Introduction
In the Unites States and China, the incidence and
mortality rates of urothelial carcinoma (UC) increases yearly
(1,2).
UC is a malignant tumor originating from urinary tract epithelium,
and primarily consists of renal pelvis, ureter and bladder cancer
(3,4).
The majority of cases of UC arise in the bladder, although renal
pelvis and ureter cancer account for ~10% of cases of UC (3). The origins and pathogenesis of these
diseases is similar; however, they possess distinct symptoms,
including microscopic and macroscopic painless hematuria and lower
urinary tract symptoms (4). UC has a
high rate of invasion, recurrence and metastasis (5), making it important to detect UC from
voided urine specimens as early as possible.
At present, radiographic testing, urine cytology,
cystoscopy and ureteroscopy are the most common methods for the
diagnosis and follow-up of patients with UC (6,7). A biopsy
performed using cystoscopy or ureteroscopy is the gold standard for
UC diagnosis; however, the procedure is invasive and causes patient
discomfort (8). Cytology is a
non-invasive method with a high specificity; however, its
sensitivity is low, making the early detection of cancer by this
method inefficient (9). Although
ultrasound or computed tomography can identify the mass and
consequential dilatation of the urinary system, the sensitivity of
these techniques depends on the quality of equipment and experience
of the clinician (6). Endoscopy is an
invasive method from which complications may arise, including
hemorrhage and infection (8). Thus,
the use of cytology and these imaging techniques are limited to the
detection of small tumors and low-stage lesions. Therefore, it is
necessary to develop a non-invasive and highly sensitive method for
the detection of UC in its early stages.
Tumor-associated chromosomal aberrations and gene
alterations are the focus of present research into tumor
pathogenesis and progression (10–12).
Previous studies have indicated that the cause of the majority of
urothelial carcinoma cases is associated with chromosomal
aberration (11,13). Aneuploidy and chromosomal aberration
(deletion or amplification), particularly on chromosomes 3, 7, 17
and 9 (the location of cyclin dependent kinase inhibitor 2A
(CDKN2A, also known as p16), are positively associated with tumor
grade (14). Fluorescence in
situ hybridization (FISH) can be used to detect the
aforementioned cytogenetic changes in voided urine samples to
achieve the early diagnosis of UC, which is a method approved by
the US Food and Drug Administration for UC detection (13). At present, this method is widely used
in Europe and the United States for the clinical detection of UC;
however, the focus of the current study is primarily on
abnormalities to chromosomes 3, 7 and 17 (deletion, haploidy or
polyploidy) and the deletion of chromosome 9 (15,16).
Additionally, the present study aimed to assess the role of p16
amplification in the development of urothelial carcinoma and the
association with clinical stage and classification.
The p16 gene, also known as multiple tumor
suppressor 1 and cyclin-dependent kinase-4 (CDK4) inhibitor, is a
multi-tumor suppressor gene associated with certain types of human
malignant tumor (10,17). p16 is located on the short arm of
human chromosomal 9 (9p21), is 8.5 kb in length and is comprises 3
exons and 2 introns. The protein encoded by p16 has a molecular
weight of 16 kDa and is composed of 148 amino acids. As an
inhibitor of CDK4, p16 is a negative regulator of cell
proliferation and a tumor suppressor gene that is directly involved
in cell cycle progression (17). The
previous diagnostic criteria only assessed the heterozygous and
homozygous deletion of p16 without considering p16 amplification
(13,16); however, it was identified that that
p16 amplification is a frequent event in ongoing surceillance by
our research group, making it necessary to investigate the
diagnostic value of detecting p16 amplification in combination with
p16 heterozygous and homozygous deletion. The present study aimed
to detect p16 aberrations combining the aforementioned targets (p16
amplification, heterozygous and homozygous deletion) by FISH assay
and to evaluate the clinical significance of genetic alteration to
p16, particularly polyploidy, in the diagnosis of UC.
Materials and methods
Patients and samples
The study was conducted at the Department of Urology
and Pathology, The First Affiliated Hospital, Sun Yat-sen
University (Guangzhou, China) between November 2009 and March 2017.
Voided urine specimens from 77 patients (45 males and 32 females;
mean age, 64.2 years; age range, 28–86 years) were collected in the
morning for analysis using FISH assays. The patients recruited were
those with urinary symptoms such as hematuria and lower urinary
tract symptoms, but the most common methods mentioned above
(radiographic testing, urine cytology, cystoscopy and ureteroscopy)
did not distinguish between benign or malignant cells. Those who
were diagnosed or treated in the past were excluded. The cohort
consisted of 65 samples from patients with urothelial carcinoma (40
males and 25 females; mean age, 64.8 years; age range, 28–86 years)
and 12 samples from patients with urinary benign disease as control
group (5 males and 7 females; mean age, 61.1 years; age range,
34–74 years; 3 with prostatic hyperplasia, 3 with cyst and 6 with
nephrotic syndrome or nephritis). Tumors were localized to the
renal pelvis (37 cases), ureter (15 cases) and bladder (13
cases).
All patients were diagnosed whether they were UC by
using pathology as the gold standard using specimens obtained via
surgical resection. In hematoxylin and eosin (H&E) staining,
the tissues were first fixed in 10% formalin for 30 min at room
temperature. Then, paraffin-embedded slides were obtained after a
series of steps including dehydration (50%, 70%, 80%, 95%, 100%,
100% alcohol, each concentration for 30 min), paraffin embedding
and sectioning. The slides were stained in hematoxylin for 5 min
and eosin for 1 min at room temperature.
The present study was approved by the Medical Ethics
Committee of Sun Yat-sen University (Guangzhou, Guangdong, China).
