27), ubiquitination
and degradation (24), and
redistribution and/or sequestration of DNMT1 away from DNA
(28) have been proposed.
We also evaluated the relationships between LINE-1
hypomethylation and clinicopathological features of the GC samples.
Our analysis revealed that LINE-1 hypomethylation of GC was
associated with Lauren's histologic classification, tumor
differentiation, and background intestinal metaplasia. This is
consistent with a previous study that demonstrated that LINE-1
methylation was significantly associated with histologic
differentiation and Lauren's histologic classification (22). Our results showed no correlation with
tumor stage, similar to previous studies (22,29). Some
studies have demonstrated that LINE-1 is a potential prognostic
biomarker (22,29); that is, LINE-1 hypomethylation in GC
is related to unfavorable prognosis. However, in our study, there
was no significant association between LINE-1 hypomethylation and
prognosis (data not shown). The mechanism by which aberrant global
DNA methylation results in poorer prognosis remains unclear.
Several studies have suggested that global DNA methylation is
associated with chromosomal instability and mitotic catastrophe
(5,6,9,18,30).
Large-scale studies are required to confirm LINE-1 methylation
status as a useful prognostic factor. Additionally, we analyzed the
association between UHRF1 gene expression and clinicopathological
features of the GC samples but did not find any relation between
them (data not shown).
GC develops by multistep progression, from chronic
gastritis, atrophic gastritis, intestinal metaplasia, dysplasia, to
GC (22). Previous studies have
evaluated the genomic hypomethylation status at various stages in
gastric tumorigenesis. One study showed that LINE-1 methylation,
measured using pyrosequencing, progressively declined from chronic
gastritis to gastric dysplasia (22).
However, there were no additional changes in LINE-1 methylation
during the progression from dysplasia to cancer (22). Meanwhile, another study based on
combined bisulphite restriction analysis demonstrated that LINE-1
methylation progressively decreased from chronic gastritis to GC
(31). In our study, LINE-1
methylation in GCs with background intestinal metaplasia was
significantly lower than in GCs without background metaplasia.
Meanwhile, the presence or absence of background atrophic gastritis
in GC was not associated with LINE-1 methylation. Helicobacter
pylori infection is closely related to premalignant lesions,
including atrophic gastritis and intestinal metaplasia, in
multistep gastric tumorigenesis. Therefore, the prevalence of H.
pylori infection can influence methylation in premalignant
gastric lesions. It has been suggested that quantitative data
regarding the methylation of specific genes may be useful for
predicting the risk of developing GC (32,33). Taken
together, it can be inferred that LINE-1 may be a useful biomarker
for understanding gastric tumorigenesis and for classifying GC
types according to pathogenesis.
There are limitations in this study. First, in
vitro study are required to elucidate the regulatory mechanism
of LINE-1 methylation in GC. Second, the sample size was relatively
small. Finally, we could not investigate the relationship between
LINE-1 methylation and H. pylori infection status, owing to
the lack of data.
In conclusion, our results suggest that LINE-1
hypomethylation, which may be regulated by UHRF1 overexpression,
may be a useful biomarker for GC. Further studies are needed to
better clarify how UHRF1 regulates LINE-1 methylation and whether
the mutation in UHRF1 gene and other methylation regulators affect
LINE-1 methylation levels in GC.
Acknowledgements
Not applicable.
Funding
This study was supported by Chungnam National
University Hospital Research fund in 2014, by Chungnam National
University research fund, by National Research Foundation of Korea
(NRF) grant funded by the Korean goverment (MSIP)
(2015R1C1A2A01052150), by the Korea Health Technology R&D
Project through the Korea Health Industry Development Institute
(KHIDI) grant funded by the Ministry of Health & Welfare
(HI14C1063), and by Basic Science Research Program through the
National Research Foundation of Korea (NRF) funded by the Ministry
of Education (NRF-2017R1D1A1B04033515).
Availability of data and materials
All data generated or analyzed during this study are
available from the corresponding author on reasonable request.
Author's contributions
JKS, JHH and EHJ designed the research; SK performed
the experiments; KSS contributed the selection of subjects and
clinical data acquisition; EHJ and JHH performed the data and
statistical analysis; JKS and EHJ contributed to writing and
revision of the manuscript; All authors read and approved final
manuscript.
Ethics approval and consent to
participate
All patients enrolled in this study provided their
written informed consent for tissue collection and use. The
Institutional Review Board of Chungnam National University Hospital
approved the study protocol (IRB No. 2014-10-031).
Consent for publication
All patients provided their written informed consent
for the publication of any associated data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
LINE-1
|
long interspersed element-1
|
|
GC
|
gastric cancer
|
|
UHRF1
|
ubiquitin-like with PHD and
ring-finger protein 1
|
|
DNMT1
|
DNA methyltransferase 1
|
|
gDNA
|
Genomic DNA
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
SD
|
standard deviation
|
|
SEM
|
standard error of the mean
|
References
|
1
|
Taby R and Issa JP: Cancer epigenetics. CA
Cancer J Clin. 60:376–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Portela A and Esteller M: Epigenetic
modifications and human disease. Nat Biotechnol. 28:1057–1068.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ehrlich M: DNA hypomethylation in cancer
cells. Epigenomics. 1:239–259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin Z, Jiang W and Wang L: Biomarkers for
gastric cancer: Progression in early diagnosis and prognosis
(Review). Oncol Lett. 9:1502–1508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eden A, Gaudet F, Waghmare A and Jaenisch
R: Chromosomal instability and tumors promoted by DNA
hypomethylation. Science. 300:4552003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karpf AR and Matsui S: Genetic disruption
of cytosine DNA methyltransferase enzymes induces chromosomal
instability in human cancer cells. Cancer Res. 65:8635–8639. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berdasco M and Esteller M: Aberrant
epigenetic landscape in cancer: How cellular identity goes awry.
Dev Cell. 19:698–711. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Howard G, Eiges R, Gaudet F, Jaenisch R
and Eden A: Activation and transposition of endogenous retroviral
elements in hypomethylation induced tumors in mice. Oncogene.
27:404–408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sharif J, Muto M, Takebayashi S, Suetake
I, Iwamatsu A, Endo TA, Shinga J, Mizutani-Koseki Y, Toyoda T,
Okamura K, et al: The SRA protein Np95 mediates epigenetic
inheritance by recruiting Dnmt1 to methylated DNA. Nature.
450:908–912. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cordaux R and Batzer MA: The impact of
retrotransposons on human genome evolution. Nat Rev Genet.
10:691–703. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yegnasubramanian S, Haffner MC, Zhang Y,
Gurel B, Cornish TC, Wu Z, Irizarry RA, Morgan J, Hicks J, DeWeese
TL, et al: DNA hypomethylation arises later in prostate cancer
progression than CpG island hypermethylation and contributes to
metastatic tumor heterogeneity. Cancer Res. 68:8954–8967. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saito K, Kawakami K, Matsumoto I, Oda M,
Watanabe G and Minamoto T: Long interspersed nuclear element 1
hypomethylation is a marker of poor prognosis in stage IA non-small
cell lung cancer. Clin Cancer Res. 16:2418–2426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baba Y, Huttenhower C, Nosho K, Tanaka N,
Shima K, Hazra A, Schernhammer ES, Hunter DJ, Giovannucci EL, Fuchs
CS, et al: Epigenomic diversity of colorectal cancer indicated by
LINE-1 methylation in a database of 869 tumors. Mol Cancer.
9:1252010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang M, Kim HS and Cho MY: Different
methylation profiles between intestinal and diffuse sporadic
gastric carcinogenesis. Clin Res Hepatol Gastroenterol. 38:613–620.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Avvakumov GV, Walker JR, Xue S, Li Y, Duan
S, Bronner C, Arrowsmith CH and Dhe-Paganon S: Structural basis for
recognition of hemi-methylated DNA by the SRA domain of human
UHRF1. Nature. 455:822–825. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bostick M, Kim JK, Esteve PO, Clark A,
Pradhan S and Jacobsen SE: UHRF1 plays a role in maintaining DNA
methylation in mammalian cells. Science. 317:1760–1764. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hashimoto H, Horton JR, Zhang X, Bostick
M, Jacobsen SE and Cheng X: The SRA domain of UHRF1 flips
5-methylcytosine out of the DNA helix. Nature. 455:826–829. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mudbhary R, Hoshida Y, Chernyavskaya Y,
Jacob V, Villanueva A, Fiel MI, Chen X, Kojima K, Thung S, Bronson
RT, et al: UHRF1 overexpression drives DNA hypomethylation and
hepatocellular carcinoma. Cancer cell. 25:196–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamura K, Baba Y, Kosumi K, Harada K,
Shigaki H, Miyake K, Kiyozumi Y, Ohuchi M, Kurashige J, Ishimoto T,
et al: UHRF1 regulates global DNA hypomethylation and is associated
with poor prognosis in esophageal squamous cell carcinoma.