Written informed consent was obtained from all patients prior to
enrollment.
FISH assay
Urine samples were collected in the morning (at
least 200 ml) for FISH analysis. The voided urine samples were
centrifuged at 2,582 × g for 5 min at room temperature and
incubated in a hypotonic solution (0.075 M KCl) at room temperature
for 20 min. The cells were fixed with mixed solution (methanol:
Acetic acid, 2:1) twice at room temperature, each for 10 min. A
total of 4 slides were produced from the re-suspended cells of each
patient and were stored until use for subsequent FISH assays.
The specific probe kit (9p21, p16 probe; cat. no.
F01008-02) used in the present study was purchased from Beijing GP
Medical Technologies, Ltd., Beijing, China. White blood cells
(WBCs) from healthy participants were utilized as controls. A probe
targeting centromere and p16 was used to ensure that the
experimental method and procedure were correct. Absence of signal
was likely to due to deletion of p16 rather than a hybridization
technical failure. According to the kit protocol, slides were
washed with 2X saline sodium citrate (SSC) and then dehydrated in a
series of ethanol washes (70, 85 and 100% for 2 min each at room
temperature). A 10-µl probe mixture was then added to each slide
and samples were denatured at 75°C for 5 min, followed by 42°C
overnight in a Denaturation & Hybridization System (StatSpin
Thermobrite, USA). Next, the slides were treated with 2X SSC for 20
min and 2X SSC/0.1% NP-40 for 5 min at 42°C. DAPI was used for
counterstaining. The fluorescent hybridization signal was observed
under a fluorescence microscope (low magnification, ×100; high
magnification, ×1,000) and analyzed by a software (VideoTesT-FISH
2.0, VideoTesT).
A uniform field of view was utilized to analyze
atypical epithelial cells with large and irregular nuclei at a low
magnification (×100). Cells that overlapped, were incomplete, had
large quantities of cytoplasm or produced weak hybridization
signals were excluded. Close intracellular signals, whose distance
was <2 hybridization points apart, were considered one signal.
Those cells with 2 signals were judged as normal cells without p16
deletion or amplification. In addition, cells that produced ≥3
signals were deemed to demonstrate triploidy or polyploidy.
Besides, cells that produced only 1 or 0 signals were considered to
exhibit heterozygous or homozygous deletions, respectively. If the
number of aberrant cells exceeded 10% of the total cell number, the
specimen was considered to have a positive FISH result. A total of
100 nuclei were studied per slide. The results of amplification,
heterozygous and homozygous deletion were combined together to
diagnose UC. If any aberration occurred in these samples, the FISH
result was considered positive. The absence of aberration was
indicative of a negative FISH result.
Statistical analysis
The significance of intergroup differences were
calculated using the χ2 test. The rate of p16
aberrations between UC and control groups was analyzed and the
association between FISH result and tumor stage, grade
(Tumor-Node-Metastasis classification by World Health
Organization/International Society of Urology Pathologists, 2004)
(18) and location was assessed. To
evaluate the auxiliary diagnostic value of p16 in UC, a receiver
operating characteristic (ROC) curve was performed to compare the
diagnostic power of p16 deletion, p16 amplification alone and the
two in combination. The ROC area under the curve (AUC) reflects the
diagnostic value of different methods. The larger the AUC is, the
more valuable the test method shows. The ROC curves were created by
3 groups of data (p16 deletion, p16 amplification and the
combination of the two). Statistical analysis was performed using
SPSS software, version 20.0 (IBM Corp., Armonk, NY, USA). For all
statistical tests, P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of p16 aberrations in
patients with UC
In the present study, 65 patients with UC and 12
patients with benign disease were analyzed. The results of FISH
clinical data analysis for all patients with UC are presented in in
Table I. According to the
pathological Tumor-Node-Metastasis classification (19) of renal pelvis, ureter and bladder
carcinoma, the 65 resected tumors were classified as
non-muscle-invasive (pTa, pT1) in 23 cases (35.4%) and
muscle-invasive (pT2-4) in 42 cases (64.6%). The tumor grade was
low in 18 cases (27.7%) and high in 47 cases (72.3%). Epithelioid
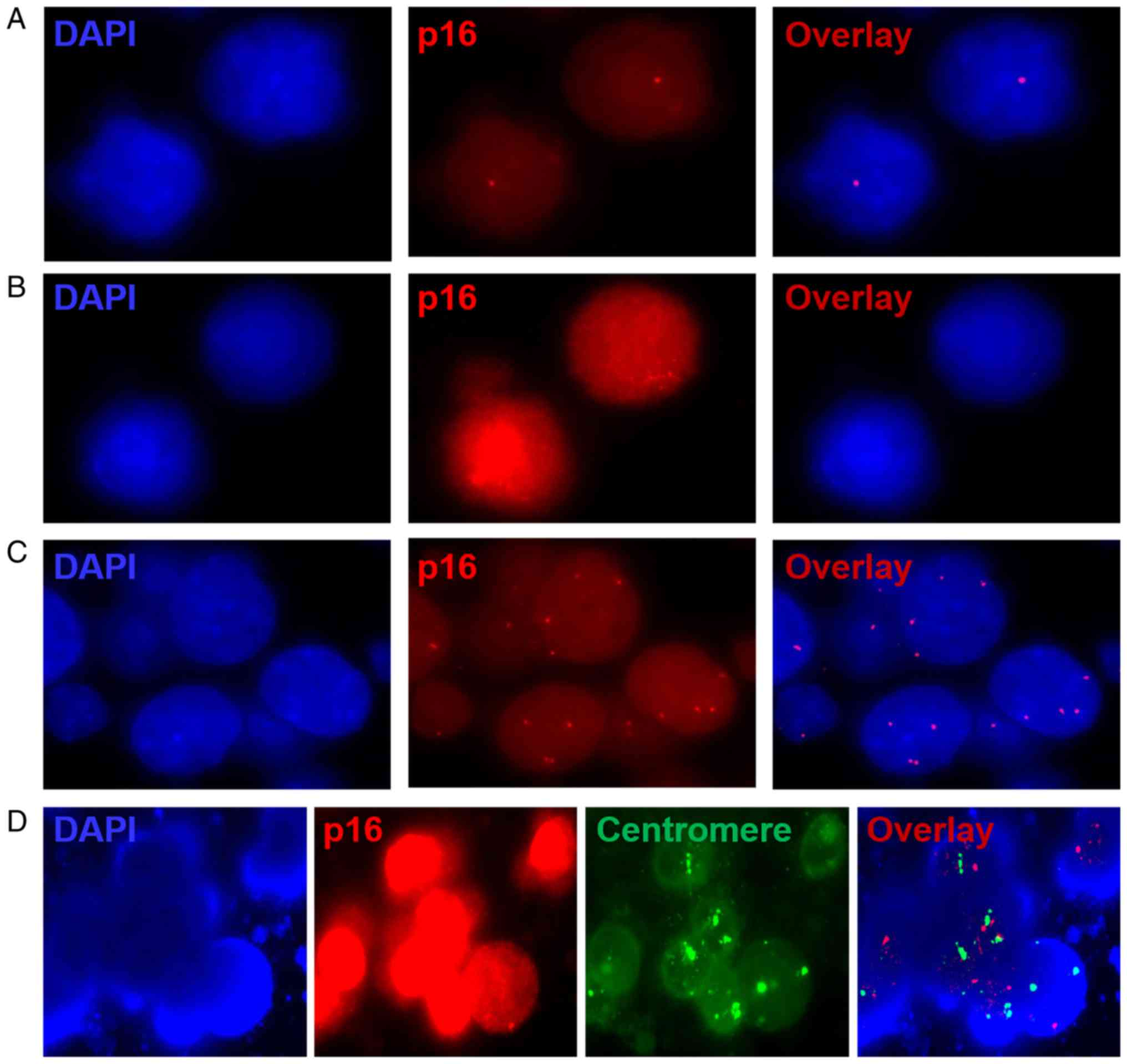
cells with 2 red signals in their nuclei were confirmed as normal.
In addition, p16 heterozygous deletions, homozygous deletions and
amplification polyploidy was detected with 1, 0 and >2 red
signals, respectively. Furthermore, the control probe targeting the
centromeres in WBCs demonstrated that the experimental method and
procedure were correct; the green signals indicated centromeres in
WBCs (Fig. 1).
 | Table I.FISH results (p16 amplification) for
patients with histologically diagnosed urothelial carcinoma. |
Table I.
FISH results (p16 amplification) for
patients with histologically diagnosed urothelial carcinoma.
| No. | Sex | Age | Tumor stage | Tumor grade | Location | FISH results | Type of
aberration |
|---|
| 1 | F | 69 | pT2 | High | Renal pelvis | Positive | Amplification |
| 2 | M | 75 | pT2 | High | Ureter | Positive | Homozygous |
| 3 | M | 46 | pT2 | High | Renal pelvis | Positive | Amplification |
| 4 | M | 67 | pT2 | High | Renal pelvis | Positive | Heterozygous |
| 5 | M | 79 | pT1 | High | Bladder | Positive | Homozygous |
| 6 | M | 49 | pT3 | High | Renal pelvis | Positive | Homozygous |
| 7 | M | 71 | pT1 | Low | Bladder | Negative | – |
| 8 | M | 45 | pT2 | High | Renal pelvis | Positive | Amplification |
| 9 | F | 74 | pT1 | Low | Renal pelvis | Positive | Homozygous |
| 10 | M | 68 | pT1 | Low | Bladder | Positive | Homozygous |
| 11 | F | 59 | pT2 | Low | Renal pelvis | Positive | Homozygous |
| 12 | M | 31 | pT1 | Low | Bladder | Negative | – |
| 13 | M | 61 | pT3 | High | Renal pelvis | Positive | Homozygous |
| 14 | F | 67 | pT1 | Low | Renal pelvis | Negative | – |
| 15 | M | 78 | pT3 | High | Ureter | Positive | Homozygous |
| 16 | M | 58 | pT4 | High | Ureter | Positive | Homozygous |
| 17 | M | 76 | pT1 | High | Ureter | Positive | Homozygous |
| 18 | F | 73 | pT1 | Low | Bladder | Negative | – |
| 19 | M | 77 | pT1 | High | Renal pelvis | Positive | Homozygous |
| 20 | M | 66 | pT2 | High | Renal pelvis | Positive | Homozygous |
| 21 | F | 72 | pT1 | Low | Renal pelvis | Positive | Amplification |
| 22 | F | 56 | pT3 | High | Ureter | Positive | Amplification |
| 23 | M | 74 | pT1 | Low | Ureter | Negative | – |
| 24 | M | 72 | pT1 | Low | Bladder | Negative | – |
| 25 | M | 74 | pT2 | Low | Renal pelvis | Positive | Heterozygous |
| 26 | M | 43 | pT2 | High | Renal pelvis | Positive | Homozygous |
| 27 | F | 86 | pT2 | High | Renal pelvis | Positive | Amplification |
| 28 | M | 67 | pT2 | High | Ureter | Positive | Amplification |