Oncotarget. 7:57821–57831. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Daskalos A, Oleksiewicz U, Filia A,
Nikolaidis G, Xinarianos G, Gosney JR, Malliri A, Field JK and
Liloglou T: UHRF1-mediated tumor suppressor gene inactivation in
nonsmall cell lung cancer. Cancer. 117:1027–1037. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Irahara N, Nosho K, Baba Y, Shima K,
Lindeman NI, Hazra A, Schernhammer ES, Hunter DJ, Fuchs CS and
Ogino S: Precision of pyrosequencing assay to measure LINE-1
methylation in colon cancer, normal colonic mucosa and peripheral
blood cells. J Mol Diagn. 12:177–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bae JM, Shin SH, Kwon HJ, Park SY, Kook
MC, Kim YW, Cho NY, Kim N, Kim TY, Kim D, et al: ALU and LINE-1
hypomethylations in multistep gastric carcinogenesis and their
prognostic implications. Int J Cancer. 131:1323–1331. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsiung DT, Marsit CJ, Houseman EA, Eddy K,
Furniss CS, McClean MD and Kelsey KT: Global DNA methylation level
in whole blood as a biomarker in head and neck squamous cell
carcinoma. Cancer Epidemiol Biomarkers Prev. 16:108–114. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin W, Leonhardt H and Spada F: Usp7 and
Uhrf1 control ubiquitination and stability of the maintenance DNA
methyltransferase Dnmt1. J Cell Biochem. 112:439–444. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim KB, Son HJ, Choi S, Hahm JY, Jung H,
Baek HJ, Kook H, Hahn Y, Kook H and Seo SB: H3K9 methyltransferase
G9a negatively regulates UHRF1 transcription during leukemia cell
differentiation. Nucleic Acids Res. 43:3509–3523. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Citterio E, Papait R, Nicassio F, Vecchi
M, Gomiero P, Mantovani R, Di Fiore PP and Bonapace IM: Np95 is a
histone-binding protein endowed with ubiquitin ligase activity. Mol
Cell Biol. 24:2526–2535. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Du Z, Song J, Wang Y, Zhao Y, Guda K, Yang
S, Kao HY, Xu Y, Willis J, Markowitz SD, et al: DNMT1 stability is
regulated by proteins coordinating deubiquitination and
acetylation-driven ubiquitination. Sci Signal. 3:ra802010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kobayakawa S, Miike K, Nakao M and Abe K:
Dynamic changes in the epigenomic state and nuclear organization of
differentiating mouse embryonic stem cells. Genes Cells.
12:447–460. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shigaki H, Baba Y, Watanabe M, Murata A,
Iwagami S, Miyake K, Ishimoto T, Iwatsuki M and Baba H: LINE-1
hypomethylation in gastric cancer, detected by bisulfite
pyrosequencing, is associated with poor prognosis. Gastric Cancer.
16:480–487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baba Y, Watanabe M and Baba H: Review of
the alterations in DNA methylation in esophageal squamous cell
carcinoma. Surg Today. 43:1355–1364. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park SY, Yoo EJ, Cho NY, Kim N and Kang
GH: Comparison of CpG island hypermethylation and repetitive DNA
hypomethylation in premalignant stages of gastric cancer,
stratified for Helicobacter pylori infection. J Pathol.
219:410–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakajima T, Maekita T, Oda I, Gotoda T,
Yamamoto S, Umemura S, Ichinose M, Sugimura T, Ushijima T and Saito
D: Higher methylation levels in gastric mucosae significantly
correlate with higher risk of gastric cancers. Cancer Epidemiol
Biomarkers Prev. 15:2317–2321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kaise M, Yamasaki T, Yonezawa J, Miwa J,
Ohta Y and Tajiri H: CpG island hypermethylation of
tumor-suppressor genes in H. pylori-infected non-neoplastic gastric
mucosa is linked with gastric cancer risk. Helicobacter. 13:35–41.
2008. View Article : Google Scholar : PubMed/NCBI
|