| 29 | M | 59 | pT1 | High | Renal pelvis | Positive | Homozygous |
| 30 | M | 76 | pT1 | High | Bladder | Positive | Amplification |
| 31 | M | 71 | pT2 | High | Ureter | Positive | Homozygous |
| 32 | M | 67 | pT1 | Low | Bladder | Negative | – |
| 33 | F | 74 | pT1 | High | Ureter | Positive | Heterozygous |
| 34 | F | 68 | pT2 | High | Renal pelvis | Positive | Amplification |
| 35 | M | 60 | pT2 | High | Renal pelvis | Positive | Amplification |
| 36 | M | 53 | pT1 | Low | Bladder | Positive | Amplification |
| 37 | F | 70 | pT2 | High | Ureter | Negative | – |
| 38 | F | 67 | pT2 | High | Renal pelvis | Positive | Amplification |
| 39 | M | 60 | pT2 | High | Renal pelvis and
ureter | Positive | Heterozygous |
| 40 | M | 77 | pT2 | High | Bladder | Positive | Amplification |
| 41 | F | 59 | pT3 | High | Ureter | Positive | Heterozygous |
| 42 | M | 55 | pT1 | Low | Bladder | Positive | Amplification |
| 43 | F | 49 | pT2 | High | Renal pelvis | Positive | Amplification |
| 44 | F | 74 | pT2 | High | Ureter | Positive | Homozygous |
| 45 | F | 73 | pT3 | High | Renal pelvis | Positive | Heterozygous |
| 46 | F | 62 | pT2 | High | Renal pelvis | Positive | Amplification |
| 47 | M | 50 | pT2 | High | Bladder | Positive | Heterozygous |
| 48 | F | 53 | pT2 | High | Renal pelvis | Positive | Homozygous |
| 49 | F | 82 | pT1 | High | Renal pelvis | Positive | Homozygous |
| 50 | M | 59 | pT1 | Low | Renal pelvis | Positive | Amplification |
| 51 | F | 58 | pT1 | Low | Ureter and
bladder | Negative | – |
| 52 | M | 77 | pT4 | High | Renal pelvis | Positive | Amplification |
| 53 | F | 60 | pT2 | High | Renal pelvis | Positive | Homozygous |
| 54 | M | 79 | pT2 | High | Renal pelvis and
ureter | Positive | Amplification |
| 55 | F | 62 | pT2 | High | Renal pelvis | Positive | Amplification |
| 56 | F | 71 | pT2 | High | Renal pelvis | Positive | Homozygous |
| 57 | M | 64 | pT2 | High | Renal pelvis | Positive | Homozygous |
| 58 | M | 85 | pT2 | High | Bladder | Positive | Heterozygous |
| 59 | M | 51 | pT1 | Low | Renal pelvis | Positive | Amplification |
| 60 | M | 71 | pT3 | High | Ureter | Positive | Heterozygous |
| 61 | F | 68 | pT2 | High | Renal pelvis | Positive | Heterozygous |
| 62 | M | 28 | pT1 | Low | Renal pelvis | Positive | Homozygous |
| 63 | F | 73 | pT2 | High | Ureter | Positive | Homozygous |
| 64 | M | 66 | pT2 | High | Renal pelvis | Positive | Heterozygous |
| 65 | M | 49 | pT3 | High | Renal pelvis | Positive | Heterozygous |
p16 gene amplification was detected in 21/65
patients (32.3%) with UC and in 2 of 12 patients (16.7%) with
benign disease, respectively (Table
II). In addition, p16 heterozygous and homozygous loss was
identified in 12 (18.5%) and 23 cases (35.4%) of UC, respectively.
The expression of p16 was normal in the remaining cases. The
results of amplification, heterozygous loss and homozygous deletion
were combined into a single diagnostic criterion. p16 aberrations
were found in 56 cases of 65 cases with urothelial carcinoma, so
the sensitivity of FISH for UC is 86.2% (56/65). And in 12 patients
with benign disease, 9 patients showed normal signals for FISH
results, so the specificity was 75.0% (9/12). Therefore, the
overall sensitivity and specificity of the FISH analysis using the
p16 probe was 86.2 and 75.0%, respectively.
 | Table II.Fluorescence in situ
hybridization results for patients with urothelial carcinoma and
benign disease. |
Table II.
Fluorescence in situ
hybridization results for patients with urothelial carcinoma and
benign disease.
|
| Chromosomal
aberration |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Type | Heterozygous | Homozygous | Amplification | Normal | Total | P-value |
|---|
| Urothelial
carcinoma | 12 | 23 | 21 | 9 | 65 | <0.05 |
| Benign urinary
disease | 1 | 0 | 2 | 9 | 12 |
|
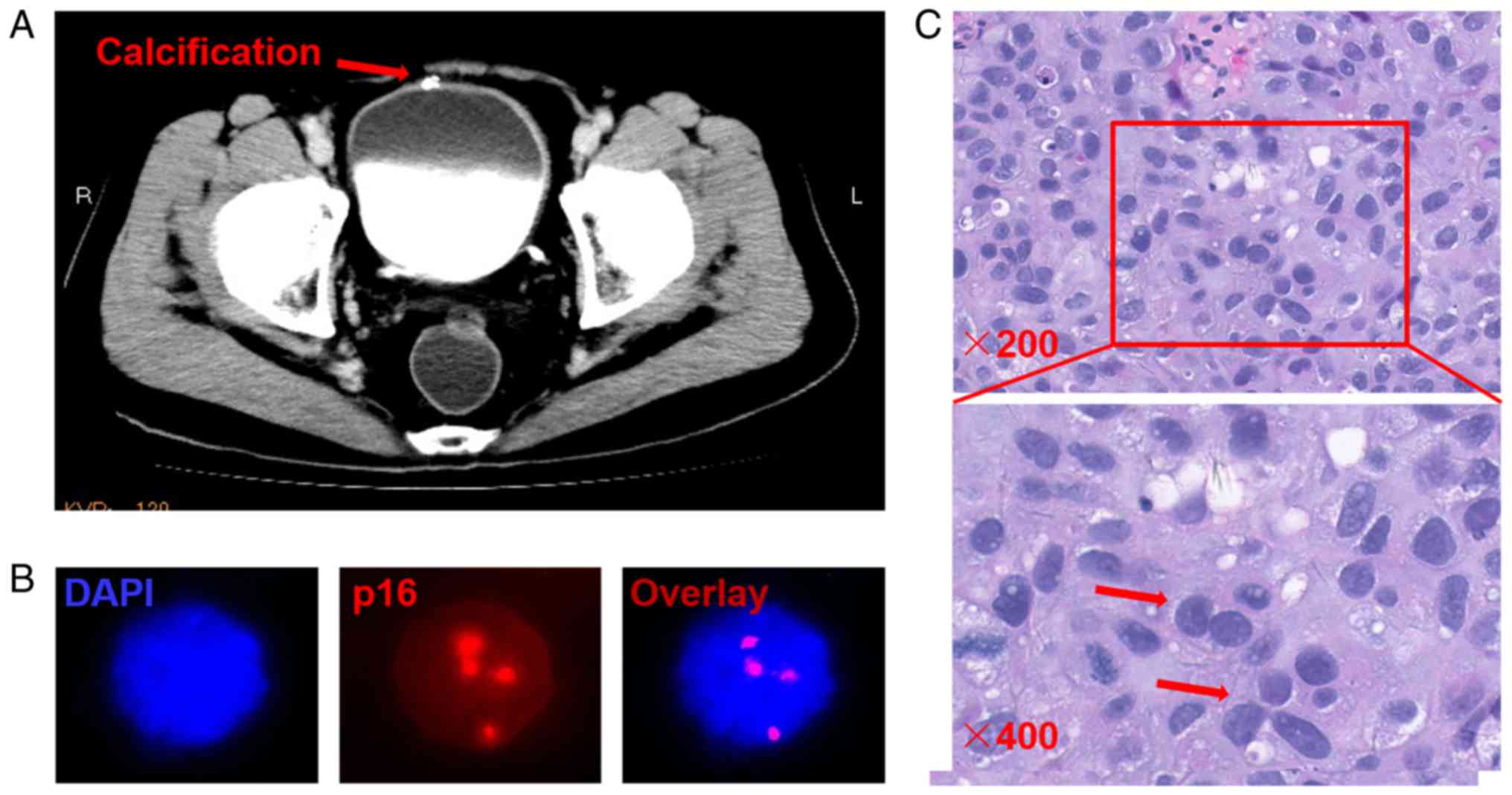
There were several incidences in which the
radiographic testing of patients with UC was normal, but p16
detection was aberrant. For instance, there was a unique case in
which the radiographic testing indicated that the disease was
benign, but FISH results demonstrated an amplification of p16. The
patient was finally diagnosed with a high-grade urothelial
carcinoma by the gold standard H&E staining method (Fig. 2).
Association between p16 FISH status
and clinical pathological parameters
The association between FISH results and
pathological parameters, including stage, grade and tumor location,
are presented in Table III.
According to the aforementioned criterion, FISH positive rates
between different stages, grades and locations were compared. As
the level of stage or grade increased, the positive rate of p16
aberrations increased significantly (P<0.05). Furthermore, the
tumors of 37 cases (56.9%) were located in the renal pelvis, 15 in
the ureter (23.1%) and 13 in the bladder (20.0%). The positive
rates among these locations were also significantly different
(P<0.05). p16 aberrations were found in 15 cases of 23 cases
with pTa-pT1, so the sensitivity of FISH for pTa-pT1 is 65.2%
(15/23). Similarly, we can calculate others' sensitivity with the
same methods. For pTa-pT1: 41/42 (97.6%), for low-grade: 10/18
(55.6%), for high-grade: 46/47(97.9%). In summary, the sensitivity
of FISH for pTa-pT1 and pT2-pT4 tumors was 65.2 and 97.6%,
respectively. The sensitivity of FISH for low- and high-grade
tumors was 55.6 and 97.9%, respectively.
 | Table III.Fluorescence in situ
hybridization results of urothelial carcinoma in various stages and
grades. |
Table III.
Fluorescence in situ
hybridization results of urothelial carcinoma in various stages and
grades.
| Pathological
parameters | Positive | Negative | Total | P-value |
|---|
| Stage |
|
|
| <0.05 |
|
pTa-pT1 | 15 | 8 | 23 |
|
|
pT2-pT4 | 41 | 1 | 42 |
|
| Grade |
|
|
| <0.05 |
|
Low | 11 | 8 | 18 |
|
|
High | 46 | 1 | 47 |
|
| Location |
|
|
| <0.05 |
| Renal
pelvis | 36 | 1 | 37 |
|
|
Ureter | 12 | 3 | 15 |
|
|
Bladder | 8 | 5 | 13 |
|
Diagnostic value of p16
aberrations
The results demonstrated that the AUC values for p16
deletion, p16 amplification and the combination were 0.728, 0.578
and 0.806, respectively. The AUC of groups were compared by SPSS
software (version 20.0) and the difference between the p16 deletion
and combination of deletion and amplification groups was
statistically significant (P<0.05). In addition, the 95%
confidence intervals of their AUCs were 0.594–0.861 and
0.654–0.957, respectively. According to these data, the diagnostic
value of p16 deletion and amplification when combined is higher
than for any indicator alone (Fig.
3).
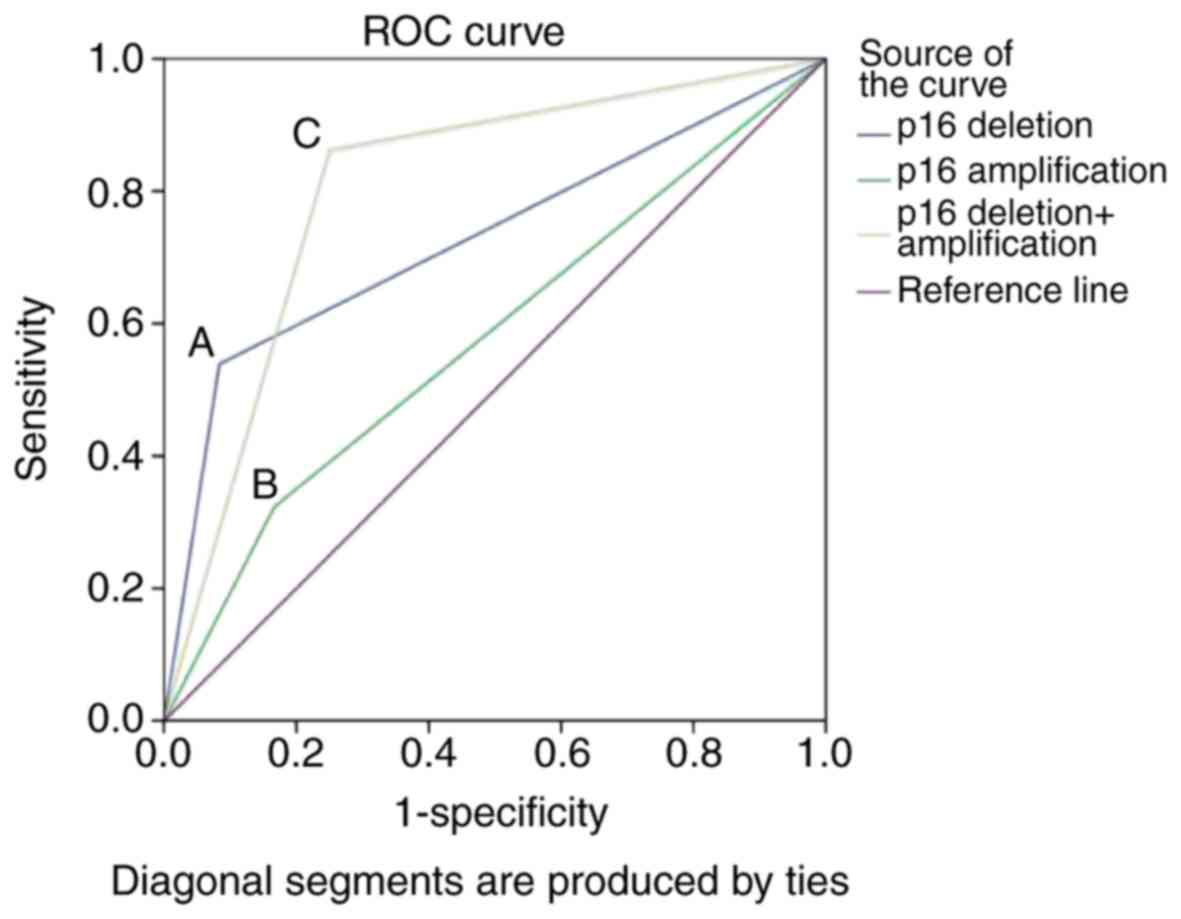 | Figure 3.ROC curve of p16 deletion, p16
amplification and the two combined for the diagnosis of urothelial
carcinoma. A, The sensitivity and specificity of p16 deletion were
53.8 and 91.6%, respectively, with an AUC of 0.728 (95% confidence
interval, 0.594–0.861). B, The sensitivity and specificity of p16
amplification were 32.3 and 83.3%, respectively, with an AUC value
of 0.578 (95% confidence interval, 0.412–0.745). C, p16 deletion in
combination with amplification produced a sensitivity and
specificity of 86.2 and 75.0%, respectively, with an AUC of 0.806
(95% confidence interval, 0.654–0.957). ROC, receiver operating
characteristic; AUC, area under the curve; p16, cyclin dependent
kinase inhibitor 2A. |
Discussion
UC is the most common malignant tumor of the urinary
system. There are ~100,000 cases diagnosed and 30,000 incidences of
mortality due to UC each year in United States alone (2). The high incidence and recurrence rate
associated with the disease make early diagnosis and follow-up
after surgery particularly important (1,2). Previous
studies (6–8) have demonstrated that chromosomes and
their fragments, including chromosomes 3, 7 and 17 with the p16
(9p21) locus, may be associated with certain non-random changes in
the development of UC. Chromosomal aberration is the primary cause
of UC genesis and development; therefore, its detection in UC cells
provides valuable information with regards to clinical diagnosis
and therapeutic action. On the basis of their high sensitivity and
specificity, FISH assays are one of the most common methods used in
molecular genetic research, and are widely used for the study of
chromosome number and structural distortion in UC. Although
chromosomal aberration is identified in UC, chromosomes 3, 7, 9 and
17 are primarily used for its clinical diagnosis (9,15).
Chromosomal aberrations consist of deletion, haploidy and
polyploidy, and the change to 9p21 (p16) is specifically associated
with deletion (13,16); however, studies assessing the role of
p16 gene amplification in UC are limited (20).
The present study utilized the p16 gene as a
specific probe to detect the status of chromosome 9 in voided urine
specimens from 65 cases of UC and 12 cases of benign disease. FISH
assays were performed to evaluate the value of p16 deletion and
polyploidy in the clinical diagnosis and degree of progression of
UC. The positive rate of p16 deletion in UC was 53.8% (18.5%
heterozygous deletion and 35.4% homozygous deletion). Chromosome 9
incorporates a number of tumor suppressor genes, including cyclin
dependent kinase inhibitor 2B, p16 and TSC complex subunit 1, and
its deletion may be an early event in the tumorigenic pathway that
leads to UC development (11,21). In addition, chromosome 9 deletion is
associated with the recurrence and progression of bladder cancer
(22). The heterozygous and
homozygous loss of the p16 locus in UC is detected using FISH
assays and its positive rate in previous studies is ~50% (16,23), which
is similar to the results of the present study.
However, in the current study, p16 gene
amplification accounted for some of the aberrations detected on
chromosome 9, which is not consistent with previous studies
(8,16). P16 polyploidy in UC was 32.3% (21/65),
which was significantly higher than that in urinary benign disease
(16.7%; 2/12; P<0.05). As tumor stage and grade increased, the
positive rate of FISH results also increased. When the tumor stage
was pT2-pT4 or the tumor grade was high, the sensitivity of FISH
assays for diagnosing UC by using a single p16 probe was >95%.
The results indicated that p16 aberrations, including polyploidy,
were closely associated with the classification and stage of UC. In
addition, chromosomal aberrations may be an initial step in UC
development, which may be valuable in the prediction of UC
progression. The results of the current study are also similar to
those of a previous study, which demonstrated the importance of
alterations to chromosomes 3, 7, 17 and p16 in UC (12). In addition, Berggren de Verdier et
al (20) demonstrated that UC
cases with multiple duplications at chromosome 9p21 were associated
with poor survival.
p16 amplification was detected in 32.3% patients
with UC in the present study. This result may be explained by two
theories. Firstly, although p16 is a tumor suppressor gene, its
amplification may only be an intermediate step in the progression
of UC and an unknown regulatory feedback mechanism may exist that
is yet to be elucidated. In addition, as a specific probe that
detects the genetic changes of chromosome 9, p16 may not be a
factor that initiates these alterations. We hypothesized that an
additional potential oncogene, also located on chromosome 9, may
result in cytogenetic aberration, combining the p16 with its probe
and indicating a positive result. The polyploidy of chromosomes 3,
7 and 17 was also previously detected using a FISH assay (23,24). It
was determined that p16 amplification may exert similar biological
behaviors to these genes located in chromosomes 3, 7 and 17.
Secondly, the present study was retrospective and the cases that
were selected may not accurately represent the population of
patients with UC. In the present study, the number of patients with
tumors located in the renal pelvis exceeded 50%, yet renal pelvis
cancer accounts for only 5% of all UC in the normal population
(16). The number cases of ureter and
bladder cancer is limited in the present study and the positive and
negative rates of FISH results in bladder cancer were similar,
which may not reflect the real condition in large sample
population. At the hospital from which samples were collected in
the present study, those that were recommended for FISH assays were
difficult to diagnose using routine methods. The majority of
patients with ureteral or bladder cancer may have been diagnosed by
biopsy under ureteroscopy or cystoscopy, and therefore would not
have undergone FISH-based assays for auxiliary diagnosis.
Consequently, the number of cases of ureter or bladder cancer
available for analysis in the current study was limited. Thus, p16
amplification appears to be significant in UC, particularly in
renal pelvis cancer, although a further prospective study must be
conducted for the verification of the differences observed in the
present study.
Previous studies and clinical practices have
combined use of chromosomes 3, 7 and 17 with the p16 (9p21) locus
to detect UC by FISH assays to improve detection sensitivity
(6,8,23).
However, in the present study, the diagnostic sensitivity of UC by
FISH using a single p16 probe was higher compared with those
studies using a combination of different probes. Furthermore, the
present study demonstrated that p16 amplification was detected in
~35% patients with UC, which may represent a key event in UC
occurrence and development, and provide a novel avenue for future
study. Despite the existence of studies from multiple different
hospitals, no consensus has been reached on the value of FISH
assays in UC diagnosis (8,13,20,25).
Therefore, a multi-center study with a large sample size is
required. Additionally, more cases detecting other loci may also be
required for future study.
In conclusion, the results of the present study
indicate that p16 aberrations, including amplification, may be a
primary event in the development of UC, particularly in renal
pelvis cancer. As a technique for detecting cytogenetic changes of
chromosomes or genes, FISH using p16 as a specific probe serves an
important role in the auxiliary diagnosis and progression of
patients with UC. However, increases to the sample size and
follow-up duration are required in further studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 30900650,
81372501, 81572260, 81773299 and 31430030), the Guangdong Natural
Science Foundation (grant nos. 2011B031800025, S2012010008378,
S2012010008270, S2013010015327, 2013B021800126, 20090171120070,
9451008901002146, 2013B021800126, 2014A030313052, 2014J4100132,
2015A020214010, 2016A020215055 and 2013B021800259), and the
Guangzhou Science and Technology Planning Program (grant no.
201704020094).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author, on reasonable
request.
Author contributions
ZK, LW and XM conceived and designed the study. BL,
YL, SL, JZ, QH, HJ, YCh, YS, YCU, WJ and HW performed the
experiments. XM and BL wrote the paper. ZK reviewed and edited the
manuscript. The final version of the manuscript has been read and
approved by all authors.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Sun Yat-sen University (Guangzhou, Guangdong, China).
Written informed consent was obtained from all patients prior to
enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yaxley JP: Urinary tract cancers: An
overview for general practice. J Family Med Prim Care. 5:533–538.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tyler A: Urothelial cancers: Ureter, renal
pelvis, and bladder. Semin Oncol Nurs. 28:154–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Milojevic B, Dzamic Z, Kajmakovic B,
Petronic Milenkovic D and Grujicic Sipetic S: Urothelial carcinoma:
Recurrence and risk factors. J BUON. 20:391–398. 2015.PubMed/NCBI
|
|
6
|
Ho CC, Tan WP, Pathmanathan R, Tan WK and
Tan HM: Fluorescence-in-situ-hybridization in the surveillance of
urothelial cancers: Can use of cystoscopy or ureteroscopy be
deferred? Asian Pac J Cancer Prev. 14:4057–4059. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mian C, Lodde M, Comploj E, Negri G,
Egarter-Vigl E, Lusuardi L, Palermo S, Marberger M and Pycha A:
Liquid-based cytology as a tool for the performance of uCyt+ and
Urovysion Multicolour-FISH in the detection of urothelial
carcinoma. Cytopathology. 14:338–342. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin T, Liu Z, Liu L, Yang L, Han P, Zhang
P and Wei Q: Prospective evaluation of fluorescence in situ
hybridization for diagnosing urothelial carcinoma. Oncol Lett.
13:3928–3934. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reynolds JP, Voss JS, Kipp BR, Karnes RJ,
Nassar A, Clayton AC, Henry MR, Sebo TJ, Zhang J and Halling KC:
Comparison of urine cytology and fluorescence in situ hybridization
in upper urothelial tract samples. Cancer Cytopathol. 122:459–467.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nobori T, Miura K, Wu DJ, Lois A,
Takabayashi K and Carson DA: Deletions of the cyclin-dependent
kinase-4 inhibitor gene in multiple human cancers. Nature.
368:753–756. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin SL, Chen XJ, Xu X, Shou JZ, Bi XG, Ji
L, Han YL, Cai Y, Wei F, Ma JH, et al: Detection of chromosomal
alterations in bladder transitional cell carcinomas from Northern
China by comparative genomic hybridization. Cancer Lett.
238:230–239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gallucci M, Guadagni F, Marzano R,
Leonardo C, Merola R, Sentinelli S, Ruggeri EM, Cantiani R,
Sperduti I, Lopez Fde L and Cianciulli AM: Status of the p53, p16,
RB1, and HER-2 genes and chromosomes 3, 7, 9, and 17 in advanced
bladder cancer: Correlation with adjacent mucosa and pathological
parameters. J Clin Pathol. 58:367–371. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Halling KC and Kipp BR: Bladder cancer
detection using FISH (UroVysion assay). Adv Anat Pathol.
15:279–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su X, Hao H, Li X, He Z, Gong K, Zhang C,
Cai L, Zhang Q, Yao L, Ding Y, et al: Fluorescence in situ
hybridization status of voided urine predicts invasive and
high-grade upper tract urothelial carcinoma. Oncotarget.
8:26106–26111. 2017.PubMed/NCBI
|
|
15
|
Bubendorf L: Multiprobe fluorescence in
situ hybridization (UroVysion) for the detection of urothelial
carcinoma-FISHing for the right catch. Acta Cytol. 55:113–119.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo B, Li W, Deng CH, Zheng FF, Sun XZ,
Wang DH and Dai YP: Utility of fluorescence in situ hybridization
in the diagnosis of upper urinary tract urothelial carcinoma.
Cancer Genet Cytogenet. 189:93–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kamb A, Gruis NA, Weaver-Feldhaus J, Liu
Q, Harshman K, Tavtigian SV, Stockert E, Day RS III, Johnson BE and
Skolnick MH: A cell cycle regulator potentially involved in genesis
of many tumor types. Science. 264:436–440. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garg M: Epithelial plasticity in
urothelial carcinoma: Current advancements and future challenges.
World J Stem Cells. 8:260–267. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amin MB: Histological variants of
urothelial carcinoma: Diagnostic, therapeutic and prognostic
implications. Mod Pathol. 22 Suppl 2:S96–S118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Verdier Berggren PJ, Kumar R, Adolfsson
J, Larsson P, Norming U, Onelöv E, Wijkström H, Steineck G and
Hemminki K: Prognostic significance of homozygous deletions and
multiple duplications at the CDKN2A (p16INK4a)/ARF (p14ARF) locus
in urinary bladder cancer. Scand J Urol Nephrol. 40:363–369. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ng SK, Casson RJ, Burdon KP and Craig JE:
Chromosome 9p21 primary open-angle glaucoma susceptibility locus: A
review. Clin Exp Ophthalmol. 42:25–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Simoneau M, LaRue HL, Aboulkassim TO,
Meyer F, Moore L and Fradet Y: Chromosome 9 deletions and
recurrence of supercial bladder cancer: Identication of four
regions of prognostic interest. Oncogene. 19:6317–6323. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Wu J, Peng L, Tu P, Li W, Liu L,
Cheng W, Wang X, Zhou S, Shi S, et al: Distinguishing urothelial
carcinoma in the upper urinary tract from benign diseases with
hematuria using FISH. Acta Cytol. 56:533–538. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun JJ, Wu Y, Lu YM, Zhang HZ, Wang T,
Yang XQ, Sun MH and Wang CF: Immunohistochemistry and fluorescence
in situ hybridization can inform the differential diagnosis of
low-grade noninvasive urothelial carcinoma with an inverted growth
pattern and inverted urothelial papilloma. PLoS One.
10:e01335302015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schwarz S, Rechenmacher M, Filbeck T,
Knuechel R, Blaszyk H, Hartmann A and Brockhoff G: Value of
multicolour fluorescence in situ hybridisation (UroVysion) in the
differential diagnosis of flat urothelial lesions. J Clin Pathol.
61:272–277. 2008. View Article : Google Scholar : PubMed/NCBI
|